Abstract
BACKGROUND/OBJECTIVES
The anti-diabetic activity of pear through inhibition of α-glucosidase has been demonstrated. However, little has been reported about the effect of pear on insulin signaling pathway in obesity. The aims of this study are to establish pear pomace 50% ethanol extract (PPE)-induced improvement of insulin sensitivity and characterize its action mechanism in 3T3-L1 cells and high-fat diet (HFD)-fed C57BL/6 mice.
MATERIALS/METHODS
Lipid accumulation, monocyte chemoattractant protein-1 (MCP-1) secretion and glucose uptake were measure in 3T3-L1 cells. Mice were fed HFD (60% kcal from fat) and orally ingested PPE once daily for 8 weeks and body weight, homeostasis model assessment of insulin resistance (HOMA-IR), and serum lipids were measured. The expression of proteins involved in insulin signaling pathway was evaluated by western blot assay in 3T3-L1 cells and adipose tissue of mice.
RESULTS
In 3T3-L1 cells, without affecting cell viability and lipid accumulation, PPE inhibited MCP-1 secretion, improved glucose uptake, and increased protein expression of phosphorylated insulin receptor substrate 1 [p-IRS-1, (Tyr632)], p-Akt, and glucose transporter type 4 (GLUT4). Additionally, in HFD-fed mice, PPE reduced body weight, HOMA-IR, and serum lipids including triglyceride and LDL-cholesterol. Furthermore, in adipose tissue, PPE up-regulated GLUT4 expression and expression ratio of p-IRS-1 (Tyr632)/IRS, whereas, down-regulated p-IRS-1 (Ser307)/IRS.
CONCLUSIONS
Our results collectively show that PPE improves glucose uptake in 3T3-L1 cells and insulin sensitivity in mice fed a HFD through stimulation of the insulin signaling pathway. Furthermore, PPE-induced improvement of insulin sensitivity was not accompanied with lipid accumulation.
Keywords: Insulin sensitivity, glucose transporter, lipid metabolism, adipose tissue, pyrus
INTRODUCTION
Obesity is associated with insulin resistance [1,2], which is defined as a reduction in the ability of insulin to activate its signaling pathway in the main target organs [3,4]. Obesity-caused dysfunction of adipose tissue is strongly associated with increased incidence of type 2 diabetes (T2D) and cardiovascular diseases [5], because adipose tissue plays a critical role in regulation of insulin action, and glucose and lipid metabolism [6,7]. Insulin increases glucose uptake in adipose tissue and muscle, and inhibits glucose output from the liver. Binding of insulin to the receptors on insulin responsive cells, leads to phosphorylation of the tyrosine residues on the insulin receptor substrates (IRSs) by tyrosine kinase. Phosphorylated IRSs (p-IRSs) serve as docking proteins for SH2-containing enzymes, leading to the activation of signaling cascades that result in Akt phosphorylation. The phosphorylation of Akt activates glucose transporter type 4 (GLUT4) and induces glycogen synthesis [8,9]. The activation of glucose transport via GLUT is the rate-limiting step during glucose uptake [10]. GLUT4, predominantly expressed in adipose tissues and muscle, plays a crucial role in glucose homeostasis [11]. The inhibition of GLUT4 expression in adipose tissue results in insulin resistance [12].
The most commonly used drugs show adverse effects and usually high doses are required to improve insulin resistance [13]. Thiazolidinediones (TZDs), a class of peroxisome proliferator-activated receptor (PPAR)-γ agonists for treating T2D, promote adipogenesis and increase insulin sensitivity [14,15]. However, the clinical use of TZDs has several adverse effects such as weight gain, fluid retention, congestive heart failure, and bone fracture [16,17]. Furthermore, TZDs-induced weight gain will deteriorate insulin resistance. Therefore, there is an urgent need for novel effective drugs to regulate adipocyte function, especially to protect obese patients against insulin resistance and other obesity-related diseases. For this reason, research efforts are directed toward development of safe and cost-effective natural alternatives to conventional drugs commonly used to improve insulin sensitivity [18,19].
Pear is consumed as a fresh fruit and fruit products worldwide. However, pomace, the solid residue produced during manufacturing, is discarded, which causes environmental pollutions and economic waste. Pear pomace, which is composed of peels, pulps, stems, cores, and seeds, is known to contain lignocellulose-rich materials [20]. Pear has important dietary components including polyphenols and triterpenes, which exhibit antioxidant and anti-inflammatory actions [21]. Furthermore, polyphenol-rich plant extracts have inhibitory actions on α-glucosidase and α-amylase enzyme activities in T2D [22,23,24]. The anti-diabetic activity of pear peel and pulp extracts through inhibition of α-glucosidase activity has been reported [25].
However, little has been reported about the insulin signaling pathway as an underlying mechanism of pear induced insulin sensitivity improvement. Therefore, further studies are required to establish the positive effects and the underlying mechanisms of action of pear pomace on insulin sensitivity. In this study, we conducted both cell-based assay (in vitro) and animal study (in vivo) with 50% ethanol extract of pear pomace (PPE) to explore the role of pear pomace in improving insulin sensitivity.
MATERIALS AND METHODS
Preparation of extracts from pears
Pears (Pyrus pyrifolia Niitaka) were washed, de-seeded, and then juices were extracted. Pear pomace, the juice extract cake obtained from pears, was freeze-dried and grinded to powder. Powdered pear pomace (100 g) was then extracted with 2 L of 50% ethanol for 24 h at room temperature. The extract was concentrated in a rotary vacuum evaporator, freeze-dried to a power, and then stored in a deep freezer (-70℃). The yield of pear pomace ethanol extract (PPE) obtained was 27.5%.
Cell culture, cell viability and differentiation of 3T3-L1 preadipocytes
Cell culture and assessments for viability and differentiation were performed as described previously [5].
Lipid accumulation, MCP-1 secretion, and glucose uptake in 3T3-L1 cells
Oil Red O (ORO) staining was performed on day 8. To measure the lipid accumulation in 3T3-L1 preadipocytes, cells were fixed with 10% formalin and stained with ORO. ORO dye was extracted by isopropanol to quantify relative TG accumulation [5]. The level of monocyte chemotactic protein-1 (MCP-1) in the supernatants were detected by using ELISA kit (Thermo Fisher Scientific Co., Rockford, IL, USA) according to the manufacture's protocol. The standard curve of MCP-1 was 16-1000 pg/mL. All standards and samples were assayed in triplicate. Cellular glucose uptake was quantified by the 2-[N-(7-notrobenz-2-oxa-1, 3-diazol-4-yl) Amino]-2-Deoxyglucose (2-NBDG, Invitrogen, Carlsbad, CA, USA) assay. 3T3-L1 cells were seeded in 96 well black plates and differentiated into mature adipocytes as reported previously [5]. On day 6, the cells treated with PPE. After 24 h, treatment medium was removed and added with 200 µL 2-NBDG solution for 30 min. Then, cells were washed twice with PBS, the cells were measured with a VICTOR™ Multilabel Counter (PerkinElmer, USA), set at an excitation wavelength of 465 nm and an emission wavelength of 540 nm [26].
Western blot analysis from 3T3-L1 cells
Mature adipocytes were treated with PPE for 24 h. Medium was removed and replaced with DMEM containing 10 nM insulin for 30 min. After washing with PBS, cells were collected in lysis buffer, followed by centrifugation at 13,000 rpm for 20 min to collect the supernatant. Protein concentrations were determined by the Bradford (Sigma, St. Louis, MO, USA) method and equal amounts of protein (25 µg for each) were separated in 10% SDS-PAGE gels and transferred onto a nitrocellulose membrane (Millipore, Billerica, MA, USA). The membrane was blocked for 1 h at 4℃ with 5% nonfat milk in TBS-T buffer [10 mM Tris-HCl (pH 6.8), 100 mM NaCl, and 0.1% Tween 20]. The membranes were then incubated with primary antibodies [p-IRS-1 (Ser307) 1:1000, IRS-1 1:1000, Akt 1:1000, p-Akt (Ser473) 1:1000, and GLUT4 1:1000; Cell Signaling Technology, Beverly, MA, USA; p-IRS-1 (Tyr632) 1:1000 and β-actin 1:1000; Santa Cruz Biotechnology Inc., Dallas, Tex, USA] overnight at 4℃ and then with horseradish peroxidase conjugated secondary antibody [goat anti-rabbit or goat anti-mouse (1:2000), Jackson ImmunoResearch Inc, West Grove, PA, USA] for 1 h. Bands were detected by chemiluminescent substrate (IMGENEX ,San Diego, CA, USA), and the protein expression was quantified using UVP (imaging system for chemiluminescent Western blot, Upland, CA, USA) and Vision Works TMLS (Analysis Software, Upland, CA, USA).
Animals
The study protocol was approved by the Animal Care and Use Committee of Mokpo National University (MNU-IACUC-2014-006). Five week old male C57BL/6 mice were obtained from Central Lab. Animal, Inc. (Seoul, Republic of Korea). All mice were maintained in a climate-controlled room (temperature and relative humidity maintained at 22 ± 2℃, 50 ± 10%, respectively) under 12 h light/dark cycle, and provided with diet and water ad libitum. At 6 weeks of age, the mice were randomly divided into three groups (n = 10/group) and fed a high-fat diet (HFD; 60% kcal fat) for 8 weeks. The mice were administrated with PPE (200 or 400 mg/kg body weight dissolved in distilled water) by oral gavage once per a day. Change in body weight of all mice was monitored weekly. At week 8, blood was collected after 8 h of fasting and serum was separated. Tissues, including liver and abdominal adipose tissue were removed, washed with phosphate-buffered saline (PBS), and weighed. Adipose tissue was snap-frozen immediately in liquid nitrogen and stored at -70℃ for further analysis.
Measurement of blood biochemical parameters
Fasting blood glucose concentration was determined using a commercial kit (Allmedicus, gyeonggi-do, Republic of Korea) at week 8. Fasting serum insulin (Shibayagi Co. Ltd, Gumma Pref, Japan) was also measured using a commercial kit. Serum lipids including triglyceride (TG), total cholesterol (TC), and LDL-cholesterol were determined using an Automated Chemistry Analyzer (Beckman Coulter Inc, Brea, Cal, USA).
Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as blood glucose (mM) * insulin concentration (mU/L)/ 22.5, utilizing serum obtained at the time the animals were euthanized [27,28].
Western blot analysis in mice
Snap frozen adipose tissues were homogenized in ice-cold radioimmune precipitation assay (RIPA) buffer (Thermo Fisher Scientific Co., Rockford, IL, USA) with 1% protease inhibitors cocktail and phosphatase inhibitor. The cell lysates centrifuged at 13,000 rpm for 20 min to collect the supernatant. The method for immunoblotting was same as described in cell-based assay.
Statistical analysis
Statistical analysis was performed by one-way ANOVA followed by post hoc Duncan's multiple comparison test. Data are presented as the mean ± SE for three independently performed experiments. P-value < 0.05 was considered as statistically significant. Statistical analysis of all data was conducted using SPSS version 23.0.
RESULTS
Effects of PPE on the viability and the differentiation of 3T3-L1 preadipocytes
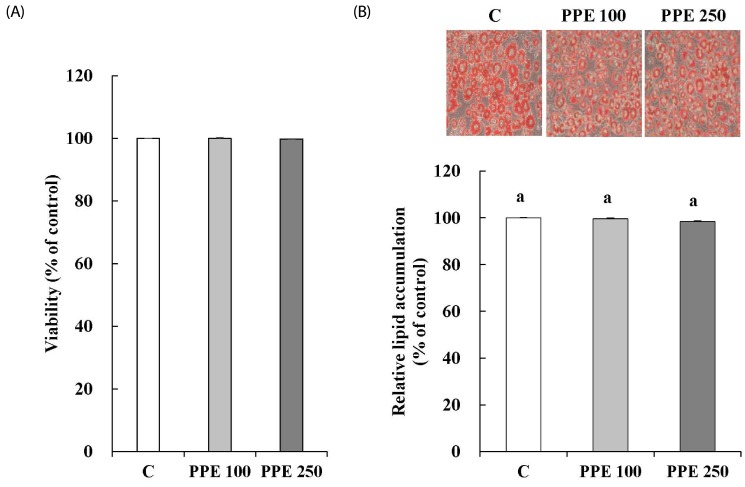
3T3-L1 cells were incubated with PPE for 24 h before MTT assay. MTT assay showed that the PPE did not affect cell viability at concentrations of 100 and 250 µg/mL (Fig. 1A). Changes in TG accumulation in response to PPE treatment were determined at 8 day. The PPE did not affect TG accumulation (Fig. 1B).
Fig. 1. Effects of pear pomace ethanol extract on viability (A) and differentiation (B) of 3T3-L1 cells.
Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05). C: Distilled water, PPE 100: Pear pomace ethanol extract 100 µg/mL, PPE 250: Pear pomace ethanol extract 250 µg/mL
Effects of PPE on MCP-1 secretion and the glucose uptake in 3T3-L1 cells
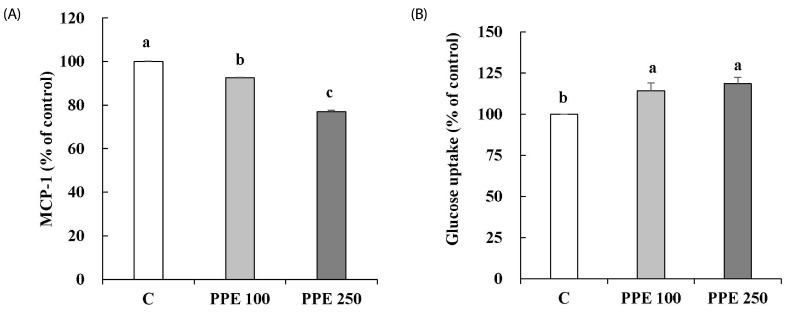
MCP-1 secretion and glucose uptake in 3T3-L1 cells treated with PPE were determined. As shown in Fig. 2, PPE significantly decreased MCP-1 secretion (A) and increased glucose uptake (B) compared with control.
Fig. 2. Effects of pear pomace ethanol extract on the MCP-1 secretion (A) and glucose uptake (B) in 3T3-L1 cells.
Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05). C: Distilled water, PPE 100: Pear pomace ethanol extract 100 µg/mL, PPE 250: Pear pomace ethanol extract 250 µg/mL
Effects of PPE on the insulin signaling pathway in 3T3-L1 cells
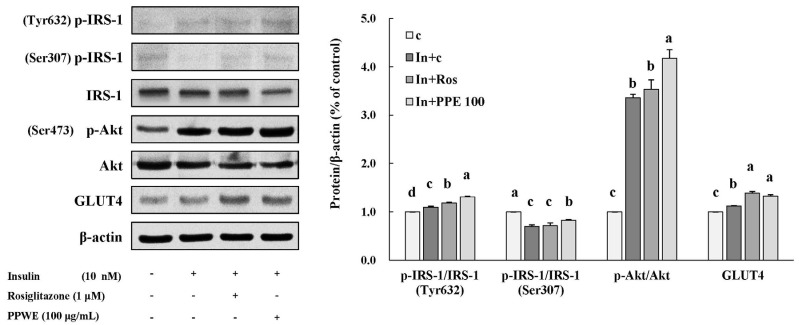
To determine the underlying mechanism of action of PPE in improving insulin sensitivity, we determined the expression of proteins involved in insulin signaling pathway such as IRS-1, p-IRS-1 (Tyr632/Ser307), Akt, p-Akt (Ser473), and GLUT4 (Fig. 3). PPE significantly increased the expression of proteins such as p-IRS-1 (Tyr632) and p-Akt, and the increasing effect of PPE was bigger than that of rosiglitazone (1 µM). PPE also increased the expression of GLUT4 to the same level as rosiglitazone did. Contrary to our expectation, PPE did not decrease the expression of p-IRS-1 (Ser307) in 3T3-L1 cells activated by insulin (Fig. 3).
Fig. 3. Effects of pear pomace ethanol extract on protein expression in 3T3-L1 cells.
Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05). C: Distilled water, In + C: Insulin 10 nM + distilled water, In + Ros: Insulin 10 nM + rosiglitazone 1 µM, In + PPE 100: Insulin 10 nM + Pear pomace ethanol extract 100 µg/mL
Effects of PPE on the account of weight gain and organ weight in high-fat diet-fed mice.
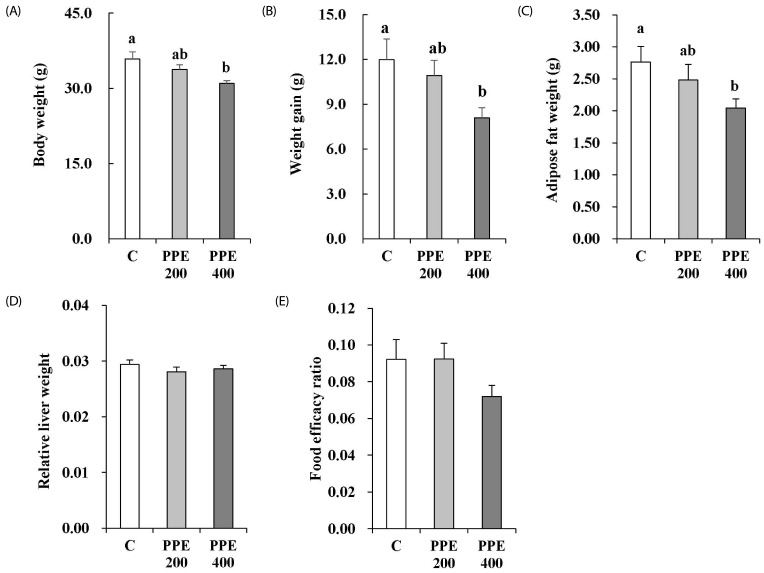
The body weight and the amount of weight gain of the mice treated with 400 mg/kg PPE significantly decreased in comparison to those of the mice in the control group (Fig. 4A and B). PPE at a dose of 400 mg/kg/day decreased intra-abdominal adipose tissue weight (Fig. 4C). The relative liver weight was calculated by dividing the weight of the liver by the body weight (liver weight/body weight). The PPE did not reduce relative liver weight (Fig. 4D). Food efficacy ratio was calculated as the weight gain/food intake during the experimental period. PPE at a dose of 400 mg/kg/day tended to reduce food efficacy ratio, but PPE-induced reduction was not statistically significant (Fig. 4E). Weight gain and adipose tissue weight in mice treated with PPE at a dose of 200 mg/kg/day tend to be lower than those in control mice, but the differences were not statistically significant.
Fig. 4. Effects of pear pomace ethanol extract on the final weight (A), weight gain (B), adipose tissue weight (C), relative liver weight (D), and food efficacy ratio (E) in high-fat diet-fed mice.
Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05). C: Distilled water, PPE 200: Pear pomace ethanol extract 200 mg/kg B.W., PPE 400: Pear pomace ethanol extract 400 mg/kg B.W.
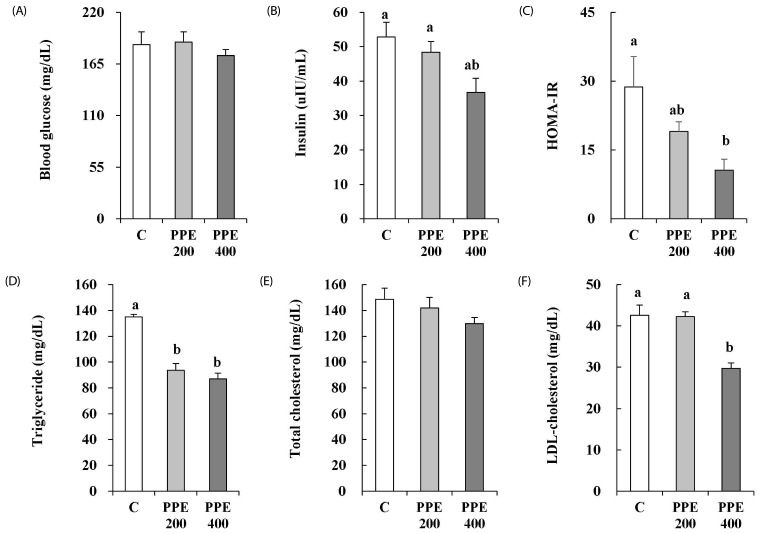
Effects of PPE on insulin resistance and levels of serum lipids in high-fat diet-fed mice
PPE at a dose of 400 mg/kg/day reduced fasting blood insulin, however, the difference was not statistically significant (Fig. 5B). Nevertheless, 400 mg/kg/day of PPE significantly decreased HOMA-IR (Fig. 5C). Serum triglyceride (TG) and LDL-cholesterol (LDL-C) concentrations in mice treated with PPE at a dose of 400 mg/kg/day significantly decreased (Fig. 5D and F). However, no significant difference was observed in serum total cholesterol (TC) when compared with that of control group mice (Fig. 5E).
Fig. 5. Effects of pear pomace ethanol extract on the fasting blood glucose (A), insulin (B), HOMA-IR (C), triglyceride (D), total cholesterol (E), and LDL-cholesterol (F) in high-fat diet-fed mice.
Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05). C: Distilled water, PPE 200: Pear pomace ethanol extract 200 mg/kg B.W., PPE 400: Pear pomace ethanol extract 400 mg/kg B.W.
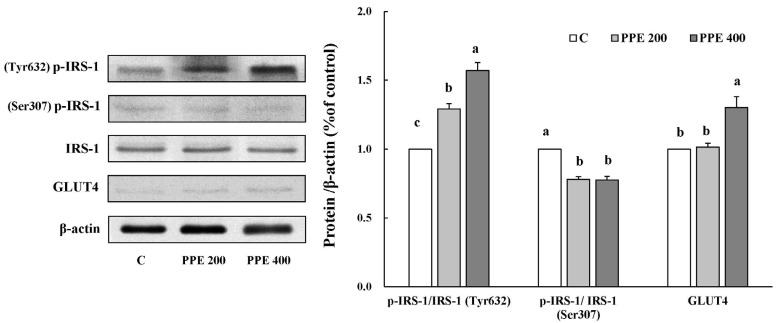
Effects of PPE on the insulin signaling pathway of adipose tissue in high-fat diet-fed mice
To identify the underlying action mechanisms of PPE in improving insulin sensitivity in high-fat diet-fed animal model, we determined the expression of proteins involved in insulin signaling pathway such as IRS-1, p-IRS-1 (Tyr632/Ser307), and GLUT4 in the adipose tissue (Fig. 6). Similar to 3T3-L1 cells, mice treated with PPE showed a significant increase in the expression of proteins such as p-IRS-1 (Tyr632), and GLUT4. Furthermore, PPE treated mice showed a decrease in the protein expression of p-IRS-1 (Ser307).
Fig. 6. Effects of pear pomace ethanol extract on the protein expression in adipose tissue.
Means with the same letter are not significantly different by Duncan's multiple range test (P < 0.05). C: Distilled water, PPE 200: Pear pomace ethanol extract 200 mg/kg B.W, PPE 400: Pear pomace ethanol extract 400 mg/kg B.W.
DISCUSSION
Owing to the adverse effects of commonly used anti-diabetic drugs, the current research trend is to identify and develop newer and safer alternatives for management of diabetes and insulin resistance. In the present study, we demonstrate the insulin sensitivity improving effects of PPE via activation of insulin signaling pathway in 3T3-L1 cells and high-fat diet-fed animals.
Adipose tissue plays an important role in the regulation of glucose and lipid metabolism, energy balance, inflammation, and insulin action [6,7]. In obesity, adipose tissue is the major site for increased macrophage infiltration. MCP-1 recruits macrophages into adipose tissue. Macrophages promote proinflammatory state and result in insulin resistance in adipocytes by inhibiting GLUT4 expression [12,29]. In the present study, PPE decreased MCP-1 secretion and increased glucose uptake in 3T3-L1 cells (Fig. 2). However, both macrophages and adipocytes produce proinflammatory cytokines and these cytokines synergize and regulate each other [12]. In our experiment, we observed PPE-induced decrease in MCP-1 secretion in 3T3-L1 cells without coculturing with macrophages or treatment of TNF-δ. Therefore we need more researches to demonstrate the triggering effect of PPE on GLUT4 expression via a downregulation of MCP-1 secretion. The anti-oxidative and anti-inflammatory effects of pear fruit eaten with peal have been also reported [21,25]. We also revealed that PPE reduced HOMA-IR in high-fat diet-fed animals, indicating that PPE can also improve insulin sensitivity in an in vivo model.
Several studies have been conducted on the insulin signaling pathway and revealed disruption of this insulin signaling cascade can induce insulin resistance [8,9,10,11,12]. Therefore, we can assume that agents that improve obesity-induced disruption of insulin signaling cascade can be developed as novel therapeutic agents. Thus, we determined whether PPE can improve insulin sensitivity through action on the insulin signaling pathway. In the present study, PPE increased tyrosine phosphorylation of IRS-1 thereby, increasing the ratio of p-IRS-1 (Tyr632)/IRS-1, which in turn increased phosphorylation of Akt in 3T3-L1 cells (Fig. 3). Furthermore, PPE increased GLUT4 expression in 3T3-L1 cells (Fig. 3). GLUT4 is the primary transporter for glucose uptake in adipose tissue [30]. Acquired insulin resistance of glucose uptake, observed in diabetes, could be explained by a variety of defects in GLUT4, including alteration in GLUT4 expression and translocation [31]. Decreased expression of GLUT4 and impaired responsiveness of GLUT4 to insulin were observed in adipocytes of obese and diabetic patients [32,33]. Therefore, our results suggest that PPE improves insulin sensitivity through the activation of insulin signaling pathway in 3T3-L1 cells. The activation of insulin signaling pathway by PPE was also demonstrated in a high-fat diet-fed animal model (Fig. 6). High-fat diet is a nutritional condition that accounts for the high incidence of metabolic syndrome in the world [34]. In animal model, a high-fat diet feeding leads to an elevation of fasting blood glucose and insulin accompanied by an increased HOMA-IR index [35]. Fraulob et al. [36] demonstrated that C57BL/6 mice fed high-fat chow (60% calories from fat) distinctly displayed an increased HOMA-IR index that is compatible with the presence of insulin resistance in these animals. In the present study, similar to its effects on 3T3-L1 cells, high-fat diet-fed animals treated with PPE showed increases in p-IRS-1 (Tyr632)/IRS-1 in adipose tissue. Moreover, PPE also inhibited serine phosphorylation of IRS-1 in adipose tissue of high-fat diet-fed animals, which was hardly revealed in 3T3-L1 cells treated with PPE. Serine phosphorylation of IRS-1 inhibits the tyripsin phosphorylation of IRS-1 and disrupts insulin signaling cascade [37]. We could not determine the protein expression of p-Akt in the adipose tissue of high-fat fed animals, because Akt phosphorylation is significantly lower in adipose tissue than live tissue without insulin stimulation [38,39]. PPE-induced increase in GLUT4 expression was also demonstrated in adipose tissue of high-fat diet-fed animals (Fig. 6). Therefore, we confirmed insulin sensitivity improving effect of PPE and demonstrate that PPE improves high-fat diet-induced insulin resistance through activation of insulin signaling pathway.
Obese individuals are assumed to ultimately develop diabetes, with insulin resistance occurring years before definite diagnosis. Therefore, agents that improve insulin resistance can prevent obese individuals from developing clinically overt diabetes. Weight gain is the one of the frequently occurring side effects of commonly used anti-diabetic drugs, indicating that these classes of drugs cannot protect obese patients against insulin resistance and diabetes. With respect to the metabolic effects of PPE, it should be noted that PPE did not affect lipid accumulation in 3T3-L1 cells (Fig. 1). Moreover, it decreased body weight and adipose tissue weight in high-fat diet-fed animals (Fig. 4). PPE also demonstrated hypolipidemic effects in high-fat diet-fed animals (Fig. 5). Further study need to elucidate two separate underlying mechanisms of PPE responsible for insulin sensitivity and adipogenesis. Some researchers have reported the therapeutic alternatives in the improvement of insulin resistance through enhancement of insulin signaling pathway without body weight gain [40]. They suggest the activation of AMPK [40], alteration of gut microbiota composition [35] and down-regulation of mTOR [41] as underlying mechanisms of anti-obesity effect.
In conclusion, PPE demonstrated insulin sensitivity improving effects via activation of insulin signaling pathway in 3T3-L1 cells (in vitro) and in high-fat diet-fed animal model (in vivo). Notably, the unique properties of PPE suggested that there might be two separate underlying mechanisms responsible for increased insulin sensitivity and adipogenesis.
Footnotes
This research was supported by the Bio-Industry Technology Development Program, Ministry of Agriculture, Food and Rural Affairs (313019-03-3-HD030).
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
References
- 1.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 2.Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 3.Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106:165–169. doi: 10.1172/JCI10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saad MJ, Araki E, Miralpeix M, Rothenberg PL, White MF, Kahn CR. Regulation of insulin receptor substrate-1 in liver and muscle of animal models of insulin resistance. J Clin Invest. 1992;90:1839–1849. doi: 10.1172/JCI116060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajala MW, Scherer PE. Minireview: the adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 7.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- 9.Pilch PF, Lee J. Insulin receptor family. In: Lennarz WJ, Lane MD, editors. Encyclopedia of Biological Chemistry. Vol. 2. Oxford: Elsevier Academic Press; 2004. pp. 436–440. [Google Scholar]
- 10.Muretta JM, Mastick CC. How insulin regulates glucose transport in adipocytes. Vitam Horm. 2009;80:245–286. doi: 10.1016/S0083-6729(08)00610-9. [DOI] [PubMed] [Google Scholar]
- 11.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Xie L, Ortega MT, Mora S, Chapes SK. Interactive changes between macrophages and adipocytes. Clin Vaccine Immunol. 2010;17:651–659. doi: 10.1128/CVI.00494-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 15.Diamant M, Heine RJ. Thiazolidinediones in type 2 diabetes mellitus: current clinical evidence. Drugs. 2003;63:1373–1405. doi: 10.2165/00003495-200363130-00004. [DOI] [PubMed] [Google Scholar]
- 16.Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R American Heart Association; American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation. 2003;108:2941–2948. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- 17.Bazelier MT, Gallagher AM, van Staa TP, Cooper C, Leufkens HG, Vestergaard P, de Vries F. Use of thiazolidinediones and risk of osteoporotic fracture: disease or drugs? Pharmacoepidemiol Drug Saf. 2012;21:507–514. doi: 10.1002/pds.3234. [DOI] [PubMed] [Google Scholar]
- 18.Chang CL, Lin Y, Bartolome AP, Chen YC, Chiu SC, Yang WC. Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compounds. Evid Based Complement Alternat Med. 2013;2013:378657. doi: 10.1155/2013/378657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh R, Kaur N, Kishore L, Gupta GK. Management of diabetic complications: a chemical constituents based approach. J Ethnopharmacol. 2013;150:51–70. doi: 10.1016/j.jep.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 20.Rabetafika HN, Bchir B, Blecker C, Paquot M, Wathelet B. Comparative study of alkaline extraction process of hemicelluloses from pear pomace. Biomass Bioenergy. 2014;61:254–264. [Google Scholar]
- 21.Li X, Wang T, Zhou B, Gao W, Cao J, Huang L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.) Food Chem. 2014;152:531–538. doi: 10.1016/j.foodchem.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Boath AS, Stewart D, McDougall GJ. Berry components inhibit α-glucosidase in vitro: synergies between acarbose and polyphenols from black currant and rowanberry. Food Chem. 2012;135:929–936. doi: 10.1016/j.foodchem.2012.06.065. [DOI] [PubMed] [Google Scholar]
- 23.Oboh G, Ademiluyi AO, Akinyemi AJ, Henle T, Saliu JA, Schwarzenbolz U. Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type-2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro. J Funct Foods. 2012;4:450–458. [Google Scholar]
- 24.Zhang L, Hogan S, Li J, Sun S, Canning C, Zheng SJ, Zhou K. Grape skin extract inhibits mammalian intestinal α-glucosidase activity and suppresses postprandial glycemic response in streptozocin-treated mice. Food Chem. 2011;126:466–471. [Google Scholar]
- 25.Wang T, Li X, Zhou B, Li H, Zeng J, Gao W. Anti-diabetic activity in type 2 diabetic mice and α-glucosidase inhibitory, antioxidant and anti-inflammatory potential of chemically profiled pear peel and pulp extracts (Pyrus spp.) J Funct Foods. 2015;13:276–288. [Google Scholar]
- 26.Kim J, Park Y, Yoon KS, Clark JM, Park Y. Permethrin alters adipogenesis in 3T3-L1 adipocytes and causes insulin resistance in C2C12 myotubes. J Biochem Mol Toxicol. 2014;28:418–424. doi: 10.1002/jbt.21580. [DOI] [PubMed] [Google Scholar]
- 27.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 28.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 29.Fernández-Veledo S, Nieto-Vazquez I, Vila-Bedmar R, Garcia-Guerra L, Alonso-Chamorro M, Lorenzo M. Molecular mechanisms involved in obesity-associated insulin resistance: therapeutical approach. Arch Physiol Biochem. 2009;115:227–239. doi: 10.1080/13813450903164330. [DOI] [PubMed] [Google Scholar]
- 30.James DE, Brown R, Navarro J, Pilch PF. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988;333:183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- 31.Zhou L, Yang Y, Wang X, Liu S, Shang W, Yuan G, Li F, Tang J, Chen M, Chen J. Berberine stimulates glucose transport through a mechanism distinct from insulin. Metabolism. 2007;56:405–412. doi: 10.1016/j.metabol.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 32.Garvey WT, Maianu L, Huecksteadt TP, Birnbaum MJ, Molina JM, Ciaraldi TP. Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J Clin Invest. 1991;87:1072–1081. doi: 10.1172/JCI115068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buettner R, Schölmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 35.Song H, Han W, Yan F, Xu D, Chu Q, Zheng X. Dietary Phaseolus vulgaris extract alleviated diet-induced obesity, insulin resistance and hepatic steatosis and alters gut microbiota composition in mice. J Funct Foods. 2016;20:236–244. [Google Scholar]
- 36.Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, Mandarim-de-Lacerda CA. A mouse model of metabolic syndrome: Insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr. 2010;46:212–223. doi: 10.3164/jcbn.09-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsai CW, Liu KL, Lin YR, Kuo WC. The mechanisms of carnosic acid attenuates tumor necrosis factor-α-mediated inflammation and insulin resistance in 3T3-L1 adipocytes. Mol Nutr Food Res. 2014;58:654–664. doi: 10.1002/mnfr.201300356. [DOI] [PubMed] [Google Scholar]
- 38.Hwang JS, Park JW, Nam MS, Cho H, Han IO. Glucosamine enhances body weight gain and reduces insulin response in mice fed chow diet but mitigates obesity, insulin resistance and impaired glucose tolerance in mice high-fat diet. Metabolism. 2015;64:368–379. doi: 10.1016/j.metabol.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Williams VL, Martin RE, Franklin JL, Hardy RW, Messina JL. Injury-induced insulin resistance in adipose tissue. Biochem Biophys Res Commun. 2012;421:442–448. doi: 10.1016/j.bbrc.2012.03.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Araújo TG, de Oliveira AG, Vecina JF, Marin RM, Franco ES, Abdalla Saad MJ, de Sousa Maia MB. Parkinsonia aculeata (Caesalpineaceae) improves high-fat diet-induced insulin resistance in mice through the enhancement of insulin signaling and mitochondrial biogenesis. J Ethnopharmacol. 2016;183:95–102. doi: 10.1016/j.jep.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 41.Yamashita H, Kusudo T, Takeuchi T, Qiao S, Tsutsumiuchi K, Wang T, Wang Y. Dietary supplementation with evodiamine prevents obesity and improves insulin resistance in ageing mice. J Funct Foods. 2015;19:320–329. [Google Scholar]