Abstract
The beginnings of laparoscopic liver resection (LLR) were at the start of the 1990s, with the initial reports being published in 1991 and 1992. These were followed by reports of left lateral sectionectomy in 1996. In the years following, the procedures of LLR were expanded to hemi-hepatectomy, sectionectomy, segmentectomy and partial resection of posterosuperior segments, as well as the parenchymal preserving limited anatomical resection and modified anatomical (extended and/or combining limited) resection procedures. This expanded range of LLR procedures, mimicking the expansion of open liver resection in the past, was related to advances in both technology (instrumentation) and technical skill with conceptual changes. During this period of remarkable development, two international consensus conferences were held (2008 in Louisville, KY, United States, and 2014 in Morioka, Japan), providing up-to-date summarizations of the status and perspective of LLR. The advantages of LLR have become clear, and include reduced intraoperative bleeding, shorter hospital stay, and - especially for cirrhotic patients-lower incidence of complications (e.g., postoperative ascites and liver failure). In this paper, we review and discuss the developments of LLR in operative procedures (extent and style of liver resections) during the first quarter century since its inception, from the aspect of relationships with technological/technical developments with conceptual changes.
Keywords: Hepatectomy, Laparoscopic surgery, Liver cancer, History, Technology, Technique, Concept, Approach, Posture, Simulation
Core tip: Laparoscopic liver resection (LLR) was introduced in early 1990s. Thereafter, LLR procedures have expanded to left lateral sectionectomy, hemi-hepatectomy, sectionectomy, segmentectomy and partial resection of posterosuperior segments, as well as parenchymal preserving limited and modified anatomical resection. This expansion is related to technological/technical developments with conceptual changes. During this period, two international consensus conferences summarized the up-to-date status and perspective of LLR. The current advantages of LLR include reduced intraoperative bleeding, shorter hospital stay, and lower incidence of complications. Here, we review and discuss the developments of LLR in operative procedures during the first quarter century since its inception.
INTRODUCTION
Although laparoscopes were first introduced in the 1960s as diagnostic tools for urological and gynecological diseases, a new technology to create pneumoperitoneum and the development of the charged-coupled device (CCD) camera, which magnifies and projects laparoscopic images onto television monitors, led to the first laparoscopic cholecystectomy performed in the current style in 1987 by Phillipe Mouret of Lyon, France[1]. The procedure gained immediate acceptance according to its related clinical experiences of less pain and rapid recovery, in addition to the cosmetic advantages[2,3]. Since then, the field of laparoscopic surgery has expanded rapidly to include surgery for other abdominal organs and more complex and technically demanding abdominal surgery.
The beginnings of laparoscopic liver resection (LLR) were at the start of the 1990s, with the initial reports[4-6] published in 1991 and 1992. These were followed by the reports of left lateral sectionectomy (LLS)[7,8] in 1996. In the years following, the procedures of LLR were expanded to hemi-hepatectomy, sectionectomy, segmentectomy and partial resection of posterosuperior segments, as well as parenchymal preserving limited anatomical resection and modified anatomical (extended and/or combining limited) resection. This expanded range of LLR procedures, mimicking the expansion of open liver resection (OLR) in the past, was related to advances in both technology (instrumentation) and technical skill with conceptual changes (Table 1).
Table 1.
Development of laparoscopic liver resection over the first 25 years
| Year | Procedure [Ref] | Related developments (technological, technical, conceptual) |
| 1991 | 1st report of LLR[4-6] (partial resection in AL) | |
| 1996 | LLS[7,8] | |
| 1997 | Hemi-hepatectomy[13-15] | Energy devices |
| (coagulating, sealing, shearing) | ||
| CUSA | ||
| HALS[19,20] and hybrid[21,22], | ||
| Inflow control[17,18] | ||
| 2000s-2010s | Sectionectomy (right posterior, right anterior, left medial) | Glissonian approach |
| (extra-[26], intra-hepatic[27]) | ||
| Caudal approach[10,31] | ||
| Postural change[29-31] | ||
| Segmentectomy and partial resection of segments 7, 8, 1 | Postural change[29-31] | |
| Caudal approach[10,31] | ||
| Lateral approach[37-39] (intercostal port) | ||
| Tracoscopic approach[40,41] | ||
| Limited anatomical resection and modified anatomical (extended and/or combining limited) resection[48-51] | Simulation and navigation[46,47] | |
| 3D endoscope[45] |
Ref: Reference number in the References section; LLR: Laparoscopic liver resection; AL: Anterolateral segments; LLS: Left lateral sectionectomy; CUSA: Cavitron ultrasonic surgical aspirator; HALS: Hand-assisted laparoscopic surgery; Hybrid: Laparoscopic-assisted LLR; 3D: Three-dimensional.
During this period of remarkable development, two international consensus conferences (ICCLLR) were held (2008 in Louisville, KY, United States[9] and 2014 in Morioka, Japan[10]), providing up-to-date summarizations of the status and perspective of LLR. The anxieties over LLR-specific complications, including gas-embolism, were eased by the cautious application of these procedures to and the long-term outcomes of selected patients for LLR, which were confirmed as similar to those for OLR. The advantages of LLR became clearly established, in particular, reduced intraoperative bleeding, shorter hospital stay, and - especially for cirrhotic patients - lower incidence of complications (e.g., postoperative ascites and liver failure).
In this review of the developments of LLR in operative procedures (extent and style of liver resections) that have occurred during the first quarter century since its inception, we discuss the relationships of these advances in technological/technical aspects of LLR with conceptual changes.
DEVELOPMENT OF LLR
Partial resection of anterolateral segments and LLS: The beginnings of LLR
The initial reports of LLR by Reich et al[4], Katkhouda et al[5], Gagner et al[6] appeared in 1991 and 1992. These were followed by reports of LLS by Azagra et al[7] and Kaneko et al[8] in 1996. Although segment level Glissonian pedicles and thick hepatic veins should be divided in LLS, the lesions located in the anterolateral segments (segments 2, 3, 4b, 5, 6) are more accessible laparoscopically than those in the posterosuperior segments (1, 4a, 7, 8). Also, the relatively small transection plane of LLS lies in a caudal-to-cranial direction and is vertical when the patient is in supine position, making it easier to handle in the natural laparoscopic view and to access with ports below the costal-arch level. Therefore, LLS is a big partial resection of anterolateral segments in some aspects; indeed, the first development of the LLR procedure involved anterolateral partial resection to LLS. LLS is the most straightforward sectionectomy procedure, as in OLR, and the standardization of this procedure has emerged recently as a topic of considerable discussion[11,12].
Hemi-hepatectomy and feasibility studies
The first report of hemi-hepatectomy was in 1997 by Hüscher et al[13], just 1 year after the LLS reports. The transection plane of hemi-hepatectomy, like the one in LLS, lies in the caudal-to-cranial direction and is vertical in supine position, making it easier to handle via the laparoscopic approach. Hemi-hepatectomies are the second-most straightforward procedure, again as in OLR, after anterolateral partial resection and LLS[14,15]. However, stable transection maneuvers were required in this step of development, since the transection plane in hemi-hepatectomies is a large area. Advances in technologies and instrumentation contributed to the step[16,17]. In the early stage of LLR development, pre-transectional coagulation via coagulating energy devices proved important in reducing the possibility of intra-operative massive bleeding. Development of transection maneuvers that mimic open maneuvers, such as crash-clamp transection and Cavitron ultrasonic surgical aspirator (CUSA; or its equivalent) transection, was accomplished by adaptation of various energy devices (to achieve coagulation, sealing and shearing) and laparoscopic CUSA, accompanied by inflow control[17,18]. Differences exist between the right and left hemi-hepatectomy forms of the major hepatectomies, these specifically involve mobilization of the liver and handling of the caudate lobe and IVC. The mobilization procedure for the left liver is relatively straightforward, except for the dissection of the roots of the middle and left hepatic veins. Also, when it is performed without resection of the Spiegel lobe, there is no need for dissection of the IVC. On the other hand, the right hemi-hepatectomy is usually performed with resection of the para-caval caudate lobe and, therefore, necessitates dissection of the IVC and right adrenal gland. During mobilization of the right liver, handling of the heavy and large-volume right liver is also much more demanding, complicating the laparoscopic surgical procedure which occurs without the surgeon’s hands being present in the operative field. As such, the procedure of laparoscopic right hemi-hepatectomy has developed more slowly than that of left[13-15].
During this and the next step of development, the hand-assisted procedure and hybrid (laparoscopic-assisted) procedure helped to reduce the technical difficulty of LLR in pure laparoscopic setting[19-22]. Also during this step of development, an encouraging feasibility study of LLR - including left hemi-hepatectomy, LLS, segementectomy and partial resection of segments 3, 4, 5, 6 - was reported by Cherqui et al[23] in 2000. This report concluded, “Laparoscopic resections are feasible and safe in selected patients with left-sided and right-peripheral lesions requiring limited resection.”
Left medial, right anterior and posterior sectionectomies
In the summary paper from the first ICCLLR[9], LLR was divided into the following three categories: I, small wedge resections; II, resections of the left lateral section or anterior segments (4b, 5, 6); III, hemi-hepatectomies, trisectionectomies and resections of posterior segments (4a, 7, 8). Category III was referred to as “major LLR”. The section on major LLR in this summary paper concluded, “Major LLR have been performed with safety and efficacy equaling OLR in highly specialized centers.” Also, in the section on hepatocellular carcinoma (HCC) treatment and LLR in this summary paper, anatomic segmental resection was recommended, instead of non-anatomical partial resection, due to the related lower rates of local recurrence. Especially for those patients with HCC and chronic liver diseases (CLDs), laparoscopic left medial, right anterior and right posterior sectionectomies were recommended as the next-step procedures after hemi-hepatectomy, in order to accomplish the preservation of residual liver function and to maintain oncological efficacy equal to that of OLR[24].
The transection planes in sectionectomies are larger in area and more difficult to handle than those in hemi-hepatectomies. Also, hilar dissection with individual vessel preparation for processing territorial vessels cannot be performed in this level. Although the Glissonian approach has been employed for hemi-hepatectomy[25] alongside hilar dissection with individual vessel preparation, the importance of the Glissonian approach is greater in sectionectomies and in more limited anatomical resections. Both extrahepatic[26] and intrahepatic[27] laparoscopic Glissonian approaches have been reported and employed widely, as in OLR, for this step in the development of LLR.
On the other hand, handling of the transection plane-especially the border between the anterior and posterior sections - is one of the key obstacles for right anterior and posterior sectionectomies[28]. Since the liver is located in the subphrenic rib cage, in OLR, surgeons open the subphrenic cage with a large subcostal incision and lifting-up of the costal arch; after which, the surgeon dissects the retro-peritoneal attachments and physically picks-up the liver with his/her left hand in order to manipulate the intact organ (Figure 1A). However, in LLR, there are no instruments as good as the surgeon’s left hand and, moreover, no anterior space available without abdominal wall incision. Therefore, laparoscopic right anterior and posterior sectionectomies are technically demanding to obtain a fine surgical field that will ensure hemostasis and an appropriate surgical margin in handling the transection plane beneath the large and heavy right liver in the small subphrenic rib cage.
Figure 1.
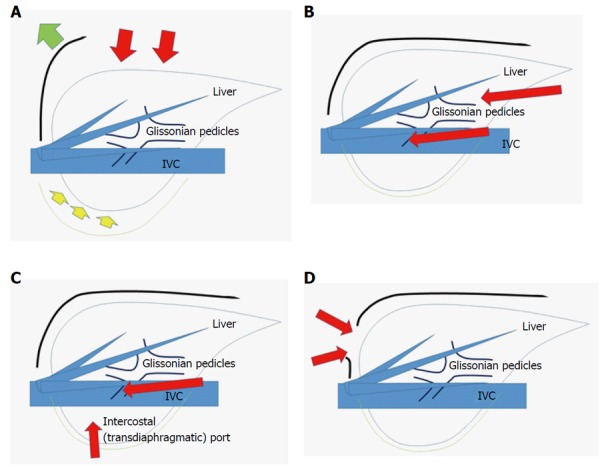
Schema of open liver resection (A), laparoscopic liver resection (regular caudal approach, B), laparoscopic liver resection (lateral approach, C) and thracoscopic liver resection (D). Red arrows indicate the directions of view and manipulation in each approach. A: In the open approach, the subcostal cage containing the liver is opened with a large subcostal incision and instruments are used to lift the costal arch, after which the liver is dissected and mobilized (lifted) from the retroperitoneum; B: In the regular laparoscopic caudal approach, the laparoscope and forceps are placed into the subcostal cage from the caudal direction, and the surgery is performed with minimal alteration and destruction of the associated structures; C: In the laparoscopic lateral approach, the intercostal (transdiaphragmatic) ports combined with total mobilization of the liver from the retroperitoneum can allow the direct lateral approach into the cage and to the posterosuperior tumors; D: Thoracoscopic approach is employed for lesions in segment 8, with direct exposure of the tumor into the pleural cavity upon incision on the diaphragm adjacent to the tumor, with the endoscope placed in the pleural cavity.
Postural changes have been employed to conquer this obstacle. Semi-prone[29,30] and left lateral[31] position LLR were reported as capable of allowing for acquirement of fine surgical view and manipulation for sectionectomies in the right liver. Also, a paper on lateral position posterior sectionectomy published by our group[31] described the new concept of “caudal approach in LLR” (Figure 1B); in this approach, the laparoscopic specific view and manipulation access is made from the caudal direction, using ports entering below the costal-arch level and going into the subphrenic rib cage. The summary paper of the second ICCLLR[10], explains this concept as follows: “The caudal approach, which relies on visual magnification, offers improved exposure around the right adrenal gland and the vena cava and greatly facilitates identification of the Laennec’s capsule and the Glissonian pedicle at the hilar plate.”
Segmentectomies and partial resections of segments 7, 8 and 1
Although LLS, segmentectomies for segments 5 and 6, and left medial sectionectomy (segment 4 segmentectomy) had been performed in the earlier stage of LLR development, segments 7, 8 and 1 remained unresolved challenge areas for segmentectomy and even for partial resection[32,33].
In LLR, and distinctive from OLR, more sectionectomies or right hepatectomies have been performed than segmentectomies or partial resections as treatment of tumors involving segments 7 and 8[9,34-36]. This trend can be explained by the fact that the straightforward transection plane of the liver, from caudal edge to the diaphragm in right hepatectomy or posterior sectionectomy, is more easily handled in LLR. In the laparoscope view from the caudal direction, the transection planes of segment 7 and 8 segmentectomies or partial resections are located in the deep small subphrenic space behind the liver, with segments 5 and 6 acting as physical obstacles to the lesions. Since surgeons need to create a precisely curved or angulated transection plane in the space, the parenchymal preserving segmentectomies or partial resections of the area are technically more difficult than performance of a posterior sectionectomy or right hepatectomy.
Adequate functional reserve of the liver after resection is as important as oncological efficacy, especially in impaired livers, as encountered in CLD patients with HCC[24]. An important consideration for LLR of this area, therefore, is how to obtain good and stable access that allow for sufficient and safe handling of the liver and tumors, so that a well-visualized transection plane can be acquired. To this end, intercostal (transdiaphragmatic) ports with total mobilization of the liver from the retroperitoneum have been applied to facilitate the direct lateral approach into the rib cage (in the abdominal cavity) and to segment 7 (Figure 1C)[37-39]. In addition, the thoracoscopic approach was employed for lesions in segment 8 (Figure 1D), with direct exposure of the tumor into the pleural cavity being achieved by incision on the diaphragm adjacent to the tumor[40,41]. Endoscopes have been placed in the abdominal cavity for the lateral approach using intercostal ports (Figure 1C), and in the pleural cavity for the thoracoscopic approach (Figure 1D).
On the other hand, postural changes, such as semi-prone positioning for tumors located in segment 7[28-30], have also been applied to solve the same problem. Although segment 7 is located in the bottom of the abdominal cavity when the patient is in supine position, that same area is located almost on the top of the abdominal cavity when the patient is in semi-prone position. Adapting those postural changes allows for the weight of the liver itself to facilitate its own mobilization, ultimately providing a good and stable surgical space above the liver. Ikeda et al[42] applied semi-prone position LLR with the use of intercostal ports to treat tumors in the anterosuperior and posterior segments.
There are still only a few reports, all with small numbers of cases, for laparoscopic isolated resection of the caudate lobe[33,43,44]. Although further experiences are needed for segment 1 LLR, especially for the total isolated resection of caudate lobe (Spiegel lobe, caudate process and paracaval portion), the fine laparoscopic caudal view to the vena cava area and behind the hilar plate, particularly from the left side with the incision on the gastro-hepatic ligament, could facilitate LLR for this area[44].
FUTURE PERSPECTIVES OF LLR
During the development of the LLR procedures, disadvantages of LLR have also been recognized. The lack of three-dimensional (3D) view was overcome by the development of the 3D laparoscope[45]. However, the lack of overview in the operative field (despite the local fine magnified view) combined with the lack of tactile sensation easily leads to disorientation on the perspective of the organs, tumors and the intrahepatic structures during LLR. Therefore, intraoperative laparoscopic ultrasonography and preoperative simulation/intraoperative navigation using reconstruction of preoperative imaging scans and the intraoperative implementation of near-infrared fluorescence scans with indocyanine green have become more important and are continued to be developed[46,47]. Based on the development of the imaging techniques, parenchymal preserving limited anatomical resection and modified (extended and/or combining limited) anatomical resection are advocated[48-51]. Robotic-assisted LLR holds the promise of facilitating a more precise surgery in certain situations, such as bile duct reconstruction[47,52,53].
On the other hand, there are specific advantages in LLR, besides those advantages common to all laparoscopic surgeries. For one, improved direct exposure with magnification could be obtained under the laparoscopic specific view to the liver inside the rib cage. This allows clearer access to the surgical field without the destruction of the surrounding environments, such as collateral vessels in patients with HCC and liver cirrhosis, and without inducing compression damage on the liver parenchyma[54,55]. Pneumoperitoneum pressure during laparoscopic surgery could reduce the amount of bleeding from the hepatic vein concomitantly with inflow control[54]. This creates a very dry surgical field, with clear visualization of the detailed internal structures of the liver. After the second ICCLLR in Morioka (2014), two important studies using propensity score analysis on about 5,000 patients’ data were published, and both showed the short-term benefits of LLR without deteriorating long-term results, compared with open procedure[56,57].
In the context of these advantages, several endeavors have now been attempted with the aim of increasing the adoption rate of LLR to clinical practice. To help ensure the safe and consistent extended application of the procedure, studies aimed at determining the learning curve of LLR were published[58,59] and a difficulty scoring system[60] (i.e., calculated according to tumor condition, resection style and liver condition) for the appropriate selection of the patient according to the surgeon’s skill set was proposed in the second ICCLLR[10]. Randomized clinical trials are underway and two have been completed[61,62], and registries have been started in several nations and areas[63,64]. It is likely that LLR will become a more standardized procedure with wider application in the second quarter century based upon the experiences in the first quarter century.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Morise Z and Wakabayashi G declare no conflicts of interest related to this publication.
Peer-review started: February 1, 2017
First decision: February 27, 2017
Article in press: April 21, 2017
P- Reviewer: Iacono C, Morris DLL S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Litynski GS. Profiles in laparoscopy: Mouret, Dubois, and Perissat: the laparoscopic breakthrough in Europe (1987-1988) JSLS. 1999;3:163–167. [PMC free article] [PubMed] [Google Scholar]
- 2.National Institutes of Health Consensus Development Conference Statement on Gallstones and Laparoscopic Cholecystectomy. Am J Surg. 1993;165:390–398. doi: 10.1016/s0002-9610(05)80929-8. [DOI] [PubMed] [Google Scholar]
- 3.Begos DG, Modlin IM. Laparoscopic cholecystectomy: from gimmick to gold standard. J Clin Gastroenterol. 1994;19:325–330. doi: 10.1097/00004836-199412000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Reich H, McGlynn F, DeCaprio J, Budin R. Laparoscopic excision of benign liver lesions. Obstet Gynecol. 1991;78:956–958. [PubMed] [Google Scholar]
- 5.Katkhouda N, Fabiani P, Benizri E, Mouiel J. Laser resection of a liver hydatid cyst under videolaparoscopy. Br J Surg. 1992;79:560–561. doi: 10.1002/bjs.1800790628. [DOI] [PubMed] [Google Scholar]
- 6.Gagner M, Rheault M, Dubuc J. Laparoscopic partial hepatectomy for liver tumor. Surg Endosc. 1992;6:97–98. [Google Scholar]
- 7.Azagra JS, Goergen M, Gilbart E, Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy-technical aspects. Surg Endosc. 1996;10:758–761. doi: 10.1007/BF00193052. [DOI] [PubMed] [Google Scholar]
- 8.Kaneko H, Takagi S, Shiba T. Laparoscopic partial hepatectomy and left lateral segmentectomy: technique and results of a clinical series. Surgery. 1996;120:468–475. doi: 10.1016/s0039-6060(96)80065-1. [DOI] [PubMed] [Google Scholar]
- 9.Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825–830. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 10.Wakabayashi G, Cherqui D, Geller DA, Buell JF, Kaneko H, Han HS, Asbun H, OʼRourke N, Tanabe M, Koffron AJ, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg. 2015;261:619–629. doi: 10.1097/SLA.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 11.Belli G, Gayet B, Han HS, Wakabayashi G, Kim KH, Cannon R, Kaneko H, Gamblin T, Koffron A, Dagher I, et al. Laparoscopic left hemihepatectomy a consideration for acceptance as standard of care. Surg Endosc. 2013;27:2721–2726. doi: 10.1007/s00464-013-2840-8. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa Y, Nitta H, Sasaki A, Takahara T, Ito N, Fujita T, Kanno S, Nishizuka S, Wakabayashi G. Laparoscopic left lateral sectionectomy as a training procedure for surgeons learning laparoscopic hepatectomy. J Hepatobiliary Pancreat Sci. 2013;20:525–530. doi: 10.1007/s00534-012-0591-x. [DOI] [PubMed] [Google Scholar]
- 13.Hüscher CG, Lirici MM, Chiodini S, Recher A. Current position of advanced laparoscopic surgery of the liver. J R Coll Surg Edinb. 1997;42:219–225. [PubMed] [Google Scholar]
- 14.O’Rourke N, Fielding G. Laparoscopic right hepatectomy: surgical technique. J Gastrointest Surg. 2004;8:213–216. doi: 10.1016/j.gassur.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Dagher I, O’Rourke N, Geller DA, Cherqui D, Belli G, Gamblin TC, Lainas P, Laurent A, Nguyen KT, Marvin MR, et al. Laparoscopic major hepatectomy: an evolution in standard of care. Ann Surg. 2009;250:856–860. doi: 10.1097/SLA.0b013e3181bcaf46. [DOI] [PubMed] [Google Scholar]
- 16.Kaneko H, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, Yamazaki K. Application of devices for safe laparoscopic hepatectomy. HPB (Oxford) 2008;10:219–224. doi: 10.1080/13651820802166831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurokawa T, Inagaki H, Sakamoto J, Nonami T. Hand-assisted laparoscopic anatomical left lobectomy using hemihepatic vascular control technique. Surg Endosc. 2002;16:1637–1638. doi: 10.1007/s00464-002-4212-7. [DOI] [PubMed] [Google Scholar]
- 18.Scatton O, Brustia R, Belli G, Pekolj J, Wakabayashi G, Gayet B. What kind of energy devices should be used for laparoscopic liver resection? Recommendations from a systematic review. J Hepatobiliary Pancreat Sci. 2015;22:327–334. doi: 10.1002/jhbp.213. [DOI] [PubMed] [Google Scholar]
- 19.Fong Y, Jarnagin W, Conlon KC, DeMatteo R, Dougherty E, Blumgart LH. Hand-assisted laparoscopic liver resection: lessons from an initial experience. Arch Surg. 2000;135:854–859. doi: 10.1001/archsurg.135.7.854. [DOI] [PubMed] [Google Scholar]
- 20.Huang MT, Lee WJ, Wang W, Wei PL, Chen RJ. Hand-assisted laparoscopic hepatectomy for solid tumor in the posterior portion of the right lobe: initial experience. Ann Surg. 2003;238:674–679. doi: 10.1097/01.sla.0000094301.21038.8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koffron AJ, Kung RD, Auffenberg GB, Abecassis MM. Laparoscopic liver surgery for everyone: the hybrid method. Surgery. 2007;142:463–468; discussion 468.e1-2. doi: 10.1016/j.surg.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 22.Nitta H, Sasaki A, Fujita T, Itabashi H, Hoshikawa K, Takahara T, Takahashi M, Nishizuka S, Wakabayashi G. Laparoscopy-assisted major liver resections employing a hanging technique: the original procedure. Ann Surg. 2010;251:450–453. doi: 10.1097/SLA.0b013e3181cf87da. [DOI] [PubMed] [Google Scholar]
- 23.Cherqui D, Husson E, Hammoud R, Malassagne B, Stéphan F, Bensaid S, Rotman N, Fagniez PL. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232:753–762. doi: 10.1097/00000658-200012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morise Z, Kawabe N, Tomishige H, Nagata H, Kawase J, Arakawa S, Yoshida R, Isetani M. Recent advances in the surgical treatment of hepatocellular carcinoma. World J Gastroenterol. 2014;20:14381–14392. doi: 10.3748/wjg.v20.i39.14381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topal B, Aerts R, Penninckx F. Laparoscopic intrahepatic Glissonian approach for right hepatectomy is safe, simple, and reproducible. Surg Endosc. 2007;21:2111. doi: 10.1007/s00464-007-9303-z. [DOI] [PubMed] [Google Scholar]
- 26.Cho A, Yamamoto H, Kainuma O, Souda H, Ikeda A, Takiguchi N, Nagata M. Safe and feasible extrahepatic Glissonean access in laparoscopic anatomical liver resection. Surg Endosc. 2011;25:1333–1336. doi: 10.1007/s00464-010-1358-6. [DOI] [PubMed] [Google Scholar]
- 27.Machado MA, Makdissi FF, Galvão FH, Machado MC. Intrahepatic Glissonian approach for laparoscopic right segmental liver resections. Am J Surg. 2008;196:e38–e42. doi: 10.1016/j.amjsurg.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 28.Morise Z. Laparoscopic liver resection for posterosuperior tumors using caudal approach and postural changes: A new technical approach. World J Gastroenterol. 2016;22:10267–10274. doi: 10.3748/wjg.v22.i47.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda T, Yonemura Y, Ueda N, Kabashima A, Shirabe K, Taketomi A, Yoshizumi T, Uchiyama H, Harada N, Ijichi H, et al. Pure laparoscopic right hepatectomy in the semi-prone position using the intrahepatic Glissonian approach and a modified hanging maneuver to minimize intraoperative bleeding. Surg Today. 2011;41:1592–1598. doi: 10.1007/s00595-010-4479-6. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda T, Mano Y, Morita K, Hashimoto N, Kayashima H, Masuda A, Ikegami T, Yoshizumi T, Shirabe K, Maehara Y. Pure laparoscopic hepatectomy in semiprone position for right hepatic major resection. J Hepatobiliary Pancreat Sci. 2013;20:145–150. doi: 10.1007/s00534-012-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tomishige H, Morise Z, Kawabe N, Nagata H, Ohshima H, Kawase J, Arakawa S, Yoshida R, Isetani M. Caudal approach to pure laparoscopic posterior sectionectomy under the laparoscopy-specific view. World J Gastrointest Surg. 2013;5:173–177. doi: 10.4240/wjgs.v5.i6.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho JY, Han HS, Yoon YS, Shin SH. Feasibility of laparoscopic liver resection for tumors located in the posterosuperior segments of the liver, with a special reference to overcoming current limitations on tumor location. Surgery. 2008;144:32–38. doi: 10.1016/j.surg.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 33.Salloum C, Lahat E, Lim C, Doussot A, Osseis M, Compagnon P, Azoulay D. Laparoscopic Isolated Resection of Caudate Lobe (Segment 1): A Safe and Versatile Technique. J Am Coll Surg. 2016;222:e61–e66. doi: 10.1016/j.jamcollsurg.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 34.Castaing D, Vibert E, Ricca L, Azoulay D, Adam R, Gayet B. Oncologic results of laparoscopic versus open hepatectomy for colorectal liver metastases in two specialized centers. Ann Surg. 2009;250:849–855. doi: 10.1097/SLA.0b013e3181bcaf63. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen KT, Laurent A, Dagher I, Geller DA, Steel J, Thomas MT, Marvin M, Ravindra KV, Mejia A, Lainas P, et al. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg. 2009;250:842–848. doi: 10.1097/SLA.0b013e3181bc789c. [DOI] [PubMed] [Google Scholar]
- 36.Cho JY, Han HS, Yoon YS, Shin SH. Outcomes of laparoscopic liver resection for lesions located in the right side of the liver. Arch Surg. 2009;144:25–29. doi: 10.1001/archsurg.2008.510. [DOI] [PubMed] [Google Scholar]
- 37.Ogiso S, Conrad C, Araki K, Nomi T, Anil Z, Gayet B. Laparoscopic Transabdominal With Transdiaphragmatic Access Improves Resection of Difficult Posterosuperior Liver Lesions. Ann Surg. 2015;262:358–365. doi: 10.1097/SLA.0000000000001015. [DOI] [PubMed] [Google Scholar]
- 38.Gumbs AA, Gayet B. Video: the lateral laparoscopic approach to lesions in the posterior segments. J Gastrointest Surg. 2008;12:1154. doi: 10.1007/s11605-007-0455-x. [DOI] [PubMed] [Google Scholar]
- 39.Schwarz L, Aloia TA, Eng C, Chang GJ, Vauthey JN, Conrad C. Transthoracic Port Placement Increases Safety of Total Laparoscopic Posterior Sectionectomy. Ann Surg Oncol. 2016;23:2167. doi: 10.1245/s10434-016-5126-2. [DOI] [PubMed] [Google Scholar]
- 40.Murakami M, Aoki T, Kato T. Video-assisted thoracoscopic surgery: hepatectomy for liver neoplasm. World J Surg. 2011;35:1050–1054. doi: 10.1007/s00268-011-0999-5. [DOI] [PubMed] [Google Scholar]
- 41.Teramoto K, Kawamura T, Takamatsu S, Noguchi N, Nakamura N, Arii S. Laparoscopic and thoracoscopic partial hepatectomy for hepatocellular carcinoma. World J Surg. 2003;27:1131–1136. doi: 10.1007/s00268-003-6936-5. [DOI] [PubMed] [Google Scholar]
- 42.Ikeda T, Toshima T, Harimoto N, Yamashita Y, Ikegami T, Yoshizumi T, Soejima Y, Shirabe K, Maehara Y. Laparoscopic liver resection in the semiprone position for tumors in the anterosuperior and posterior segments, using a novel dual-handling technique and bipolar irrigation system. Surg Endosc. 2014;28:2484–2492. doi: 10.1007/s00464-014-3469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dulucq JL, Wintringer P, Stabilini C, Mahajna A. Isolated laparoscopic resection of the hepatic caudate lobe: surgical technique and a report of 2 cases. Surg Laparosc Endosc Percutan Tech. 2006;16:32–35. doi: 10.1097/01.sle.0000202183.27042.63. [DOI] [PubMed] [Google Scholar]
- 44.Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246:385–392; discussion 392-394. doi: 10.1097/SLA.0b013e318146996c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Velayutham V, Fuks D, Nomi T, Kawaguchi Y, Gayet B. 3D visualization reduces operating time when compared to high-definition 2D in laparoscopic liver resection: a case-matched study. Surg Endosc. 2016;30:147–153. doi: 10.1007/s00464-015-4174-1. [DOI] [PubMed] [Google Scholar]
- 46.Hallet J, Gayet B, Tsung A, Wakabayashi G, Pessaux P. Systematic review of the use of pre-operative simulation and navigation for hepatectomy: current status and future perspectives. J Hepatobiliary Pancreat Sci. 2015;22:353–362. doi: 10.1002/jhbp.220. [DOI] [PubMed] [Google Scholar]
- 47.Giulianotti PC, Bianco FM, Daskalaki D, Gonzalez-Ciccarelli LF, Kim J, Benedetti E. Robotic liver surgery: technical aspects and review of the literature. Hepatobiliary Surg Nutr. 2016;5:311–321. doi: 10.21037/hbsn.2015.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ho CM, Wakabayashi G, Nitta H, Takahashi M, Takahara T, Ito N, Hasegawa Y. Total laparoscopic limited anatomical resection for centrally located hepatocellular carcinoma in cirrhotic liver. Surg Endosc. 2013;27:1820–1825. doi: 10.1007/s00464-012-2624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morise Z, Kawabe N, Tomishige H, Nagata H, Kawase J, Arakawa S, Isetani M. How Far Can We Go with Laparoscopic Liver Resection for Hepatocellular Carcinoma? Laparoscopic Sectionectomy of the Liver Combined with the Resection of the Major Hepatic Vein Main Trunk. Biomed Res Int. 2015;2015:960752. doi: 10.1155/2015/960752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Isetani M, Morise Z, Kawabe N, Tomishige H, Nagata H, Arakawa S, Ikeda M, Kamio K, Mizoguchi Y. A case of deeply located small hepatocellular carcinoma in cirrhotic liver treated with laparoscopic small anatomic liver resection. Fujita Med J. 2015;1:15–19. [Google Scholar]
- 51.Wakabayashi G. What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? Hepatobiliary Surg Nutr. 2016;5:281–289. doi: 10.21037/hbsn.2016.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ho CM, Wakabayashi G, Nitta H, Ito N, Hasegawa Y, Takahara T. Systematic review of robotic liver resection. Surg Endosc. 2013;27:732–739. doi: 10.1007/s00464-012-2547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ji WB, Wang HG, Zhao ZM, Duan WD, Lu F, Dong JH. Robotic-assisted laparoscopic anatomic hepatectomy in China: initial experience. Ann Surg. 2011;253:342–348. doi: 10.1097/SLA.0b013e3181ff4601. [DOI] [PubMed] [Google Scholar]
- 54.Wakabayashi G, Cherqui D, Geller DA, Han HS, Kaneko H, Buell JF. Laparoscopic hepatectomy is theoretically better than open hepatectomy: preparing for the 2nd International Consensus Conference on Laparoscopic Liver Resection. J Hepatobiliary Pancreat Sci. 2014;21:723–731. doi: 10.1002/jhbp.139. [DOI] [PubMed] [Google Scholar]
- 55.Morise Z, Ciria R, Cherqui D, Chen KH, Belli G, Wakabayashi G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J Hepatobiliary Pancreat Sci. 2015;22:342–352. doi: 10.1002/jhbp.215. [DOI] [PubMed] [Google Scholar]
- 56.Takahara T, Wakabayashi G, Beppu T, Aihara A, Hasegawa K, Gotohda N, Hatano E, Tanahashi Y, Mizuguchi T, Kamiyama T, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22:721–727. doi: 10.1002/jhbp.276. [DOI] [PubMed] [Google Scholar]
- 57.Beppu T, Wakabayashi G, Hasegawa K, Gotohda N, Mizuguchi T, Takahashi Y, Hirokawa F, Taniai N, Watanabe M, Katou M, et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for colorectal liver metastases with propensity score matching: a multi-institutional Japanese study. J Hepatobiliary Pancreat Sci. 2015;22:711–720. doi: 10.1002/jhbp.261. [DOI] [PubMed] [Google Scholar]
- 58.Vigano L, Laurent A, Tayar C, Tomatis M, Ponti A, Cherqui D. The learning curve in laparoscopic liver resection: improved feasibility and reproducibility. Ann Surg. 2009;250:772–782. doi: 10.1097/SLA.0b013e3181bd93b2. [DOI] [PubMed] [Google Scholar]
- 59.Hasegawa Y, Nitta H, Takahara T, Katagiri H, Baba S, Takeda D, Makabe K, Wakabayashi G, Sasaki A. Safely extending the indications of laparoscopic liver resection: When should we start laparoscopic major hepatectomy? Surg Endosc. 2017;31:309–316. doi: 10.1007/s00464-016-4973-z. [DOI] [PubMed] [Google Scholar]
- 60.Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, Hasegawa Y, Katagiri T, Takagi C, Itano O, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21:745–753. doi: 10.1002/jhbp.166. [DOI] [PubMed] [Google Scholar]
- 61.Fretland ÅA, Kazaryan AM, Bjørnbeth BA, Flatmark K, Andersen MH, Tønnessen TI, Bjørnelv GM, Fagerland MW, Kristiansen R, Øyri K, Edwin B. Open versus laparoscopic liver resection for colorectal liver metastases (the Oslo-CoMet Study): study protocol for a randomized controlled trial. Trials. 2015;16:73. doi: 10.1186/s13063-015-0577-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Dam RM, Wong-Lun-Hing EM, van Breukelen GJ, Stoot JH, van der Vorst JR, Bemelmans MH, Olde Damink SW, Lassen K, Dejong CH. Open versus laparoscopic left lateral hepatic sectionectomy within an enhanced recovery ERAS® programme (ORANGE II-trial): study protocol for a randomised controlled trial. Trials. 2012;13:54. doi: 10.1186/1745-6215-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wakabayashi G, Kaneko H. Can major laparoscopic liver and pancreas surgery become standard practices? J Hepatobiliary Pancreat Sci. 2016;23:89–91. doi: 10.1002/jhbp.293. [DOI] [PubMed] [Google Scholar]
- 64.Aldrighetti L, Belli G, Boni L, Cillo U, Ettorre G, De Carlis L, Pinna A, Casciola L, Calise F. Italian experience in minimally invasive liver surgery: a national survey. Updates Surg. 2015;67:129–140. doi: 10.1007/s13304-015-0307-2. [DOI] [PubMed] [Google Scholar]


