Abstract
Acute pancreatitis (AP) is one of the most common diseases. AP is associated with significant morbidity and mortality, but it lacks specific and effective therapies. Traditional Chinese medicine (TCM) is one of the most popular complementary and alternative medicine modalities worldwide for the treatment of AP. The current evidence from basic research and clinical studies has shown that TCM has good therapeutic effects on AP. This review summarizes the widely used formulas, single herbs and monomers that are used to treat AP and the potential underlying mechanisms of TCM. Because of the abundance, low cost, and safety of TCM as well as its ability to target various aspects of the pathogenesis, TCM provides potential clinical benefits and a new avenue with tremendous potential for the future treatment of AP.
Keywords: Acute pancreatitis, Traditional Chinese medicine, Alternative therapy, Pancreas protection, Anti-inflammatory
Core tip: Specific and effective therapies for the treatment of AP are lacking. Traditional Chinese medicine (TCM) exhibits beneficial, curative effects in basic research and clinical studies of AP treatment. Because of its abundance, low cost, safety and ability to target various aspects of AP pathogenesis, TCM provides a promising complementary and alternative therapy for the treatment of AP.
INTRODUCTION
Acute pancreatitis (AP) is characterized by the activation of trypsinogen and the establishment of a local inflammatory response in the pancreas, with the risk of developing into severe acute pancreatitis (SAP), characterized by systemic inflammatory response syndrome (SIRS) and multiple organ dysfunction syndrome (MODS)[1-3]. The pathogenesis of AP is not clear. Research in recent decades has focused on trypsinogen activation, pancreatic microcirculation malfunction, calcium overload and inflammatory pathways[4]. Pancreatic exocrine secretion inhibitors, such as octreotide, demonstrate a modest preventative role in the treatment of AP[5]; however, specific and effective therapies are lacking. Given the limited treatment of option, patients seek additional therapies to improve the therapeutic effect, which leading many to focus on complementary and alternative therapies.
Based on the unique traditional Chinese medicine (TCM) theoretical system and effective treatment methods, people have used TCM to prevent and address diseases for centuries, and more attention has been directed to this medicinal approach in recent studies[6-9]. TCM has demonstrated its superiority in the management of AP and other inflammatory diseases in China for many years[10]. Under the guidance of TCM pharmaceutical theory, AP is categorized as epigastric pain, splenic precordial pain, splenopyretic disease and knotted chest disease. The principle of treatment in TCM is to clear away the heat-evil (heat as a pathogenic factor that causes heat pattern/syndrome) and expelling superficial evils, supplementing qi (vital energy) and nourishing yin (body fluid), activating blood circulation to dissipate blood stasis and inner communication and purgation.
The philosophical basis of TCM is influenced by a holistic view that aligns with a theory of organism balance regulation in modern medicine. The theory of TCM in AP treatment is localized not only in the pancreas but also in the integrity and functional regulation of the organism. TCM coincides with the concepts of modern medicine and has attracted increasing attention as a combination therapy for AP[11]. As a complementary therapy, TCM that uses formulas and single herbs is increasingly considered to be effective and safe for treating AP[12]. Recently, the published guidelines for AP treatment in China stated that by using formulas and single herbs, TCM can be used as an alternative therapy for AP, and they acknowledged that TCM has exhibited good clinical effects. Accumulating evidence has demonstrated that TCM reduces the levels of serum and urinary amylase, decreases the permeability of capillaries, depresses the production of inflammatory cytokines, inhibits neutrophilic granulocyte activation and attenuates pancreatic injuries. These benefits block multiple steps in the development of AP according to experimental and clinical studies. Based on its characteristics of improved symptoms, reduced medical costs and increased satisfaction of AP patients, TCM appears to be a promising complementary and alternative therapy for the treatment of AP[7,13,14]. This review will provide a new understanding of the properties of TCM with an emphasis on the regulation of important molecular targets in AP in the context of basic and clinical research and the representative TCM approaches that can be combined with classic treatments of AP.
TCM FORMULAS
A TCM formula is the combination of several types of medicinal herbs or minerals that can amplify the therapeutic efficacies of each agent. The theory of the compatibility of medicinal ingredients is the principle of formula prescriptions. A formula commonly comprises various medicines, which are usually named sovereign (jun), minister (chen), assistant (zuo) and messenger (shi) ingredient drugs because of their different roles in the formula; these ingredients affect multiple targets and exert synergistic therapeutic effects, which lead to maximal therapeutic efficacy with minimal adverse effects[15]. The frequently used formulas for AP treatment are liquid medicines that are termed “decoction”, such as dachengqi decoction, qingyi decoction, yinchenchengqi decoction, chaiqinchengqi decoction, huoxueqingyi decoction and dahuangfuzi decoction.
Dachengqi decoction
Dachengqi decoction, one of the famous formulas in China for purgation, comprises rheum, immature bitter orange, natni sulfas natura, and Magnolia officinalis bark. Dachengqi decoction was first recorded in the classic TCM masterpiece Shang Han Lun (Treatise on Febrile Diseases) and has been widely used for acute abdominal pain throughout China[16-18]. Animal experiments have shown that dachengqi decoction increases cell viability, induces pancreatic acinar cell apoptosis, reduces acinar necrosis and protects from injuries to the pancreas in vivo and in vitro. The likely therapeutic mechanisms of action of dachengqi decoction may operate through reducing ROS generation and regulating the nitric oxide pathway in a rat SAP model[19]. Randomized controlled trials have reported that dachengqi decoction decreases serum resistin levels, significantly reduces mortality and exerts a beneficial therapeutic effect in SAP patients[20,21]. Concerning intestinal mucosal permeability, Chen et al[18] found that dachengqi decoction promoted the recovery of intestinal mucosal permeability and decreased the incidence of MODS and pancreatic infection in patients with SAP, which is supported by other studies[22]. Regarding abdominal compartment syndrome in SAP patients, Zhang et al[23] found that the combination of dachengqi decoction and conventional therapy decreased the intra-abdominal pressure of SAP patients and provided therapeutic effects on the abdominal compartment syndrome of SAP. Wan et al[24] investigated the effects of combined therapy with a modified dachengqi decoction and dexamethasone in the treatment of SAP patients on survival, acute respiratory distress syndrome (ARDS), renal failure, haemorrhage, sepsis, pancreatic pseudocyst, pancreatic abscess, operability, and days of hospitalization. Their data demonstrated that the modified dachengqi decoction combined with dexamethasone can decrease the risk of developing ARDS in SAP patients with SIRS, and it shortened their length of hospitalisation. This finding suggests that the modified dachengqi decoction is a safe and beneficial treatment option for SAP patients with SIRS.
Qingyi decoction
Qingyi decoction is the most famous formula for AP treatment and consists of rheum, Chinese thorowax root, white peony root, baikal skullcap root, Coptis chinenesis and other herbs. Qingyi decoction was invented by Doctor Boyu Zhang of the Shanghai University of Traditional Chinese Medicine and has exhibited good therapeutic effects for AP, as demonstrated in many preclinical and clinical studies. By targeting the inflammatory reaction in AP, qingyi decoction down-regulated the levels of serum endotoxin, α-amylase and tumour necrosis factor-α and reduced the expression of inflammatory factors in SAP rats. Moreover, the formula can ameliorate AP-induced intestinal barrier injury and lung injury. The mechanism may operate through inhibiting the overexpression of intestinal- and lung-secreted phospholipase A2[25,26]. Concerning bacterial translocation, qingyi decoction increased the density, altitude and area of intestinal mucosa and up-regulated the level of intestinal histone, which inhibited intestine permeability and bacterial translocation from the intestine. Wu et al[27] found that qingyi decoction combined with sodium sulfate was significantly superior to sodium sulfate alone in improving clinical symptoms; it also shortened hospitalizations and reduced the recovery time in SAP patients. Combined with enteral nutrition, qingyi decoction displayed improved clinical benefits in promoting the recovery of intestinal function and in regulating the balance of inflammatory cytokines[28].
Other formulas
Yinchenchengqi decoction is another frequently used formula in China for the treatment of AP. This decoction consists of Artemisia scoparia, Gardenia jasminoides, Magnolia officinalis bark, immature bitter orange, rheum and natni sulfas natura. Yinchenchengqi decoction may protect the pancreas by up-regulating Bax gene expression to induce apoptosis in the pancreatic acinar cells that are already injured; this treatment prevented cell necrosis in haemorrhagic necrotizing pancreatitis in rats[29]. In addition to the induction of apoptosis, this formula may down-regulate the expression of inflammatory mediators by inhibiting nuclear factor-kappa B (NF-κB)activation[30].
Consistent with its use in AP therapy for thousands of years in China, chaiqinchengqi decoction has been shown to inhibit the pancreatic enzymes and anti-inflammatory activity in patients with AP. Wang et al[10] found that chaiqinchengqi decoction significantly decreased the level of the serum pro-inflammatory cytokine interleukin-6 (IL-6) within the first 48 h of AP onset; it also improved symptoms and shortened hospitalization times in 107 AP patients.
Huoxueqingyi decoction, which comprises Salvia miltiorrhiza and qingyi decoction, is a modified qingyi decoction that has been widely used in the treatment of AP. It has been demonstrated that huoxueqingyi decoction that is administered rectally, intragastrically or orally shortens the hospital stay, reduces hospitalization costs and decreases the duration of SIRS and hyperamylasemia in patients with SAP. Furthermore, the formula did not induce any adverse effects such as liver injury. Therefore, huoxueqingyi decoction provides an effective, safe and economic therapeutic option[31].
Dahuangfuzi decoction, which consists of rheum, aconite, and Asarum sieboldii, is a famous traditional Chinese prescription with strong anti-inflammatory effects. Wu et al[32] found that dahuangfuzi decoction reduced the serum alanine aminotransferase (ALT) level and attenuated pancreas and liver injuries that are induced by SAP, and the anti-inflammatory mechanism of dahuangfuzi decoction operates by inhibiting the JAK/STAT signal pathway in SAP rats.
Although the decoctions have evident effects on AP, the applications of most decoctions are oral, which limits their utility because patients in the acute stage of AP have no access to food or water. In addition, the multiplicity of formulations also leads to issues with TCM standardization. This dilemma has led to the prevalence of studies on single Chinese medicinal herbs and monomers.
SINGLE CHINESE MEDICINAL HERB
Single Chinese medicinal herbs such as rheum, Salvia miltiorrhiza, natrii sulfas, baikal skullcap root, Saiko and Gardenia jasminoides have also been applied to AP treatment.
Rheum
As a classic TCM purgative, rheum has been widely used and has commonly served as the principal component in many traditional Chinese formulas for AP treatment. Rheum has been found to trigger enterokinesia, prevent translocation of intestinal bacteria, regulate intestinal flora, repair the intestinal mucosal barrier, and have an obvious therapeutic effect in SAP rats. Furthermore, rheum can inhibit the intestinal inflammatory response and ultimately improve the prognosis and outcome in SAP rats by down-regulating the signalling of the toll-like receptors (TLR)-2 and -4[33]. Currently, rheum is widely used as an adjunctive treatment in China’s guidelines for AP therapy and demonstrates good clinical effects. Wan et al[34] investigated the effects of a combined therapy using early enteral nutrition (EEN) and rhubarb. A randomized controlled trial showed that combined EEN and rhubarb significantly decreased white blood cell counts, plasma C-reactive proteins and IL-6 levels and increased plasma IL-11 levels, thus inhibiting systemic inflammation. Furthermore, the trial found that the time of abnormal bowel movements, recuperation from high fever, periods in intensive care units and duration of hospital stays were all shortened in the combined EEN and rhubarb group. In addition, combined EEN and rhubarb can reduce abnormally high levels of plasma alanine aminotransferase, aspartate aminotransferase, and creatinine (Cr) and mitigates SAP-related liver and kidney dysfunction. Zhou et al[35] investigated the combined effect of rhubarb and somatostatin in AP patients and found that it significantly reduced the total complications and APACHE II scores in patients with AP; this finding reveals that rhubarb can serve as an adjunctive therapeutic tool in AP treatment.
Salvia miltiorrhizae
Salvia miltiorrhizae is a commonly used traditional Chinese herb to activate blood and remove stasis. It has been proven that Salvia miltiorrhizae has anti-inflammatory properties, and in SAP rats, it cleared reactive oxygen species, induced apoptosis, and improved microcirculation, thus demonstrating some protective effects[36]. Salvia miltiorrhizae can protect multiple organs, strengthen immune function and thereby decrease the mortality of SAP rats. The mechanism may be through a reduction in plasma endotoxin levels, the inhibition of of intercellular cell adhesion molecule-1, TLR4, and NF-κB expression, and the regulation of the protein levels of apoptosis-related gene Bax[37,38].
Natrii sulfas
Natrii sulfas plastering therapy is a common adjunctive treatment of AP therapy that has been used in recent years with a rheum application and conventional therapy. The data from clinical research that was conducted in 60 AP patients showed that conventional therapy combined with intragastric rhubarb administration and natrii sulfas plastering therapy can relieve the symptoms of abdominal pain and distention, decrease serum and urine amylase levels, and reduce the APACHE II score compared with a control group[39]. Because sodium sulfate is the major component of natrii sulfas and has diuretic, detumescent and anti-inflammatory properties, natrii sulfas plastering therapy can adsorb moisture from the abdomen and thus disperse the swelling of the abdominal wall and intestinal canal.
TCM MONOMERS
Emodin
Emodin (1,3,8-trihydroxy-6-methyl-anthraquinone), an anthraquinone, with the molecular formula of C15H10O5 and a molecular mass of 270.23, is isolated from the traditional Chinese herb of rheum. Emodin has exhibited excellent biological activities in inflammatory diseases, such as antibacterial, anti-inflammatory, antioxidant, antitumour and immunomodulatory properties, and it inhibits trypsinogen secretion and improves the microcirculation[40,41]. Regarding the systemic inflammatory responses in AP, researchers have investigated the anti-inflammatory pharmacological mechanism that is induced by emodin in AP rats. The data showed that emodin reduced serum trypsogen, serum pro-inflammatory factor tumour necrosis factor-α (TNF-α), and IL-6 and IL-1β levels, and it inhibited NF-κB DNA-binding activity and enhanced peritoneal macrophage phagocytosis and apoptotic cell clearance. Emodin attenuated pancreatic damage through the inhibition of the TLR4 signal pathway, NF-κB and endoplasmic reticulum stress[42-45]. Emodin has also been reported to inhibit the abnormal metabolism of gadoleic acid and to improve pancreatic ischaemia in SAP. Concerning lung injury in AP, emodin intervention has been shown to up-regulate the expression of aquaporin-1, aquaporin-5, claudin-4, claudin-5 and occludin in lung tissue and decrease the histopathologic score. Emodin has also been reported to improve blood gas indexes, pulmonary oedema, vascular leakage, and alveolar epithelial barrier function, which ameliorated the acute lung injury that was induced by SAP[46,47]. Emodin has also been shown to up-regulate the mRNA expression of the apoptosis-related gene Bax, induce apoptosis in pancreatic acinar cells, and reduce cell necrosis in the pancreas. Its underlying mechanisms may operate through the inhibition of the TLR2 and TLR4 signal pathways and immune inflammation regulation[48,49]. Moreover, emodin has induced NO liberation, improved microcirculation of the pancreas, promoted cell regeneration and prevented pancreatic fibrosis. Gong et al[50] reported that emodin increased transforming growth factor β1 and epidermal growth factor gene expression, which subsequently increased DNA synthesis and protein content and thereby accelerated pancreatic repair and regeneration[51]. In addition, Wang et al[52,53] investigated the combined effect of emodin and EEN on SAP. Their data showed that the combination of emodin and EEN reduced the severity of experimental SAP in rats, and the combined strategy was rational, safe and more effective than the use of either EEN or emodin alone. In our previous study, we similarly confirmed the therapeutic effects of emodin in vivo[49,54].
Baicalein
Baicalein (5,6,7-trihydroxyflavone-7-O-D-glucuronic acid) is a flavonoid that is extracted from baikal skullcap root, a traditional Chinese herb. Baicalein has excellent antioxidant and anti-inflammatory activities and can be an anti-inflammatory agent[55]. In our previous study, we found that baicalein exerted an anti-inflammatory capability and showed a therapeutic effect in SAP rats. We investigated changes in pancreatic histopathology, ascites fluid and serum inflammatory mediators after baicalein treatment and found that baicalein was effective in decreasing the pancreatic histopathology score, reducing ascites fluid production and balancing the network between pro-inflammatory mediators and anti-inflammatory mediators. In addition, our study indicated that baicalein protected against pancreatic injury and led to improved survival in SAP rats[56]. Moreover, based on the theory of TCM, modern medicine, and the theory of the compatibility of medicinal ingredients, we have refined the classic qingyi decoction and selected baicalein and emodin for a combination treatment approach. We propose that the combined use of baicalein and emodin blocks multiple steps in the development of AP and exerts more profound therapeutic effects on pancreatic injuries in SAP rats without adverse effects[54,57]. The glycoside of baicalein that was mentioned above, which is called baicalin (5,6-dihydroxyflavon-7-yl β-D-glucopyranosiduronic acid), also has many biological properties, including antioxidant, anti-bacterial, antiviral, and anti-inflammatory effects[58]. Zhang et al[59] showed that baicalin inhibited serum P-selectin expression, decreased serum inflammatory cytokine levels and induced apoptosis of thymocytes in SAP rats.
Scutellarin
Scutellarin is extracted from the plants of the Scutellaria genus and has effective bioactivity. Scutellarin has been reported to dilate blood vessels, improve cardiovascular and cerebrovascular ischaemia, and inhibit activation of NF-κB from acute lung injury in mice[60,61]. Chen et al[62] investigated the pharmacological mechanisms of serum amylase inhibition and the protection of multiple organs (pancreas, liver, kidneys and lungs) by scutellarin in SAP rats. The data showed that scutellarin decreased serum ALT, Cr and amylase levels and relieved the pathologic changes of multiple organs. Furthermore, acute and subacute toxicity studies were performed to evaluate the safety of scutellarin. These data showed that scutellarin has a sufficient margin of safety for therapeutic use in rodents[63].
Ligustrazine
Ligustrazine is an alkaloid that is isolated from the traditional Chinese herb Szechuan lovage rhizome. Ligustrazine is a new type of calcium antagonist that has antiplatelet properties, improves microcirculation and enhances cerebral blood flow[64]. Ligustrazine has been shown to effectively induce pancreatic acinar cell apoptosis and prevent the apoptosis of cells in the liver and kidneys, which decreased the pathological score of these organs in SAP rats[65]. The mechanism of ligustrazine may operate by suppressing the p38 and ERK/MAPK pathways[66]. Moreover, ligustrazine has been shown to effectively decrease serum amylase levels and inflammatory cytokines and alleviate pathological changes in the pancreas, liver, kidney, small intestinal mucosa, thymus and spleen, which protect the body from multiple organ injuries[66,67].
Resveratrol
Resveratrol is a polyphenol that is isolated from the herb Polygonum cuspidatum, and it has high bioactivity, such as anti-inflammatory, antioxidative and anti-platelet aggregation activities. Resveratrol has been shown to effectively induce apoptosis in the pancreas, inhibit serum amylase release and inflammatory reactions, suppress microcirculatory disturbances, and alleviate pancreatic pathological injuries through the up-regulation of FasL expression and the down-regulation of the levels of angiotensin II, endothelin, nitric oxide and TNF-α[68-70]. In addition, the antioxidant and immunomodulatory properties of resveratrol may supply a promising chemopreventative approach in AP prevention[71], which coincides with the core belief of TCM as a preventive treatment.
Artemisinin
Artemisinin is a sesquiterpenes that is isolated from the traditional Chinese herb sweet wormwood. Artemisinin is a specific antimalarial that also has antileukaemic and immunoloregulation properties. Researchers have studied the effect of artemisinin on AP rats and found that artemisinin reduced trypsinogen excretion, inhibited the activation of neutrophilic granulocytes, and induced pancreatic acinar cell apoptosis. These results suggest that artemisinin alleviated the severity of AP through the caspase-3 signalling pathway and by inducing intrinsic apoptosis[72].
The chemical structures of TCM monomers mentioned above are presented in Figure 1.
Figure 1.
Chemical structures of Traditional Chinese medicine monomers.
CONCLUSION
Presently, TCM exhibits good curative effects in AP treatment (Table 1 and Figure 2). However, the ambiguity of the mechanism is an obstacle to the internationalization and generalization of TCM. Better designed trials are needed to make significant advances in the management of AP. Both pre-clinical and clinical studies have shown promising uses for TCM as a complementary and alternative therapeutic strategy for the treatment of AP that can even supplement conventional treatments, but TCM remains an alternative therapy in AP treatment. The inconvenience of the decoctions has limited the application of TCM. In most formulas, the essential compounds have not been identified. The main obstacle to the internationalization of TCM is the difficulty of standardizing the large number of herbs in one decoction. Along with the further exploration of the precise mechanisms of TCM action, it is hoped that a thorough understanding of the use of TCM in AP treatment strategies and its ability to target various aspects of the pathogenesis of AP will reveal the profound therapeutic benefits of TCM in the future.
Table 1.
Commonly used traditional Chinese medicine for AP treatment and their action targets/mechanisms
| TCM | Targets/mechanisms | Ref. |
| Dachengqi decoction | Induce apoptosis, protect from pancreas injury, recover intestinal mucosal permeability | [16-24] |
| Qingyi decoction | Anti-inflammation, inhibit pancreatic enzymes, inhibit intestinal bacterial translocation, protect from organ injury | [25-28] |
| Yinchenchengqi decoction | Induce apoptosis | [29] |
| Chaiqinchengqi decoction | Inhibit pancreatic enzymes, anti-inflammation | [10] |
| Huoxueqingyi decoction | Shorten hospital stay, reduce hospitalization cost, decrease duration of SIRS, alleviate hyperamylasemia | [31] |
| Dahuangfuzi decoction | Anti-inflammatory, protect from organ injury | [32] |
| Rheum | Anti-inflammation, inhibiting intestinal bacterial translocation, protect from organ injury, accelerating pancreatic repair and regeneration | [33-35] |
| Salvia miltiorrhizae | Anti-inflammation, induce apoptosis, improve microcirculation, clean reactive oxygen species, protect from organ injury, strengthen the immunity function | [36-38] |
| Natrii sulfas | Relieve symptoms, inhibit pancreatic enzymes, reduce APACHE II score | [39] |
| Emodin | Anti-inflammation, inhibit endoplasmic reticulum stress, protect from organ injury, induce apoptosis, improve pancreas microcirculation, accelerate pancreatic repair and regeneration | [40-54] |
| Baicalin | Anti-inflammation | [54, 56-57] |
| Baicalein | Induce apoptosis | [58-59] |
| Scutellarin | Inhibit pancreatic enzymes, protect from organ injury | [60-63] |
| Ligustrazine | Induce apoptosis, anti-inflammation, inhibit pancreatic enzymes, protect from organ injury | [64-67] |
| Resveratrol | Induce apoptosis, anti-inflammation, inhibit pancreatic enzymes, antioxidant, immunoregulation | [68-71] |
| Artemisinin | Induce apoptosis, anti-inflammation, inhibit pancreatic enzymes | [72] |
TCM: Traditional Chinese medicine.
Figure 2.
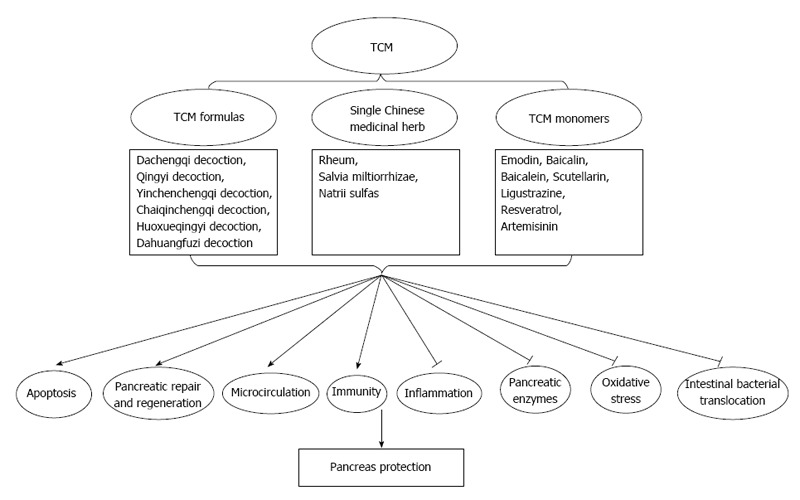
Diagram showing proposed mechanisms of Traditional Chinese medicine for acute pancreatitis treatment. Traditional Chinese medicine (TCM) induces apoptosis, accelerates pancreatic repair and regeneration, improves pancreas microcirculation and strengthens the immunity function and results in the pancreas protection. In addition, TCM mediates inhibition of inflammatory reaction, pancreatic enzymes, oxidative stress and intestinal bacterial translocation, which may contributes to the pancreas protection in AP treatment.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: No potential conflicts of interest.
Peer-review started: February 2, 2017
First decision: February 23, 2017
Article in press: May 4, 2017
P- Reviewer: Alexander JS, Kanda T, Vorobjova T S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
References
- 1.Dambrauskas Z, Giese N, Gulbinas A, Giese T, Berberat PO, Pundzius J, Barauskas G, Friess H. Different profiles of cytokine expression during mild and severe acute pancreatitis. World J Gastroenterol. 2010;16:1845–1853. doi: 10.3748/wjg.v16.i15.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinosoglou K, Gogos C. Immune-modulating therapy in acute pancreatitis: fact or fiction. World J Gastroenterol. 2014;20:15200–15215. doi: 10.3748/wjg.v20.i41.15200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J, Zhou R, Zhang J, Li ZF. Calcium signaling of pancreatic acinar cells in the pathogenesis of pancreatitis. World J Gastroenterol. 2014;20:16146–16152. doi: 10.3748/wjg.v20.i43.16146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawra R, Sah RP, Dudeja V, Rishi L, Talukdar R, Garg P, Saluja AK. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology. 2011;141:2210–2217.e2. doi: 10.1053/j.gastro.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kambhampati S, Park W, Habtezion A. Pharmacologic therapy for acute pancreatitis. World J Gastroenterol. 2014;20:16868–16880. doi: 10.3748/wjg.v20.i45.16868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Guo Y, Li GL. Current Status of Standardization of Traditional Chinese Medicine in China. Evid Based Complement Alternat Med. 2016;2016:9123103. doi: 10.1155/2016/9123103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu X, Xiao W, Kang X, Yu J, Fan Z. The effect of Chinese herbal medicine on non-biliogenic severe acute pancreatitis: a systematic review and meta-analysis. J Ethnopharmacol. 2014;155:21–29. doi: 10.1016/j.jep.2014.05.040. [DOI] [PubMed] [Google Scholar]
- 8.Zhu SF, Chen WW, Xiang J, Zhao XL, Wan MH, Yu Q, Liang MZ, Tang WF. Pharmacokinetic and pharmacodynamic comparison of chinese herbal ointment liu-he-dan and micron liu-he-dan ointment in rats with acute pancreatitis. Evid Based Complement Alternat Med. 2014;2014:389576. doi: 10.1155/2014/389576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiong W, Yiping W, Jinlin Y, Tao G, Zhen G, Pengcheng Z. Chinese medicinal herbs for acute pancreatitis. Cochrane Database Syst Rev. 2005;(1):CD003631. doi: 10.1002/14651858.CD003631.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Li Y, Ma Q, Liu Y, Rui YY, Xue P, Zhou ZG. Chaiqin Chengqi Decoction decreases IL-6 levels in patients with acute pancreatitis. J Zhejiang Univ Sci B. 2011;12:1034–1040. doi: 10.1631/jzus.B1000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia Q, Deng LH. [Hot issues on the treatment of severe acute pancreatitis by Integrated Traditional Chinese and Western Medicine] Sichuan Daxue Xuebao Yixueban. 2013;44:962–965. [PubMed] [Google Scholar]
- 12.Zhang SD, Lei RQ. Orientations on the Guideline for Severe Acute Pancreatitis proposed by Chinese Association of Pancreatic Surgery. Zhonghua Waike Zazhi. 2009;47:1441–1443. [PubMed] [Google Scholar]
- 13.Chen WW, Wan MH, Lin ZQ, Yang XN, Guo J, Wen Q, Xia Q. [Health economic evaluation of clinical pathway of traditional Chinese medicine in the treatment of mild acute pancreatitis] Sichuan Daxue Xuebao Yixueban. 2013;44:966–969. [PubMed] [Google Scholar]
- 14.Gao Q, Liang N. Integrated traditional Chinese medicine improves acute pancreatitis via the downregulation of PRSS1 and SPINK1. Exp Ther Med. 2015;9:947–954. doi: 10.3892/etm.2015.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Zhou GB, Liu P, Song JH, Liang Y, Yan XJ, Xu F, Wang BS, Mao JH, Shen ZX, et al. Dissection of mechanisms of Chinese medicinal formula Realgar-Indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA. 2008;105:4826–4831. doi: 10.1073/pnas.0712365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katakai M, Akamaru T, Tani T. [An analysis of the frequency of formulations and crude drugs described in Shan-Han-Lun] Yakushigaku Zasshi. 2002;37:28–35. [PubMed] [Google Scholar]
- 17.Li J, Zhu SF, Zhao XL, Liu YX, Wan MH, Guo H, Liu YL, Gong HL, Chen GY, Tang WF. Metabolomic profiles illuminate the efficacy of Chinese herbal Da-Cheng-Qi decoction on acute pancreatitis in rats. Pancreatology. 2015;15:337–343. doi: 10.1016/j.pan.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Chen H, Li F, Jia JG, Diao YP, Li ZX, Sun JB. Effects of traditional Chinese medicine on intestinal mucosal permeability in early phase of severe acute pancreatitis. Chin Med J (Engl) 2010;123:1537–1542. [PubMed] [Google Scholar]
- 19.Zhao J, Tang W, Wang J, Xiang J, Gong H, Chen G. Pharmacokinetic and pharmacodynamic studies of four major phytochemical components of Da-Cheng-Qi decoction to treat acute pancreatitis. J Pharmacol Sci. 2013;122:118–127. doi: 10.1254/jphs.13037fp. [DOI] [PubMed] [Google Scholar]
- 20.Ma Y, Huang ZW, Xia Q, Xue P, Guo J, Wei HQ, Chen Y, He FQ, Cheng ZY, Lin ZQ. [Influence of integrated traditional Chinese and Western medicine therapy on serum resistin levels in patients with severe acute pancreatitis: a randomized controlled trial] Zhong Xi Yi Jiehe Xuebao. 2009;7:1134–1138. doi: 10.3736/jcim20091208. [DOI] [PubMed] [Google Scholar]
- 21.Xia Q, Huang ZW, Jiang JM, Chen GY, Yang XN, Tang WF, Zhang ZD, Liu XB, Hu WM, Tian BL, et al. Yi-Huo-Qing-Xia method as the main therapy in integrated traditional Chinese and western medicine on severe acute pancreatitis, a report of 1161 cases. Zhongguo Zhongxiyijiehe Zhongzhengjianhu Zazhi. 2006;13:131–134. [Google Scholar]
- 22.Gu XD, Zhang Q. Clinical progress in the treatment of severe acute pancreatitis with integrative Chinese and Western medicine. Chin J Integr Med. 2007;13:235–240. doi: 10.1007/s11655-007-0235-1. [DOI] [PubMed] [Google Scholar]
- 23.Zhang MJ, Zhang GL, Yuan WB, Ni J, Huang LF. Treatment of abdominal compartment syndrome in severe acute pancreatitis patients with traditional Chinese medicine. World J Gastroenterol. 2008;14:3574–3578. doi: 10.3748/wjg.14.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan MH, Li J, Gong HL, Xue P, Zhu L, Chen GY, Xia Q, Wen-Fu T. Clinical observation on the effect of dexamethasone and Chinese herbal decoction for purgation in severe acute pancreatitis patients. Chin J Integr Med. 2011;17:141–145. doi: 10.1007/s11655-011-0630-5. [DOI] [PubMed] [Google Scholar]
- 25.Zhang JW, Zhang GX, Chen HL, Liu GL, Owusu L, Wang YX, Wang GY, Xu CM. Therapeutic effect of Qingyi decoction in severe acute pancreatitis-induced intestinal barrier injury. World J Gastroenterol. 2015;21:3537–3546. doi: 10.3748/wjg.v21.i12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang XM, Chen HL, Wang ZH. Expression of secretory type II phospholipase A2 in acute lung injury following acute pancreatitis and interventional effect of Qingyi decoction on it. Zhongguo Weizhongbing Jijiu Yixue. 2010;22:518–521. [PubMed] [Google Scholar]
- 27.Wu RQ, Chen Y, Liu LP. [Clinical observation on treatment of severe acute pancreatitis by combined administration of Qingyi Decoction and Glauber’s salt] Zhongguo Zhongxiyi Jiehe Zazhi. 2007;27:924–926. [PubMed] [Google Scholar]
- 28.Zhang B, Huang HT, Wang ZG, Rong YP, Yu B, Gong Y. The effects of Qing Yi decoction in combination with enteral nutrition on inflammatory cytokine balance of patients with severe acute pancreatitis. Fubu Waike Zazhi. 2010;2:108–110. [Google Scholar]
- 29.Shang D, Guan FL, Chen HL, Yang PM, Xin Y, Liu Z, Nie FY, Hu AP. Effect of Yinchenchengqi Decoction on Severe Acute Pancreatitis for Apoptosis of Acinar Cells and Expression of Bax. Zhonghua Zhongxiyi Jiehe Waike Zazhi. 2002;8:70–73. [Google Scholar]
- 30.Wu LQ, Zhang CL, Luo ZQ, Shao JH, Fu HQ. Effect of Yinchenchengqi Decoction on Severe Acute Pancreatitis for the Activeness of NF-κB and the Expression. Jiangxi Yixueyuan Xuebao. 2008;48:29–31. [Google Scholar]
- 31.Ji CH, Tang CW, Feng WM, Bao Y, Yao LQ. A Chinese Herbal Decoction, Huoxue Qingyi Decoction, Promotes Rehabilitation of Patients with Severe Acute Pancreatitis: A Retrospective Study. Evid Based Complement Alternat Med. 2016;2016:3456510. doi: 10.1155/2016/3456510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu L, Li H, Zheng SZ, Liu X, Cai H, Cai BC. Da-Huang-Fu-Zi-Tang attenuates liver injury in rats with severe acute pancreatitis. J Ethnopharmacol. 2013;150:960–966. doi: 10.1016/j.jep.2013.09.051. [DOI] [PubMed] [Google Scholar]
- 33.Yao P, Cui M, Li Y, Deng Y, Wu H. Effects of rhubarb on intestinal flora and toll-like receptors of intestinal mucosa in rats with severe acute pancreatitis. Pancreas. 2015;44:799–804. doi: 10.1097/MPA.0000000000000339. [DOI] [PubMed] [Google Scholar]
- 34.Wan B, Fu H, Yin J, Xu F. Efficacy of rhubarb combined with early enteral nutrition for the treatment of severe acute pancreatitis: a randomized controlled trial. Scand J Gastroenterol. 2014;49:1375–1384. doi: 10.3109/00365521.2014.958523. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Wang L, Huang X, Li H, Xiong Y. Add-on effect of crude rhubarb to somatostatin for acute pancreatitis: A meta-analysis of randomized controlled trials. J Ethnopharmacol. 2016;194:495–505. doi: 10.1016/j.jep.2016.09.053. [DOI] [PubMed] [Google Scholar]
- 36.Xiping Z, Jun F, Chengjun W, Meili M, Ping Y, Jing Y, Qijun Y, Feibo Z, Rongcao Y. Effect of salvia miltiorrhizae on pulmonary apoptosis of rats with severe acute pancreatitis or obstructive jaundice. Inflammation. 2009;32:287–295. doi: 10.1007/s10753-009-9132-4. [DOI] [PubMed] [Google Scholar]
- 37.Xiping Z, Dijiong W, Jianfeng L, Qihui C, Jing Y, Penghui J, Meijuan Y, Ninni Z. Effects of Salvia miltiorrhizae on ICAM-1, TLR4, NF-kappaB and Bax proteins expression in multiple organs of rats with severe acute pancreatitis or obstructive jaundice. Inflammation. 2009;32:218–232. doi: 10.1007/s10753-009-9124-4. [DOI] [PubMed] [Google Scholar]
- 38.Zhang R, Zhang X, Zhang J, Wu J, Ye Q, Xu R, Ye J, Fang X, Jin L, He J, et al. Efficacy and mechanism of Salvia miltiorrhizae injection in the treatment of rats with severe acute pancreatitis. Inflammation. 2009;32:109–119. doi: 10.1007/s10753-009-9109-3. [DOI] [PubMed] [Google Scholar]
- 39.Zhou K, Wang YL. Clinical Observation on Rhubarb Enema and Glauber’s Salt Topical Treating Acute Pancreatitis. Journal of Zhejiang Chinese Medical University. 2015;(7):545–547, 554. [Google Scholar]
- 40.Xia XM, Li BK, Xing SM, Ruan HL. Emodin promoted pancreatic claudin-5 and occludin expression in experimental acute pancreatitis rats. World J Gastroenterol. 2012;18:2132–2139. doi: 10.3748/wjg.v18.i17.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan L, Wang Z, Zhou L. Protecting effects of emodin on multiorgan failure of rats with severe acute pancreatitis. Pancreas. 2009;38:835–836. doi: 10.1097/MPA.0b013e3181b2bd31. [DOI] [PubMed] [Google Scholar]
- 42.Yao WY, Zhou YF, Qian AH, Zhang YP, Qiao MM, Zhai ZK, Yuan YZ, Yang SL. Emodin has a protective effect in cases of severe acute pancreatitis via inhibition of nuclear factorκB activation resulting in antioxidation. Mol Med Rep. 2015;11:1416–1420. doi: 10.3892/mmr.2014.2789. [DOI] [PubMed] [Google Scholar]
- 43.Ni Q, Zhang W, Sun K, Yin C, An J, Shang D. In vitro effects of emodin on peritoneal macrophage intercellular adhesion molecule-3 in a rat model of severe acute pancreatitis/systemic inflammatory response syndrome. Biomed Rep. 2014;2:63–68. doi: 10.3892/br.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ni Q, Sun K, Chen G, Shang D. In vitro effects of emodin on peritoneal macrophages that express membrane-bound CD14 protein in a rat model of severe acute pancreatitis/systemic inflammatory response syndrome. Mol Med Rep. 2014;9:355–359. doi: 10.3892/mmr.2013.1771. [DOI] [PubMed] [Google Scholar]
- 45.Wu L, Cai B, Zheng S, Liu X, Cai H, Li H. Effect of emodin on endoplasmic reticulum stress in rats with severe acute pancreatitis. Inflammation. 2013;36:1020–1029. doi: 10.1007/s10753-013-9634-y. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Huang B, Wang Y, Tong C, Xie P, Fan R, Gao Z. Emodin ameliorates acute lung injury induced by severe acute pancreatitis through the up-regulated expressions of AQP1 and AQP5 in lung. Clin Exp Pharmacol Physiol. 2016;43:1071–1079. doi: 10.1111/1440-1681.12627. [DOI] [PubMed] [Google Scholar]
- 47.Xia XM, Wang FY, Wang ZK, Wan HJ, Xu WA, Lu H. Emodin enhances alveolar epithelial barrier function in rats with experimental acute pancreatitis. World J Gastroenterol. 2010;16:2994–3001. doi: 10.3748/wjg.v16.i24.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Y, Xiong W, Yang J, Zhong J, Zhang L, Zheng J, Liu H, Zhang Q, Ouyang X, Lei L, et al. Attenuation of Inflammation by Emodin in Lipopolysaccharide-induced Acute Kidney Injury via Inhibition of Toll-like Receptor 2 Signal Pathway. Iran J Kidney Dis. 2015;9:202–208. [PubMed] [Google Scholar]
- 49.Li Z, Xia X, Zhang S, Zhang A, Bo W, Zhou R. Up-regulation of Toll-like receptor 4 was suppressed by emodin and baicalin in the setting of acute pancreatitis. Biomed Pharmacother. 2009;63:120–128. doi: 10.1016/j.biopha.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 50.Gong Z, Yuan Y, Lou K, Tu S, Zhai Z, Xu J. Mechanisms of Chinese herb emodin and somatostatin analogs on pancreatic regeneration in acute pancreatitis in rats. Pancreas. 2002;25:154–160. doi: 10.1097/00006676-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 51.Lou KX, Gong ZH, Yuan YZ. [Study on effect of emodin on TGF beta 1 expression in pancreatic tissue of rats suffering from acute pancreatitis] Zhongguo Zhongxiyi Jiehe Zazhi. 2001;21:433–436. [PubMed] [Google Scholar]
- 52.Wang G, Sun B, Gao Y, Meng QH, Jiang HC. An experimental study of emodin assisted early enteral nutrition for the treatment of severe acute pancreatitis. Hepatogastroenterology. 2008;55:33–40. [PubMed] [Google Scholar]
- 53.Wang G, Sun B, Gao Y, Meng QH, Jiang HC. The effect of emodin-assisted early enteral nutrition on severe acute pancreatitis and secondary hepatic injury. Mediators Inflamm. 2007;2007:29638. doi: 10.1155/2007/29638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li ZF, Xia XM, Huang C, Zhang S, Zhang J, Zhang AJ. Emodin and baicalein inhibit pancreatic stromal derived factor-1 expression in rats with acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2009;8:201–208. [PubMed] [Google Scholar]
- 55.Chen S, Yang Y, Feng H, Wang H, Zhao R, Liu H. Baicalein inhibits interleukin-1β-induced proliferation of human rheumatoid arthritis fibroblast-like synoviocytes. Inflammation. 2014;37:163–169. doi: 10.1007/s10753-013-9725-9. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Wu Y, Zhang S, Zhang J, Ji F, Bo W, Guo X, Li Z. Baicalein protect pancreatic injury in rats with severe acute pancreatitis by inhibiting pro-inflammatory cytokines expression. Biochem Biophys Res Commun. 2015;466:664–669. doi: 10.1016/j.bbrc.2015.09.094. [DOI] [PubMed] [Google Scholar]
- 57.Zhang XP, Li ZF, Liu XG, Wu YT, Wang JX, Wang KM, Zhou YF. Effects of emodin and baicalein on rats with severe acute pancreatitis. World J Gastroenterol. 2005;11:2095–2100. doi: 10.3748/wjg.v11.i14.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin JM, Kang JH, Lee SA, Park IH, Lee HM. Baicalin Down-Regulates IL-1β-Stimulated Extracellular Matrix Production in Nasal Fibroblasts. PLoS One. 2016;11:e0168195. doi: 10.1371/journal.pone.0168195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang XP, Feng GH, He JX, Weng WH, Xu RJ, Zhu W, Ye J, Yang QJ, Yuan MJ, Wang Q, et al. Baicalin protects thymus of rats with severe acute pancreatitis. Inflammation. 2010;33:157–165. doi: 10.1007/s10753-009-9169-4. [DOI] [PubMed] [Google Scholar]
- 60.Tan ZH, Yu LH, Wei HL, Liu GT. Scutellarin protects against lipopolysaccharide-induced acute lung injury via inhibition of NF-kappaB activation in mice. J Asian Nat Prod Res. 2010;12:175–184. doi: 10.1080/10286020903347906. [DOI] [PubMed] [Google Scholar]
- 61.Lin LL, Liu AJ, Liu JG, Yu XH, Qin LP, Su DF. Protective effects of scutellarin and breviscapine on brain and heart ischemia in rats. J Cardiovasc Pharmacol. 2007;50:327–332. doi: 10.1097/FJC.0b013e3180cbd0e7. [DOI] [PubMed] [Google Scholar]
- 62.Chen HQ, Zhang XP, Ou JM, Jiang J, Wu DJ. Research on scutellarin parenteral solution’s protective effects in rats with severe acute pancreatitis and multiple organ injuries. Inflammation. 2012;35:1005–1014. doi: 10.1007/s10753-011-9404-7. [DOI] [PubMed] [Google Scholar]
- 63.Li X, Wang L, Li Y, Bai L, Xue M. Acute and subacute toxicological evaluation of scutellarin in rodents. Regul Toxicol Pharmacol. 2011;60:106–111. doi: 10.1016/j.yrtph.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 64.Gao HJ, Liu PF, Li PW, Huang ZY, Yu FB, Lei T, Chen Y, Cheng Y, Mu QC, Huang HY. Ligustrazine monomer against cerebral ischemia/reperfusion injury. Neural Regen Res. 2015;10:832–840. doi: 10.4103/1673-5374.156991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang XP, Wang C, Wu DJ, Ma ML, Ou JM. Protective effects of ligustrazine, kakonein and Panax notoginsenosides on multiple organs in rats with severe acute pancreatitis. Methods Find Exp Clin Pharmacol. 2010;32:631–644. doi: 10.1358/mf.2010.32.9.1444768. [DOI] [PubMed] [Google Scholar]
- 66.Chen J, Chen J, Wang X, Wang C, Cao W, Zhao Y, Zhang B, Cui M, Shi Q, Zhang G. Ligustrazine alleviates acute pancreatitis by accelerating acinar cell apoptosis at early phase via the suppression of p38 and Erk MAPK pathways. Biomed Pharmacother. 2016;82:1–7. doi: 10.1016/j.biopha.2016.04.048. [DOI] [PubMed] [Google Scholar]
- 67.Zhang XP, Jiang J, Cheng QH, Ye Q, Li WJ, Zhu H, Shen JY. Protective effects of Ligustrazine, Kakonein and Panax Notoginsenoside on the small intestine and immune organs of rats with severe acute pancreatitis. Hepatobiliary Pancreat Dis Int. 2011;10:632–637. doi: 10.1016/s1499-3872(11)60107-0. [DOI] [PubMed] [Google Scholar]
- 68.Li ZD, Ma QY, Luo YH. [Effect of resveratrol-induced FasL up-regulation on the apoptosis of pancreatic acinar cells in rats with severe acute pancreatitis] Nanfang Yike Daxue Xuebao. 2009;29:454–457. [PubMed] [Google Scholar]
- 69.Sha H, Ma Q, Jha RK, Wu Z, Qingyuan Z, Wang Z, Ma Z, Luo X, Liu C. Resveratrol suppresses microcirculatory disturbance in a rat model of severe acute pancreatitis. Cell Biochem Biophys. 2013;67:1059–1065. doi: 10.1007/s12013-013-9604-x. [DOI] [PubMed] [Google Scholar]
- 70.Jha RK, Ma Q, Lei Z, Sha H. Resveratrol ameliorates the deleterious effect of severe acute pancreatitis. Cell Biochem Biophys. 2012;62:397–402. doi: 10.1007/s12013-011-9313-2. [DOI] [PubMed] [Google Scholar]
- 71.Carrasco C, Holguín-Arévalo MS, Martín-Partido G, Rodríguez AB, Pariente JA. Chemopreventive effects of resveratrol in a rat model of cerulein-induced acute pancreatitis. Mol Cell Biochem. 2014;387:217–225. doi: 10.1007/s11010-013-1887-0. [DOI] [PubMed] [Google Scholar]
- 72.Zhao M, Xue DB, Zheng B, Zhang WH, Pan SH, Sun B. Induction of apoptosis by artemisinin relieving the severity of inflammation in caerulein-induced acute pancreatitis. World J Gastroenterol. 2007;13:5612–5617. doi: 10.3748/wjg.v13.i42.5612. [DOI] [PMC free article] [PubMed] [Google Scholar]


