Abstract
AIM
To perform a systematic review and meta-analysis on minimally vs conventional invasive techniques for harvesting grafts for living donor liver transplantation.
METHODS
PubMed, Web of Science, EMBASE, and the Cochrane Library were searched comprehensively for studies comparing MILDH with conventional living donor hepatectomy (CLDH). Intraoperative and postoperative outcomes (operative time, estimated blood loss, postoperative liver function, length of hospital stay, analgesia use, complications, and survival rate) were analyzed in donors and recipients. Articles were included if they: (1) compared the outcomes of MILDH and CLDH; and (2) reported at least some of the above outcomes.
RESULTS
Of 937 articles identified, 13, containing 1592 patients, met our inclusion criteria and were included in the meta-analysis. For donors, operative time [weighted mean difference (WMD) = 20.68, 95%CI: -6.25-47.60, P = 0.13] and blood loss (WMD = -32.61, 95%CI: -80.44-5.21, P = 0.18) were comparable in the two groups. In contrast, analgesia use (WMD = -7.79, 95%CI: -14.06-1.87, P = 0.01), postoperative complications [odds ratio (OR) = 0.62, 95%CI: 0.44-0.89, P = 0.009], and length of hospital stay (WMD): -1.25, 95%CI: -2.35-0.14, P = 0.03) significantly favored MILDH. No differences were observed in recipient outcomes, including postoperative complications (OR = 0.93, 95%CI: 0.66-1.31, P = 0.68) and survival rate (HR = 0.96, 95%CI: 0.27-3.47, P = 0.95). Funnel plot and statistical methods showed a low probability of publication bias.
CONCLUSION
MILDH is safe, effective, and feasible for living donor liver resection with fewer donor postoperative complications, reduced length of hospital stay and analgesia requirement than CLDH.
Keywords: Living donor hepatectomy, Graft harvesting, Minimally invasive techniques, Conventional invasive approaches, Meta-analysis
Core tip: Minimally invasive procedures have been increasingly used in liver resection, as they are considered safe and effective. Concerns have been raised, however, about the feasibility and donor safety of minimally invasive living donor hepatectomy. We analyzed 13 articles, containing 1592 patients, to compare two techniques for harvesting grafts for living donor liver transplantation. Finally, we concluded that minimally invasive procedures are safe, effective, and feasible for living donor liver resection, with fewer donor postoperative complications and reduced length of hospital stay and analgesia requirement than conventional approaches.
INTRODUCTION
Since the first reported successful human liver transplantation in 1967[1], this technique has gained worldwide acceptance, becoming the best and most common treatment for patients with end-stage liver disease. Because of the shortage of deceased donor organs, especially in East Asian countries, living donor liver transplantation (LDLT) has become an established treatment modality for patients with end-stage liver disease[2]. In 1990, the first successful pediatric LDLT, using a left lateral section graft from a mother to her son, was reported in Australia[3]. Since then, the feasibility and safety of pediatric LDLT have been well documented[4]. Donor safety is considered paramount, as donor hepatectomy is a major surgery for healthy individuals[5]. However, the large permanent abdominal incision scar resulting from conventional open surgery may cause mental and physical stress among some putative living donors, especially young unmarried women, resulting in hesitation or unwillingness to donate liver tissue[4,6].
Although conventional living donor hepatectomy (CLDH) is safe, approximately 40% of donors have experienced postoperative complications[7-9]. Minimally invasive liver surgery has been widely used to treat patients with various liver diseases. Although laparoscopic liver surgery has resulted in lower rates of surgical morbidity and reduced postoperative pain and recovery time when compared with standard liver surgery[10,11], minimally invasive approaches to living donor hepatectomy are not generally performed. Minimally invasive living donor hepatectomy (MILDH), involving either a laparoscopic approach or a hybrid technique, has been compared with CLDH in several centers.
Although studies have compared outcomes following MILDH and CLDH, most of these studies were small series with unclear results[12-15]. Thus, their relative benefits for donors have not been investigated. This systematic review and meta-analysis analyzed studies comparing MILDH with CLDH to evaluate the safety, efficacy, and potential advantages of MILDH.
MATERIALS AND METHODS
Objective and groups
This meta-analysis was performed to compare the feasibility and donor safety of MILDH with CLDH, including evaluations of recipient survival rates. Outcomes compared included perioperative complications, estimated blood loss (EBL), requirement for analgesics, overall survival, operative time, postoperative liver function and hospital costs. MILDH in this study included fully laparoscopic and laparoscopy-assisted approaches, upper midline incision with or without laparoscopic assistance, and a hybrid approach with incision length ≤ 15 cm. CLDH included standard open donation with a large subcostal incision, Mercedes incision, L-shaped incision, and a large J-shaped or midline skin incision.
Search strategy and criteria
PubMed, EMBASE, the Web of Science, and Cochrane Library were searched for studies comparing MILDH with CLDH published through December 2015. There were no restrictions on publication date, type or language. Search terms included “donor hepatectomy” OR “liver transplantation” OR “donor liver resection” OR “donor sectionectomy” AND “open surgery” OR “right subcostal incision” OR “regular surgery” OR “conventional surgery” AND “laparoendoscopic” OR “laparoscopic”. The reference lists of all selected articles were manually searched to determine if they should be included.
The literature search identified 937 articles, of which 288 from PubMed, 434 from EMBASE, 213 from Web of Science, and two from the Cochrane Library (Figure 1). Two reviewers browsed the titles and abstracts independently. Articles were included if they: (1) compared the outcomes of MILDH and CLDH; and (2) reported at least some of the above outcomes. Articles were excluded if were submitted by the same authors or the same institutions to avoid duplication of patient populations.
Figure 1.
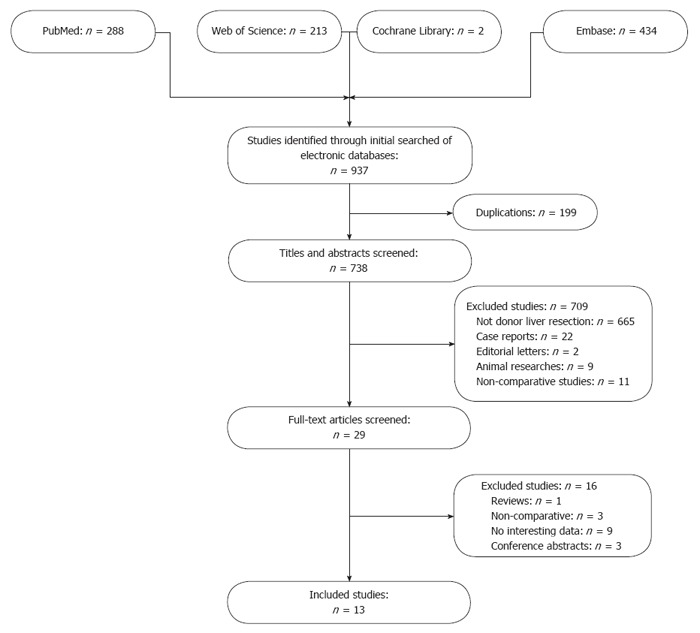
Flow diagram of study identification, inclusion and exclusion.
Of the 937 identified articles, 199 were duplications; 665 did not focus on donor liver resection; nine were in animals; 11 did not compare MILDH with CLDH; two were editorials; and 22 were case reports. The full texts of the remaining 29 articles were carefully reviewed. Of these, three did not compare MILDH with CLDH; nine did not include outcomes of interest; one was a review article; and three were conference abstracts. Finally, 13 articles[12-24] were included in this meta-analysis.
Data management
Data were analyzed by three authors (Li H, Zhang JB and Chen XL) independently. These reviewers were blinded to the authors, institutions, and journals of publication of all selected articles. Any disagreements between the reviewers were settled by the senior author (Wang GS).
Donor outcomes of interest included operative time, EBL, hospital costs, length of hospital stay, postoperative complications, analgesic use, graft weight, and postoperative liver function. Liver function was evaluated based on peak serum levels of aspartate transaminase (AST), alanine aminotransferase (ALT), and total bilirubin (TB). Recipient outcomes of interest included postoperative complications, postoperative liver function, and survival rate. If survival rate did not appear directly in an article, it was determined using Engauge software.
Quality assessment
The methodological quality of retrospective studies was assessed using the modified Newcastle-Ottawa scale, which consists of three factors: patient selection, comparability of the study groups, and assessment of outcome[25,26]. As the maximum total score on this scale is 9, studies with scores ≥ 7 were defined as high-quality studies (Tables 1 and 2).
Table 1.
Quality of cohort studies evaluated with modified Newcastle-Ottawa scale
| Ref. | Case definition |
Selection |
Definition of controls |
Comparability |
Outcomes |
Quality score | |||
| Represen- tativeness | Selection of controls | Comparable for 1, 2, 3 | Comparable for 4, 5 | Assessment of outcomes | Integrity of follow-up | ||||
| Choi et al[16], 2012 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Choi et al[17], 2014 | Yes | No | Yes | Yes | No | No | Yes | Yes | 5 |
| Makk et al[19], 2014 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 8 |
| Marubashi et al[20], 2013 | Yes | No | Yes | Yes | 2, 3 | 4 | Yes | Yes | 7 |
| Nagai et al[21], 2012 | Yes | No | Yes | Yes | 1, 3 | 4 | Yes | Yes | 7 |
| Samstein et al[22], 2015 | Yes | No | Yes | Yes | Yes | No | Yes | Yes | 7 |
| Soubrane et al[12], 2006 | Yes | No | Yes | Yes | 1,3 | 4 | Yes | Yes | 7 |
| Suh et al[23], 2015 | Yes | No | Yes | Yes | No | No | Yes | Yes | 5 |
1 = gender; 2 = body max index; 3 = graft generation; 4 = age; 5 = haemoglobin.
Table 2.
Quality of case-controlled studies evaluated with modified Newcastle-Ottawa scale
| Ref. | Case definition |
Selection |
Definition of controls |
Comparability |
Outcomes |
Quality score | |||
| Represen- tativeness | Selection of controls | Comparable for 1, 2, 3 | Comparable for 4, 5 | Ascertainment of exposure | Non-response | ||||
| Baker et al[13], 2009 | Yes | No | Yes | Yes | Yes | 4 | Yes | Yes | 7 |
| Kim et al[14], 2009 | Yes | No | Yes | Yes | Yes | 4 | Yes | Yes | 7 |
| Kim et al[18], 2011 | Yes | No | Yes | Yes | 1, 3 | Yes | Yes | Yes | 6 |
| Thenappan et al[15], 2011 | Yes | No | Yes | Yes | No | No | Yes | Yes | 7 |
| Zhang et al[24], 2014 | Yes | No | Yes | Yes | Yes | 4 | Yes | Yes | 7 |
1 = gender; 2 = body max index; 3 = graft generation; 4 = age; 5 = haemoglobin.
Data were pooled with the Cochrane Collaboration’s Review Manager 5.3 (Cochrane Collaboration, Oxford, United Kingdom). Mean differences and 95%CIs were calculated to pool functional outcomes. Statistical heterogeneity among studies was assessed using the χ2 test with significance set at P < 0.1, and heterogeneity was quantified using the I2 statistic. A random-effects model was used if there was heterogeneity among studies; otherwise, a fixed-effects model was used[27].
Subgroups and publication bias
Grafts harvested from the left and right sides of the liver differ in weight, vascularity, and bile duct distribution, affecting outcomes in both donors and recipients. Therefore, subgroup analyses were performed on donors who underwent left hepatectomy (LH) and right hepatectomy (RH). Operative time, postoperative complications, and EBL were analyzed in these subgroups.
The publication bias of selected articles was analyzed by funnel plots, which were produced by Review Manager 5.3. If outcomes were associated with significant heterogeneity among studies, a random-effects model was used to minimize bias resulting from this heterogeneity.
Characteristics of selected articles
The meta-analysis included 13 articles[12-24] involving 1592 patients; the characteristics of the selected articles are shown in Table 3. Primary outcomes of interest included donor safety, as determined by perioperative complications and EBL; donor requirement for analgesics after hepatectomy; and recipient survival rate. Secondary outcomes included postoperative liver function, length of hospital stay, and total hospital cost. The level of evidence of these articles was estimated using the United Kingdom Cochrane Centre of Evidence (2001)[28]. Six articles described cohort studies comparing contemporary series of patients (level of evidence: 2b)[12,16,19-22]; five articles were retrospective case-control studies (level of evidence: 3b)[13-15,18,24], and two articles were retrospective studies using historical series as controls (level of evidence: 4)[17,23].
Table 3.
Characteristics of included studies
| Ref. | Level of evidence |
Patient no. |
Left/right | Recipients | TMI | TCI | Matching | Quality score | |
| MILDH | CLDH | ||||||||
| Baker et al[13], 2009 | 3b | 33 | 33 | Right | W | LA | Midline epigastric | 1, 2, 3, 4 | 7 |
| Choi et al[16], 2012 | 2b | 60 | 90 | Right | W/O | LA | Right subcostal | 1, 2, 3, 4, 5 | 8 |
| Choi et al[17], 2014 | 4 | 25 | 484 | Right | W/O | HAL or LA | Mercedes-Benz or L-shaped | NA | 5 |
| Kim et al[14], 2009 | 3b | 23 | 23 | Right | W | Upper midline | J-shaped | 1, 2, 3, 4 | 7 |
| Kim et al[18], 2011 | 3b | 11 | 11 | Left | W | L | J-shaped or midline | 1, 3, 4, 5 | 7 |
| Makk et al[19], 2014 | 2b | 26 | 24 | Right | W | LA | Right subcostal with midline extension | 1, 2, 3, 4, 5 | 8 |
| Marubashi et al[20], 2013 | 2b | 31 | 79 | Left | W | LA | Mercedes | 2, 3, 4 | 7 |
| Nagai et al[21], 2012 | 2b | 28 | 30 | Right | W | Hal or upper midline | Mercedes | 1, 3, 4 | 7 |
| Samstein et al[22], 2015 | 2b | 22 | 20 | Left | W | L | Midline | 1, 2, 3 | 7 |
| Soubrane et al[12], 2006 | 2b | 16 | 14 | Left | W | L | Subcostal | 1, 3, 4 | 7 |
| Suh et al[23], 2015 | 4 | 161 | 268 | Un | W | LA or Upper midline | L-shaped | NA | 5 |
| Thenappan et al[15], 2011 | 3b | 15 | 15 | Un | W | LA or Minimally-access | Midline epigastric with subcostal | NA | 6 |
| Zhang et al[24], 2014 | 3b | 25 | 25 | Right | W | LA | Right subcostal | 1, 2, 3, 4 | 7 |
MILDH: Minimally invasive living donor hepatectomy; CLDH: Conventional living donor hepatectomy; Left/right: Graft from left or right liver lobe of donors; Recipients: With or without analyzing recipients; TMI: Type of minimally incisions; TCI: Type of conventional incisions; W: With; W/O: Without; Un: Unclear or not only one kind; L: Laparoscopic approach; LA: Laparoscopy-assisted; HAL: Hand-assisted laparoscopic; Matching: 1 = gender; 2 = body max index; 3 = graft generation; 4 = age; 5 = haemoglobin; NA: No data available.
RESULTS
Donor outcomes
Of the 1592 donors included in the 13 articles, 476 underwent MILDH and 1116 underwent CLDH (Table 4)[12-24]. Operative times were similar in the two groups [weighted mean difference (WMD) = 20.68, 95%CI: -6.25-47.60, P = 0.13] (Figure 2). Twelve studies[12-18,20-24] analyzed EBL among 1542 donors, finding no significant difference between those who underwent MILDH and CLDH (WMD = -32.61, 95%CI: -80.44-5.21, P = 0.18). Hospital costs were reported by only two articles[18,24], finding no significant difference between the two donor groups (WMD = 0.56, 95%CI: -0.62-0.74, P = 0.35). Ten studies[12,14-16,18,20-24], including 967 patients, evaluated the length of hospital stay, finding that donors who underwent MILDH group had a significantly shorter hospital stay than those who underwent CLDH (WMD: -1.25, 95%CI: -2.35-0.14, P = 0.03). Twelve articles[12-16,18-24] analyzed postoperative complications, finding that the rate of postoperative complications was significantly lower in the MILDH than in the CLDH group (OR = 0.62, 95%CI: 0.44-0.89, P = 0.009). Ten articles[12-16,18-24] compared postoperative complications of donors between the two groups, finding no statistical difference (WMD = 0.56, 95%CI: 0.27-1.18, P = 0.13) (Figure 3). Five articles[12,14-16,24] reported analgesic use, finding that the total analgesic use among donors was significantly lower in the MILDH than in the CLDH group (WMD = -7.79, 95%CI: -14.06-1.87, P = 0.01) (Figure 4). Five studies[12-14,19,24] reported graft weight, finding no significant difference between the two groups (WMD = -3.32, 95%CI: -22.25-15.61, P = 0.73). Seven articles[14,16,18,19,21,23,24] compared postoperative liver function, finding no significant difference between the two groups in peak AST (WMD = 6.41, 95%CI: -3.79-16.60, P = 0.50), ALT (WMD = 11.86, 95%CI: -10.84-34.56, P = 0.031), and TB (WMD = -0.10, 95%CI: -0.26-0.06, P = 0.21) concentrations (Figure 5).
Table 4.
Results of meta-analysis comparison of minimally invasive living donor hepatectomy and conventional living donor hepatectomy
| Outcome of interest | Study (n) | MILDH (n) | CLDH (n) | WMD/OR (95%CI) | P value |
Study heterogeneity |
P value | ||
| I2 | df | I2, % | |||||||
| Graft weight (g) | 5 | 123 | 119 | -3.32 (-22.25,15.61) | 0.73 | 6.56 | 4 | 39 | 0.16 |
| Donor outcomes | |||||||||
| Operative time (min) | 13 | 476 | 1116 | 20.68 (-6.25,47.60) | 0.13 | 147.62 | 12 | 92 | < 0.01 |
| Estimated blood loss (mL) | 12 | 450 | 1092 | -32.61 (-80.44,15.21) | 0.18 | 61.26 | 11 | 82 | < 0.01 |
| Hospital cost (dollar) | 2 | 36 | 36 | 0.56 (-0.62,1.74) | 0.35 | 4.24 | 1 | 76 | 0.04 |
| Length of hospital stay (d) | 10 | 392 | 575 | -1.25 (-2.35,-0.14) | 0.03 | 99.31 | 9 | 91 | < 0.01 |
| Post complications | 12 | 451 | 632 | 0.62 (0.44,0.89) | 0.009 | 4.40 | 11 | 0 | 0.96 |
| Analgesic use (h) | 5 | 139 | 167 | -7.97 (-14.06,-1.87) | 0.01 | 7.50 | 4 | 47 | 0.11 |
| Liver function | |||||||||
| Post AST peak (IU/L) | 7 | 334 | 471 | 6.41 (-3.79.16.60) | 0.22 | 13.60 | 6 | 56 | 0.03 |
| Post ALT peak (IU/L) | 8 | 350 | 485 | 11.86 (-10.84,34.57) | 0.31 | 15.39 | 7 | 55 | 0.03 |
| Post TB peak (mg/dL) | 7 | 324 | 461 | -0.10 (-0.26,0.06) | 0.21 | 2.10 | 6 | 0 | 0.91 |
| Recipient outcomes | |||||||||
| Liver function | |||||||||
| Post AST peak (IU/L) | 3 | 59 | 59 | -28.73 (-86.76,29.31) | 0.33 | 0.90 | 2 | 0 | 0.64 |
| Post ALT peak (IU/L) | 3 | 59 | 59 | -29.98 (-87.65,27.7) | 0.31 | 0.31 | 2 | 0 | 0.86 |
| Post TB peak (mg/dL) | 3 | 59 | 59 | -0.96 (-2.57,0.65) | 0.24 | 1.26 | 2 | 0 | 0.53 |
| Surviving | 3 | 0.96 (0.27,3.47) | 0.95 | 0.11 | 2 | 0 | 0.95 | ||
| Post complications | 6 | 272 | 375 | 0.93 (0.66,1.31) | 0.68 | 3.28 | 5 | 0 | 0.66 |
MILDH: Minimally invasive living donor hepatectomy; CLDH: Conventional living donor hepatectomy; WMD/OR: Weight mean difference/odds ratio; df: Degree of freedom; Post: Postoperative.
Figure 2.
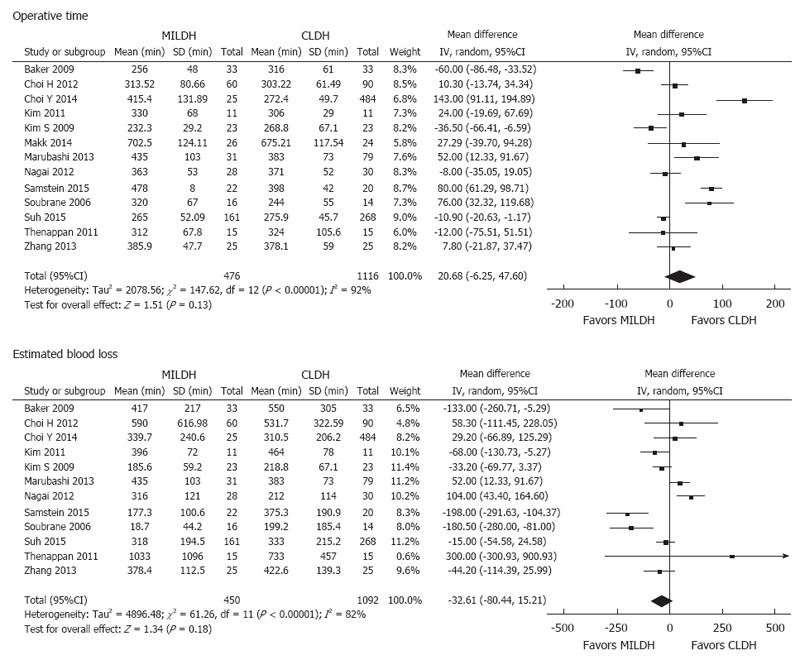
Forest plots and meta-analysis of intraoperative outcomes of donors.
Figure 3.
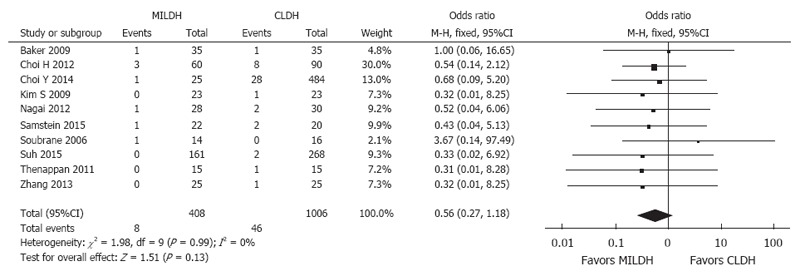
Forest plot and meta-analysis of postoperative biliary complications for donors.
Figure 4.
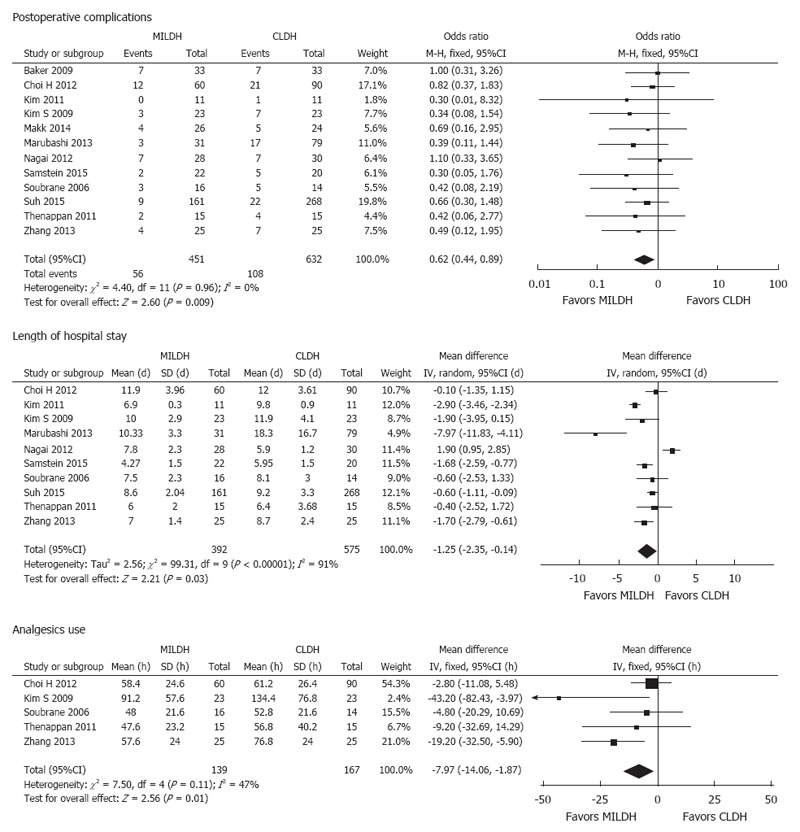
Forest plots and meta-analysis of postoperative outcomes of donors.
Figure 5.
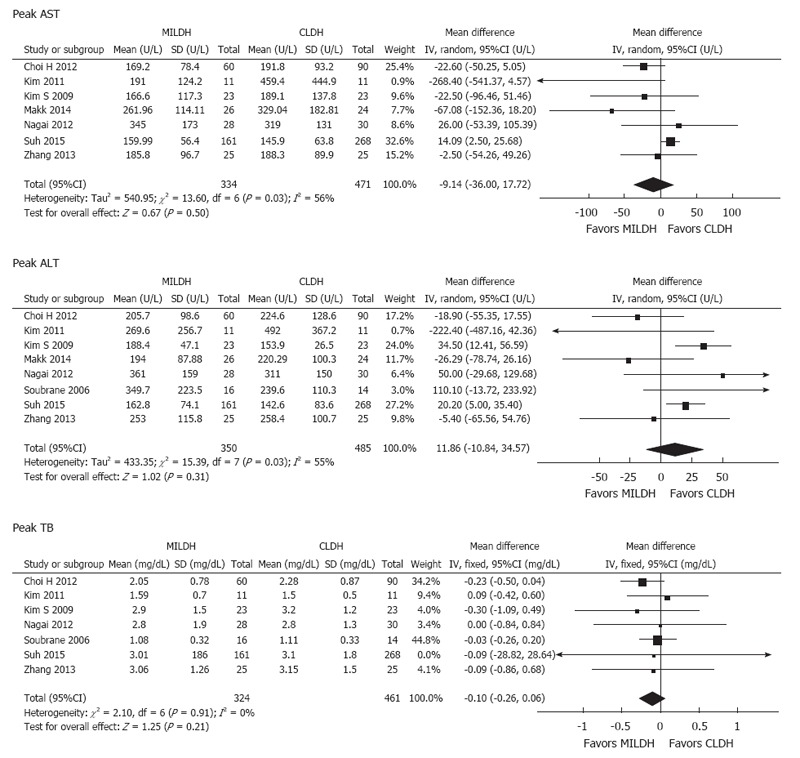
Forest plots and meta-analysis of postoperative liver function of donors.
Recipient outcomes
Six studies[12,13,18,19,23,24] compared postoperative complications in recipients, finding no significant difference in postoperative complication rates between the two groups (OR = 0.93, 95%CI: 0.66-1.31, P = 0.68). Seven studies[12,13,18,19,23,24] analyzed postoperative biliary complications for recipients, showing no significant difference between the two groups (WMD = 1.10, 95%CI: 0.73-1.66, P = 0.65) (Figure 6). Three studies[14,18,24] compared postoperative liver function in recipients, finding no significant differences between the two groups in peak AST (WMD = -28.73, 95%CI: -86.76-29.31, P = 0.33), ALT (WMD = -29.98, 95%CI: -87.65-27.7, P = 0.31), and TB (WMD = -0.96, 95%CI: -2.57-0.65, P = 0.24) concentrations. Three articles[13,20,22] compared overall recipient survival, finding no significant difference between the two groups in recipient survival rate (HR = 0.96, 95%CI: 0.27-3.47, P = 0.95) (Figure 7).
Figure 6.
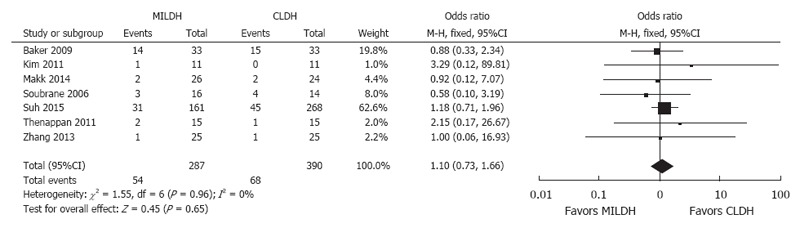
Forest plot and meta-analysis of postoperative biliary complications for recipients.
Figure 7.
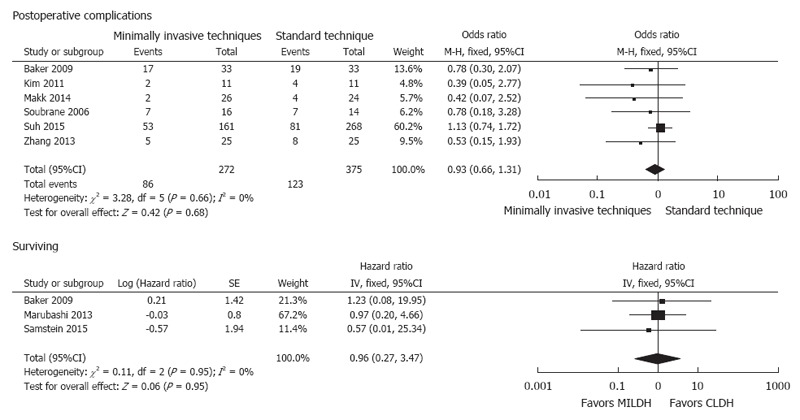
Forest plots and meta-analysis of postoperative outcomes of recipients.
Subgroup analysis
Postoperative complications, operative time, and EBL were analyzed in donors who underwent RH and LH. In assessing postoperative complications, three studies[15,17,23] were excluded, as data were unmatched. Pooled data of six studies[13,14,16,19,21,24] showed no significant difference in postoperative complication rates in donors who underwent RH by MILDH and CLDH, but did favor MILDH (OR = 0.73, 95%CI: 0.45-1.19, P = 0.21). In contrast, pooled data of four studies[12,18,20,22] that evaluated donors who underwent LH showed that the postoperative complication rate was significantly lower in patients who underwent MILDH (OR = 0.37, 95%CI: 0.16-0.87, P = 0.02) (Figure 8).
Figure 8.
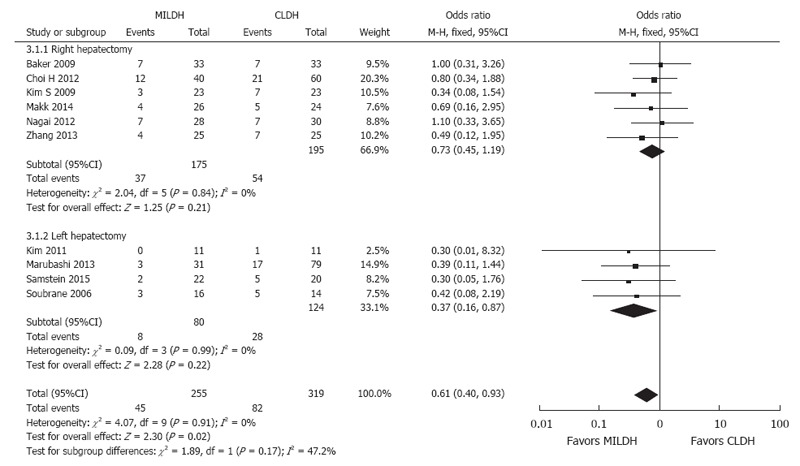
Forest plot and meta-analysis of postoperative complication rates for donors.
Seven studies[13,14,16,17,19,21,24] compared operative time for RH, finding no significant difference between MILDH and CLDH (WMD = 14.99, 95%CI: -22.52-52.50, P = 0.43). In contrast, four studies[12,18,20,22] that compared operative time for LH found that this time was significantly shorter for CLDH (WMD = 62.04, 95%CI: 37.04-87.03, P < 0.0001). The remaining two studies[15,23] were excluded (Figure 9).
Figure 9.
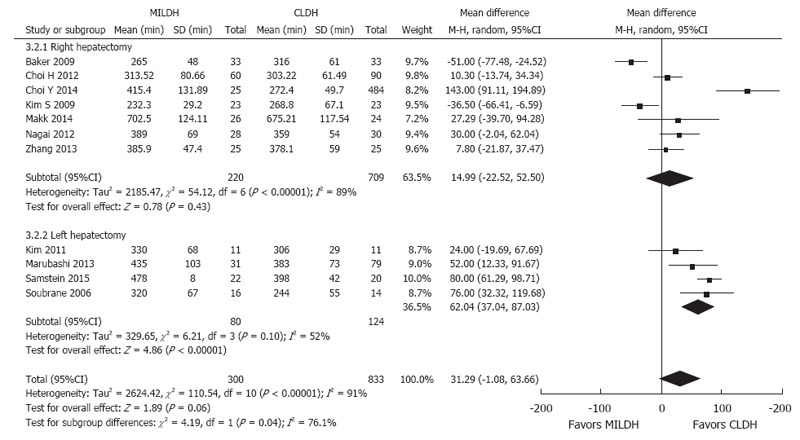
Forest plot and meta-analysis of operative time for donors.
Six studies[13,14,16,17,21,24] reported EBL in donors who underwent RH, finding no significant difference between the MILDH and CLDH groups (WMD = -1.67, 95%CI: -66.05-62.72, P = 0.96). Similarly, four studies[12,18,20,22] compared EBL in donors who underwent LH, finding no significant difference between the two groups (WMD = -93.04, 95%CI: -215.56-29.48, P = 0.14) (Figure 10).
Figure 10.
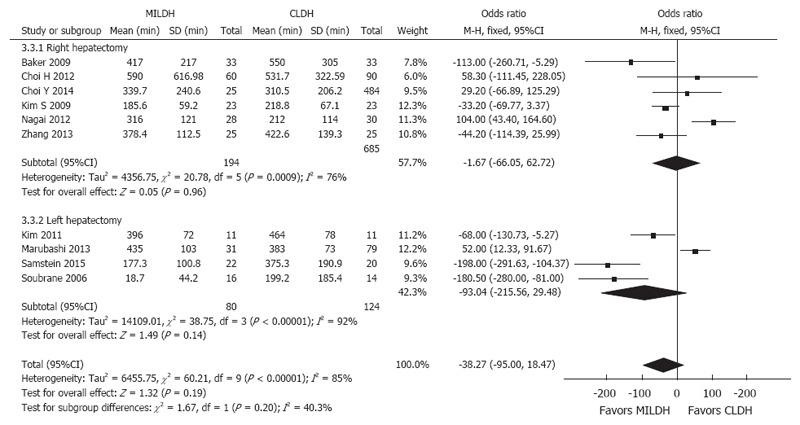
Forest plot and meta-analysis of estimated blood loss for donors.
Publication bias
The funnel plot of postoperative complications showed that all articles included in this meta-analysis were symmetrically distributed around the center line, indicating a lack of obvious publication bias (Figure 11).
Figure 11.
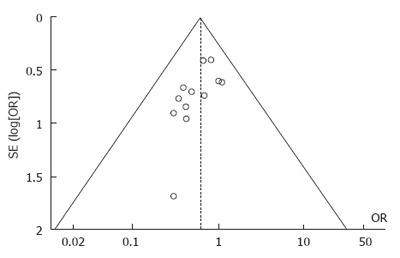
Funnel plot of postoperative complication rates.
DISCUSSION
Primary laparoscopic living donor hepatectomy was introduced in 2002 to reduce the impact of open hepatectomy on donors[29]. Since then, minimally invasive approaches have been considered safe and effective, reducing postoperative pain and surgical morbidity and providing a faster recovery time[9,11,30,31]. Despite these findings, minimally invasive approaches to living donor hepatectomy have not been accepted by consensus guidelines. A hybrid technique for donor hepatectomy was introduced in 2006[32], and subsequent studies have compared minimally invasive and conventional donor liver resection[33]. To date, however, these two methods have not been systematically analyzed in large donor populations.
This systematic review and meta-analysis of 13 studies, involving 1592 patients, compared minimally invasive with conventional methods for living donor hepatectomy, finding that MILDH was not less safe than CLDH. MILDH was associated with a significantly lower postoperative complication rate, a significantly lower analgesic requirement, and a significantly shorter hospital stay for donors than CLDH. However, operative time, EBL, graft weight, hospital costs, and postoperative liver function for donors were similar in the two groups. Moreover, comparisons of postoperative recipient liver function, complication rate, and survival rate showed no differences between these two groups.
Donor safety is of paramount importance during LDLT, regardless of the technique used. Our pooled data on perioperative outcomes indicated that MILDH was as safe and effective for LDLT as CLDH.
Our finding that operative times were comparable in the two groups is inconsistent with several studies suggesting that MILDH was associated with a shorter average operative time[13,14]. This may have been owing to the dissimilarity of operative procedures in different institutions. Nevertheless, the operative time for making an upper midline incision was generally shorter in the MILDH group, as the incision was shorter. Although the small incision reduced the time spent in opening and closing the abdomen, it was apparently balanced by the additional time required to mobilize grafts laparoscopically, as this approach required frequent installation and removal of laparoscopic devices, application of the hanging maneuver, and dissection of the deep parenchyma.
EBL did not differ significantly between the MILDH and CLDH groups, although it was lower in the MILDH group. Laparoscopic parenchymal dissection and the high intra-abdominal pressure attained by pneumoperitoneum use apparently resulted in lower blood loss in the MILDH group[34]. Furthermore, laparoscopy provided a magnified view of the liver, which was good for bleeding control.
Unlike intraoperative indices, postoperative outcomes favored MILDH. Our meta-analysis showed that postoperative complications (including wound-related, biliary, and vascular complications) occurred in 164 of 1093 patients in 12 studies, with a significantly lower postoperative donor complication rate in the MILDH group (12.4%) than in the CLDH group (17.1%). Few patients in either group experienced severe complications, including death or need for retransplantation. Our study also showed no statistical difference in the donor biliary complication rate between the MILDH group (1.96%) and CLDH group (4.57%), but favored the MILDH group. The incidence of postoperative biliary complications of donors was closely related with the preoperative assessment of the biliary system and intraoperative anatomical techniques[35]. In laparoscopic liver resection, hepatic bile duct and artery could be identified more precisely with the amplification effect of laparoscopy, and the probability of bile duct injury would be reduced. We considered them as the main reasons for lower donor biliary complication rate in the MIDH group. In addition, preoperative magnetic resonance cholangiopancreatography (MRCP), intraoperative cholangiography and marking bile duct cut line would help to reduce postoperative biliary complications in donors[16].
Vascular complications (including postoperative bleeding and vascular embolization) of donors were related to the preoperative assessment of hepatic vascular system and intraoperative anatomical techniques. Preoperative accurate assessment of hepatic vascular structures and careful intraoperative dissection techniques could reduce postoperative vascular complications effectively. Dissecting the liver precisely by minimally invasive approaches would also help to reduce donor vascular complications.
The rate of incision complications of the CLDH group was obviously higher than that of the MILDH group. The CLDH group adopted the “J” and “L” shape and “Mercedes” incision, which were larger compared to the other group. Large incision might cut off more abdominal nerves and was a high risk factor for incision complications. The smaller incision employed for MILDH, especially during laparoscopic surgery, could minimize surgical tissue trauma and abdominal nerve injury, thus reducing the rate of wound-related complications (infections, hematomas, and incisional hernias) and abdominal injuries. This finding is in good agreement with previous results[30]. Laparoscopic graft harvesting, in particular, could reduce incision hernia effectively. Smaller incision also contributes to reducing postoperative analgesia drug dose. Furthermore, minimally invasive approaches could result in earlier postoperative recovery of donors, thus minimizing other complications such as pleural effusion and intestinal obstruction.
Our study also found that the duration of continuous intravenous analgesic use was shorter in donors who underwent MILDH than in those who underwent CLDH. Minimally invasive approaches can reduce postoperative pain, as these approaches avoid cutting the subcostal muscle that is cut by conventional incisions, as well as minimizing surgical tissue trauma. Length of hospital stay was also significantly shorter for donors who underwent MILDH than in those who underwent CLDH, enabling the former to return to their normal lives earlier after surgery. A meta-analysis of 112 studies evaluating six laparoscopic surgical procedures showed a more rapid return to work after minimally invasive surgery[36]. Shorter hospital stay also contributed to lower hospital costs, increasing donor satisfaction with operative procedures. These outcomes were consistent with several studies of laparoscopic-assisted living donor hepatectomy, which found less pain, improved postoperative symptoms, and faster recovery compared with conventional open surgery[33,37].
Postoperative donor liver function was evaluated by measuring peak levels of serum AST, ALT, and TB. The pooled data showed no significant difference between the two groups. Reduced liver volume after donor liver resection may result in immediate, but transient, increases in peak AST, ALT, and TB. As the liver regenerates, all three indices would decline gradually. MILDH was a more difficult surgical procedure, but did not worsen liver function. Recovery time would be similar in donors who underwent MILDH and CLDH.
In subgroup analysis, we separately analyzed outcomes, including operative time, EBL, and postoperative complication rates, in patients who underwent LH or RH, to minimize any bias resulting from the side of liver resection. Donors who underwent minimally invasive LH had a lower rate of postoperative complications than those who underwent conventional LH; however, there was no between-group difference in donors who underwent RH. EBL and operative time were similar in donors who underwent minimally invasive and conventional LH and RH.
Evaluation of recipients showed no statistically significant differences in postoperative complication rate, postoperative liver function, or survival rate, although the complication rate was lower in the MILDH than in the CLDH group. There were also no significant differences in recipient postoperative liver function, as determined by peak serum AST, ALT, and TB levels. These findings indicate that the method of procuring liver grafts would have little effect on postoperative recipient liver function. Recovery times are similar in recipients who received grafts procured through MILDH and CLDH. The three studies[13,20,22] that evaluated recipient survival rate found no significant between-group difference. Other reports[38] evaluated several of the studies included in our meta-analysis, reporting survival rate but not postoperative liver function.
The biliary tree manipulation and identification have key impacts on the functions of graft. Our results showed no significant difference in the rate of recipient biliary complications between the MILDH group (18.8%) and CLDH group (17.4%). The recipient biliary complications include bile leakage and biliary stenosis and are closely related to the quality of liver graft[39]. Dissecting the liver precisely by minimally invasive approaches would help to harvest high-quality liver grafts and reduce the recipient biliary complications. In addition, comprehensive preoperative assessment of the biliary tract for donors (clear whether there are anomalies), familiarity with the hepatic biliary anatomy, cutting off donor bile duct precisely and feasible measures such as intraoperative cholangiography could minimize the risk of postoperative biliary complications[15].
This meta-analysis had several limitations, including the quality of the included studies. No randomized controlled trials were included, increasing the risk of bias owing to inadequate random sequence generation and blinding. In addition, all included articles were single-center studies, but differences in surgeons’ experiences with the two techniques may have influenced patient outcomes. Moreover, within each study comparing MILDH with CLDH, not all operations were performed by a single surgeon, which may have introduced selection bias. Third, the follow-up period was generally short; therefore, long-term donor outcomes could not be evaluated. Finally, only three of the 13 included studies evaluated recipient survival rate. Because these recipients underwent LDLT with curative intent, their survival rate would be an important indicator of the safety and efficacy of these surgical procedures.
Nevertheless, the results of this meta-analysis are encouraging, as MILDH, which is more challenging to perform than CLDH, was always performed by experienced liver surgeons with a commitment to minimally invasive surgery[1]. Moreover, sufficient data on a large patient cohort that had undergone MILDH had accumulated, allowing evaluation by meta-analytical methods. Multiple strategies were used to identify applicable studies, with strict criteria used for study inclusion and evaluation. Subgroup analysis was performed to minimize heterogeneity. Future studies comparing MILDH and CLDH should include larger numbers of patients, with more data about recipients and a longer follow-up period.
In conclusion, the results of this meta-analysis comparing MILDH to CLDH show that MILDH could result in lower postoperative complication rate and analgesics requirement and shorter hospital stay with similar recipient outcomes. MILDH is safe, effective, and feasible for living donor liver resection. Nevertheless, MILDH, especially fully laparoscopic approach for the right lobe harvesting, is still an immature procedure with uncertain risk and effect, and should be performed cautiously.
COMMENTS
Background
Living donor liver transplantation has become an established treatment modality for patients with end-stage liver disease. With the wide use of minimally invasive techniques in hepatic surgery in recent years, more importance has been attached to minimally invasive living donor hepatectomy. Several centers considered minimally invasive approaches as safe and efficient techniques for graft harvested compared to conventional techniques. Despite this, no consensus is available in the literature about which of these two approaches is more beneficial to the patient.
Research frontiers
Nowadays living donor liver harvesting is performed with minimally invasive approaches in a growing number of centers. The worldwide research is directed towards a type of technique to guarantee the safety of donors.
Innovations and breakthroughs
In the present study, the authors investigated the outcomes of minimally invasive living donor liver resection and conventional approaches by pooling results from different centers. This is the first report of a meta-analysis comparing these two kinds of surgical approaches with concluding satisfactory results.
Applications
This report allows understanding the role of two surgical techniques for living donor hepatectomy.
Peer-review
This systematic review and meta-analysis of retrospective studies adds useful information for practice and research, and probably for policy.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: The authors declare that there is no conflict of interest related to this report.
Data sharing statement: No additional data are available.
Peer-review started: October 28, 2016
First decision: December 29, 2016
Article in press: April 12, 2017
P- Reviewer: Bramhall S, Sanada Y, Wang CC S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Starzl TE, Groth CG, Brettschneider L, Penn I, Fulginiti VA, Moon JB, Blanchard H, Martin AJ, Porter KA. Orthotopic homotransplantation of the human liver. Ann Surg. 1968;168:392–415. doi: 10.1097/00000658-196809000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim KH, Yu YD, Jung DH, Ha TY, Song GW, Park GC, Hwang S, Lee SG. Laparoscopic living donor hepatectomy. Korean J Hepatobiliary Pancreat Surg. 2012;16:47–54. doi: 10.14701/kjhbps.2012.16.2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strong RW, Lynch SV, Ong TH, Matsunami H, Koido Y, Balderson GA. Successful liver transplantation from a living donor to her son. N Engl J Med. 1990;322:1505–1507. doi: 10.1056/NEJM199005243222106. [DOI] [PubMed] [Google Scholar]
- 4.Park JI, Kim KH, Lee SG. Laparoscopic living donor hepatectomy: a review of current status. J Hepatobiliary Pancreat Sci. 2015;22:779–788. doi: 10.1002/jhbp.288. [DOI] [PubMed] [Google Scholar]
- 5.Rao PP, Routh D, Naidu CS, Sharma S, Sharma AK, Priyaranjan V, Gaur A. Donor outcome in live-related liver transplantation. Med J Armed Forces India. 2014;70:100–104. doi: 10.1016/j.mjafi.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suh KS, Yi NJ, Kim T, Kim J, Shin WY, Lee HW, Han HS, Lee KU. Laparoscopy-assisted donor right hepatectomy using a hand port system preserving the middle hepatic vein branches. World J Surg. 2009;33:526–533. doi: 10.1007/s00268-008-9842-z. [DOI] [PubMed] [Google Scholar]
- 7.Ghobrial RM, Freise CE, Trotter JF, Tong L, Ojo AO, Fair JH, Fisher RA, Emond JC, Koffron AJ, Pruett TL, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology. 2008;135:468–476. doi: 10.1053/j.gastro.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trotter JF, Talamantes M, McClure M, Wachs M, Bak T, Trouillot T, Kugelmas M, Everson GT, Kam I. Right hepatic lobe donation for living donor liver transplantation: impact on donor quality of life. Liver Transpl. 2001;7:485–493. doi: 10.1053/jlts.2001.24646. [DOI] [PubMed] [Google Scholar]
- 9.Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246:385–392; discussion 392-394. doi: 10.1097/SLA.0b013e318146996c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montalti R, Berardi G, Laurent S, Sebastiani S, Ferdinande L, Libbrecht LJ, Smeets P, Brescia A, Rogiers X, de Hemptinne B, et al. Laparoscopic liver resection compared to open approach in patients with colorectal liver metastases improves further resectability: Oncological outcomes of a case-control matched-pairs analysis. Eur J Surg Oncol. 2014;40:536–544. doi: 10.1016/j.ejso.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Simillis C, Constantinides VA, Tekkis PP, Darzi A, Lovegrove R, Jiao L, Antoniou A. Laparoscopic versus open hepatic resections for benign and malignant neoplasms--a meta-analysis. Surgery. 2007;141:203–211. doi: 10.1016/j.surg.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 12.Soubrane O, Cherqui D, Scatton O, Stenard F, Bernard D, Branchereau S, Martelli H, Gauthier F. Laparoscopic left lateral sectionectomy in living donors: safety and reproducibility of the technique in a single center. Ann Surg. 2006;244:815–820. doi: 10.1097/01.sla.0000218059.31231.b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker TB, Jay CL, Ladner DP, Preczewski LB, Clark L, Holl J, Abecassis MM. Laparoscopy-assisted and open living donor right hepatectomy: a comparative study of outcomes. Surgery. 2009;146:817–823; discussion 823-825. doi: 10.1016/j.surg.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, Cho SY, Lee KW, Park SJ, Han SS. Upper midline incision for living donor right hepatectomy. Liver Transpl. 2009;15:193–198. doi: 10.1002/lt.21677. [DOI] [PubMed] [Google Scholar]
- 15.Thenappan A, Jha RC, Fishbein T, Matsumoto C, Melancon JK, Girlanda R, Shetty K, Laurin J, Plotkin J, Johnson L. Liver allograft outcomes after laparoscopic-assisted and minimal access live donor hepatectomy for transplantation. Am J Surg. 2011;201:450–455. doi: 10.1016/j.amjsurg.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Choi HJ, You YK, Na GH, Hong TH, Shetty GS, Kim DG. Single-port laparoscopy-assisted donor right hepatectomy in living donor liver transplantation: sensible approach or unnecessary hindrance? Transplant Proc. 2012;44:347–352. doi: 10.1016/j.transproceed.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Choi Y, Yi NJ, Lee KW, Suh KS. Section 17. Laparoscopic and minimal incisional donor hepatectomy. Transplantation. 2014;97 Suppl 8:S69–S75. doi: 10.1097/01.tp.0000446281.89546.f8. [DOI] [PubMed] [Google Scholar]
- 18.Kim KH, Jung DH, Park KM, Lee YJ, Kim DY, Kim KM, Lee SG. Comparison of open and laparoscopic live donor left lateral sectionectomy. Br J Surg. 2011;98:1302–1308. doi: 10.1002/bjs.7601. [DOI] [PubMed] [Google Scholar]
- 19.Makki K, Chorasiya VK, Sood G, Srivastava PK, Dargan P, Vij V. Laparoscopy-assisted hepatectomy versus conventional (open) hepatectomy for living donors: when you know better, you do better. Liver Transpl. 2014;20:1229–1236. doi: 10.1002/lt.23940. [DOI] [PubMed] [Google Scholar]
- 20.Marubashi S, Wada H, Kawamoto K, Kobayashi S, Eguchi H, Doki Y, Mori M, Nagano H. Laparoscopy-assisted hybrid left-side donor hepatectomy. World J Surg. 2013;37:2202–2210. doi: 10.1007/s00268-013-2117-3. [DOI] [PubMed] [Google Scholar]
- 21.Nagai S, Brown L, Yoshida A, Kim D, Kazimi M, Abouljoud MS. Mini-incision right hepatic lobectomy with or without laparoscopic assistance for living donor hepatectomy. Liver Transpl. 2012;18:1188–1197. doi: 10.1002/lt.23488. [DOI] [PubMed] [Google Scholar]
- 22.Samstein B, Griesemer A, Cherqui D, Mansour T, Pisa J, Yegiants A, Fox AN, Guarrera JV, Kato T, Halazun KJ, et al. Fully laparoscopic left-sided donor hepatectomy is safe and associated with shorter hospital stay and earlier return to work: A comparative study. Liver Transpl. 2015;21:768–773. doi: 10.1002/lt.24116. [DOI] [PubMed] [Google Scholar]
- 23.Suh SW, Lee KW, Lee JM, Choi Y, Yi NJ, Suh KS. Clinical outcomes of and patient satisfaction with different incision methods for donor hepatectomy in living donor liver transplantation. Liver Transpl. 2015;21:72–78. doi: 10.1002/lt.24033. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Yang J, Yan L, Li B, Wen T, Xu M, Wang W, Zhao J, Wei Y. Comparison of laparoscopy-assisted and open donor right hepatectomy: a prospective case-matched study from china. J Gastrointest Surg. 2014;18:744–750. doi: 10.1007/s11605-013-2425-9. [DOI] [PubMed] [Google Scholar]
- 25.Fan X, Lin T, Xu K, Yin Z, Huang H, Dong W, Huang J. Laparoendoscopic single-site nephrectomy compared with conventional laparoscopic nephrectomy: a systematic review and meta-analysis of comparative studies. Eur Urol. 2012;62:601–612. doi: 10.1016/j.eururo.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 26.Taggart DP, D’Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. Lancet. 2001;358:870–875. doi: 10.1016/S0140-6736(01)06069-X. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. The Cochrane Library, Issue 3, 2005 Chichester, UK: John Wiley & Sons, Ltd 2005. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcmr/articles/CMR-7606/frame.html. [Google Scholar]
- 28.Manterola C, Asenjo-Lobos C, Otzen T. [Hierarchy of evidence: levels of evidence and grades of recommendation from current use] Rev Chilena Infectol. 2014;31:705–718. doi: 10.4067/S0716-10182014000600011. [DOI] [PubMed] [Google Scholar]
- 29.Cherqui D, Soubrane O, Husson E, Barshasz E, Vignaux O, Ghimouz M, Branchereau S, Chardot C, Gauthier F, Fagniez PL, et al. Laparoscopic living donor hepatectomy for liver transplantation in children. Lancet. 2002;359:392–396. doi: 10.1016/S0140-6736(02)07598-0. [DOI] [PubMed] [Google Scholar]
- 30.Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825–830. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 31.Scatton O, Katsanos G, Boillot O, Goumard C, Bernard D, Stenard F, Perdigao F, Soubrane O. Pure laparoscopic left lateral sectionectomy in living donors: from innovation to development in France. Ann Surg. 2015;261:506–512. doi: 10.1097/SLA.0000000000000642. [DOI] [PubMed] [Google Scholar]
- 32.Koffron AJ, Kung R, Baker T, Fryer J, Clark L, Abecassis M. Laparoscopic-assisted right lobe donor hepatectomy. Am J Transplant. 2006;6:2522–2525. doi: 10.1111/j.1600-6143.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- 33.Kurosaki I, Yamamoto S, Kitami C, Yokoyama N, Nakatsuka H, Kobayashi T, Watanabe T, Oya H, Sato Y, Hatakeyama K. Video-assisted living donor hemihepatectomy through a 12-cm incision for adult-to-adult liver transplantation. Surgery. 2006;139:695–703. doi: 10.1016/j.surg.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Eiriksson K, Fors D, Rubertsson S, Arvidsson D. High intra-abdominal pressure during experimental laparoscopic liver resection reduces bleeding but increases the risk of gas embolism. Br J Surg. 2011;98:845–852. doi: 10.1002/bjs.7457. [DOI] [PubMed] [Google Scholar]
- 35.Braun HJ, Ascher NL, Roll GR, Roberts JP. Biliary complications following living donor hepatectomy. Transplant Rev (Orlando) 2016;30:247–252. doi: 10.1016/j.trre.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Roumm AR, Pizzi L, Goldfarb NI, Cohn H. Minimally invasive: minimally reimbursed? An examination of six laparoscopic surgical procedures. Surg Innov. 2005;12:261–287. doi: 10.1177/155335060501200313. [DOI] [PubMed] [Google Scholar]
- 37.Soyama A, Takatsuki M, Hidaka M, Adachi T, Kitasato A, Kinoshita A, Natsuda K, Baimakhanov Z, Kuroki T, Eguchi S. Hybrid procedure in living donor liver transplantation. Transplant Proc. 2015;47:679–682. doi: 10.1016/j.transproceed.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Bekheit M, Khafagy PA, Bucur P, Katri K, Elgendi A, Abdel-Salam WN, Vibert E, El-Kayal el-S. Donor safety in live donor laparoscopic liver procurement: systematic review and meta-analysis. Surg Endosc. 2015;29:3047–3064. doi: 10.1007/s00464-014-4045-1. [DOI] [PubMed] [Google Scholar]
- 39.Braun HJ, Dodge JL, Roll GR, Freise CE, Ascher NL, Roberts JP. Impact of Graft Selection on Donor and Recipient Outcomes After Living Donor Liver Transplantation. Transplantation. 2016;100:1244–1250. doi: 10.1097/TP.0000000000001101. [DOI] [PMC free article] [PubMed] [Google Scholar]


