Abstract
Rationale and objectives
The clinical utility of supine in-magnet bicycling in combination with 31P Magnetic Resonance Spectroscopy to evaluate quadriceps muscle metabolism was examined in four children with Juvenile Dermatomyositis in remission and healthy age and gender matched controls.
Materials and Methods
Two identical maximal supine bicycling tests were performed using a Magnetic Resonance-compatible ergometer. During the first test, cardiopulmonary performance was established in the exercise laboratory. During the second test, quadriceps energy balance and acid/base balance during incremental exercise and phosphocreatine recovery were determined using 31P Magnetic Resonance Spectroscopy.
Results
During the first test, no significant differences were found between patients with Juvenile Dermatomyositis and their healthy peers regarding cardiopulmonary performance. The outcomes of the first test indicate that both groups attained maximal performance. During the second test, quadriceps phosphocreatine and pH time courses were similar in all but one patient experiencing idiopathic post-exercise pain. This patient demonstrated faster phosphocreatine depletion and acidification during exercise, yet post-exercise mitochondrial ATP synthesis rate measured by phosphocreatine recovery kinetics was approximately twofold faster than control (time constant 23 seconds versus 43±7 seconds, respectively).
Conclusion
These results highlight the utility of in-magnet cycle ergometry in combination with 31P Magnetic Resonance Spectroscopy to assess and monitor muscle energetic patterns in pediatric patients with inflammatory myopathies.
Keywords: human, dermatomyositis, exercise, bicycle ergometry, 31P MRS
Introduction
Exercise is increasingly utilized as a non-pharmacological intervention in the clinical management of patients with chronic inflammatory conditions. Evaluation of the impact of exercise on systemic health commonly involves periodic testing with concomitant measurement of direct and indirect physiological outcome parameters (e.g. maximal oxygen uptake) [1]. These, however, are all macroscopic outcome measures that greatly rely on voluntary effort. Availability of more objective outcome measures that provide functional information at the cellular, or even molecular, level would significantly strengthen ongoing efforts to tailor exercise interventions to individual patient needs and limitations. While muscle biopsies before and after exercise may provide relevant microscopic information [2], the procedure is highly invasive and therefore undesirable, particularly in pediatric care. Therefore, we explored the potential of an in vivo MR-based methodology to serve as a sensitive and non-invasive tool that guides the development of personalized exercise interventions.
In vivo phosphorus magnetic resonance spectroscopy (31P MRS) has long offered a non-invasive window into the metabolic capacity of skeletal muscle to support exercise [3]. Recordings of 31P MR spectra from human muscle during exercise and metabolic recovery have been commonly used to evaluate cellular ATP synthetic function on the basis of dynamic changes in phosphocreatine (PCr), inorganic phosphate (Pi) and intracellular pH [3]. In a more general sense, these recordings yield information on the in vivo homeostatic capacity of the metabolic networks in muscle with respect to myocellular energy balance (MEB) and proton balance (MPB) during exercise.
In vivo, this homeostatic capacity is a function of intracellular metabolic and transport networks in the skeletal muscle (e.g. mitochondrial ATP synthesis and lactate transport, respectively) as well their interactions with the supracellular network of organ systems in the body, which control among others, oxygen supply while eradicating carbon dioxide and lactic acid from the working muscle (i.e. the muscle-cardiovascular-pulmonary network) [4]. For example, the rate and magnitude of PCr depletion and acidification during exercise can be altered easily by obstructing blood flow to the working muscle [5]. Conversely, increasing blood flow and ventilation, as seen during strenuous exercise will also boost MEB and MPB homeostasis [4]. This empirical knowledge prompted the current pilot study to investigate the use of dynamic 31P MRS in the measurement of MEB and MPB homeostasis in the quadriceps muscle during maximal exercise. A major challenge in this type of measurements has been performing dynamic and maximal exercise within a clinical MR scanner that is similar to common exercise behaviors (e.g. walking or bipedal cycling) and can elicit a significant cardiovascular-ventilatory response. Recently, we overcame this challenge using a novel human MR whole-body exercise platform in the form of a mechanically-braked MR-compatible bicycle ergometer in healthy adults [6].
The current pilot study examined the clinical utility of this custom-designed platform to evaluate quadriceps muscle metabolic fitness during in-magnet bicycling exercise in a population of pediatric patients with Juvenile Dermatomyositis (JDM) and their healthy peers. JDM is the most common type of juvenile inflammatory myopathy, representing about 85% of the idiopathic inflammatory myopathies (IIM) in children [7]. The condition is classified as a systemic autoimmune vasculopathy characterized by inflammation of the capillaries in skeletal muscle and pathognomonic skin rashes [8]. Significant proximal muscle weakness and exercise intolerance are prominent clinical features of JDM during treatment and even in disease remission [9], indicating that immunosuppressive treatment promotes only partial recovery of the muscle tissue. The use of dynamic maximal exercise testing in combination with 31P MRS may therefore provide additional insight into the underlying microscopic causes of muscle weakness and exercise intolerance in this population. As such the specific aim of this pilot study was to evaluate the clinical utility of MR spectroscopic evaluation of leg muscle energy and acid balance in vivo during dynamic bicycling exercise in patients with JDM.
Materials and Methods
Subjects
Eight children (mean age 15.9 ±1.5 SD years), 4 of whom were diagnosed with JDM in clinical remission (1F/3M) and 4 healthy age- and gender-matched peers, participated in the study. The female patient presented with idiopathic pain complaints following strenuous exercise. Patients with JDM were recruited from the JDM outpatient clinic in the department of Paediatric Immunology at the Wilhelmina Children's Hospital, UMC Utrecht, the Netherlands. All healthy age- and gender-matched peers were friends or family of the participating patients. Informed consent was obtained from all participants and parents/guardians before the start of this study, which was approved by the Medical Ethics Committee of the University Medical Center Utrecht, the Netherlands.
Ergometer
Details of the mechanically-braked bicycle ergometer constructed from non-ferrous materials as well as its interface with a Gyroscan S15/ACS 1.5T scanner (Philips Healthcare, Best, the Netherlands) have been described previously [6]. For the clinical purposes of this study, the ergometer was refitted with customized clipless pedals (LOOK, France) and used in combination with high density plastic binding cleats (LOOK, France) mounted on bicycle racing shoes (Shimano, Japan) (Figure 1). This improved both patient safety (i.e., quick release in the unlikely event of a magnet quench) as well as standardization of the exercise task. A range of shoe sizes were available for use by the participants.
Figure 1.
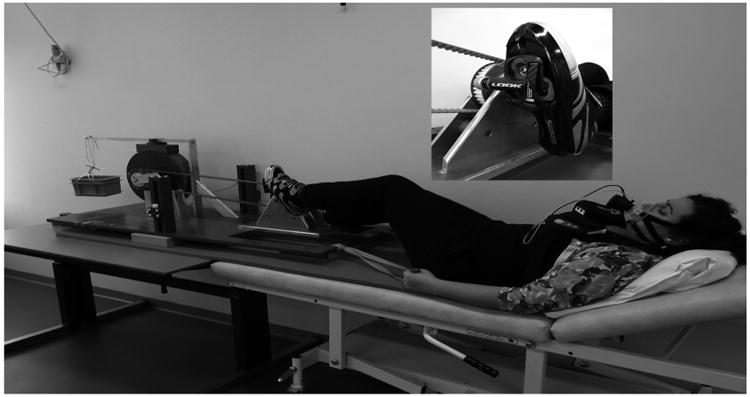
Test subject performing CPET test on the MR-compatible bicycle ergometer in the exercise Laboratory.
Cardiopulmonary exercise testing (CPET)
All subjects performed two incremental supine cycling tests to volitional exhaustion using a MR-compatible ergometer on separate days. The first test was completed outside the MRI as a form of habituation as well as to establish the cardiopulmonary demand of the exercise (Figure 1 and Table 1). The upper body was positioned under an upright angle (typically 40°), using a wedge-shaped support cushion. Seat height was adjusted to participant's comfort. Participants were then asked to perform a short bout of unloaded bicycling to familiarize themselves with the type of exercise and with stabilizing their upper bodies by pulling the handlebar ropes during exercise. The CPET started with unloaded bicycling; every 60 seconds, the resistance was increased by 0.2 kg and 0.4 kg brass weights for girls and boys, respectively, until volitional exhausting, despite strong verbal encouragement by the test leader. Considerations for gender differences in the test protocol were necessary to allow the participant to reach their limit of tolerance in a 10±2 minutes timeframe. To standardize the cycling speed, all patients were instructed to maintain an operational pedaling frequency of 70 revolutions per minute (RPM). Throughout the test, participants breathed through a facemask (Hans Rudolph Inc, Kansas city, MO), which was connected to a calibrated respiratory gas-exchange system (Cortex Metamax B3, Cortex medical, Leipzig, Germany). Minute ventilation (Ve), oxygen uptake (VO2), carbon dioxide production (VCO2) and respiratory exchange ratio (RER) were measured. Heart rate was measured continuously during the exercise test with a heart rate monitor (Polar, Kempele, Finland).
Table 1.
Anthropometrics and measurements during 1st CPET (outside MRS) including gas-analysis.
| Children with JDM in remission (N=4) Median (+Range) | Children in the control group (N=3) Median (+Range) | Sig. (2-tailed) | |
|---|---|---|---|
| Age (years) | 15.7 (3.5) | 15.5 (2.6) | 0.71 |
| Height (cm) | 169.5 (21.0) | 180.5 (26.5) | 0.86 |
| Weight (kg) | 60.6 (20.4) | 67.7 (24.9) | 0.86 |
| BMI (kg/m2) | 20.6 (3.0) | 19.7 (3.6) | 0.86 |
| HRpeak (bpm) | 182 (19.0) | 187 (30.0) | 1.00 |
| RERpeak | 1.05(0.07) | 1.04 (0.23) | 0.94 |
| VO2peak (L/min) | 2.23 (1.9) | 2.75 (1.4) | 0.63 |
| VO2peak/kg (ml/kg/min) | 35.9 (24.4) | 38.8 (10.5) | 0.63 |
| Peak work load (kg) | 2.9 (2.3) | 2.3 (1.8) | 0.97 |
| VO2/HRpeak | 12.5 (9.4) | 14.1 (6,5) | 0.86 |
1H and 31P MRS
The participants completed the second CPET in a 1.5-Tesla whole-body scanner (Gyroscan S15/ACS, Philips Healthcare, Best, NL) as described previously [6]. Briefly, a 6-cm diameter Philips 31P surface coil was fastened over the medial head of the quadriceps muscle of the right leg. Subjects were then moved into the magnet in supine position and their feet were fastened to the pedals of the ergometer. Next, a custom-built rectangular 1H surface coil (20×10cm) was positioned over the quadriceps muscle for shimming of the magnet. After an H2O line width of 30 Hz or better was obtained, the 1H coil was removed and 31P data acquisition commenced. All spectra were acquired using an adiabatic 60o pulse (duration: 5 ms). First, a 31P MR spectrum was obtained from the quadriceps muscle in resting state (16 free induction decays (FIDs), 640 points, sweep width 1 kHz; TR 3 seconds). Next, subjects performed a short bout of unloaded bicycling to test proper functioning of gated data acquisition during exercise. The spectrometer gating delay in the pulse program was set at 2.5 seconds to obtain a desired TR of 3 seconds. Two FIDs were averaged per spectrum yielding 6 s time resolutions during exercise and recovery. Subjects then repeated the incremental exercise protocol until exhaustion as described above, however without respiratory gas analyses. The spectrometer triggering software on a stand-alone personal computer (Labview, National instruments) was then switched from bicycle triggering to automatic triggering [6] and metabolic recovery was measured over a 10 minute period.
Statistics & data processing
For statistical analysis of the anthropometrical and exercise testing data (Table 1) SPSS version 15.0 was used. All statistical tests were performed two-tailed and a p-value less than 0.05 was considered significant. Due to the small sample size, differences between the JDM group and control groups were analyzed using a nonparametric test for independent variables (Mann-Whitney test). MRS data were analyzed as follows: Phosphocreatine (PCr), inorganic phosphate (Pi), ATP and phosphomonoester (PME) resonances were fitted in the time domain using the AMARES algorithm in the jMRUI software package and a prior knowledge library. Absolute concentrations were calculated after correction for partial saturation and assuming adenine nucleotide and creatine pool sizes of 8.2 and 42.7 mM, respectively [10]. Intracellular pH was calculated from the chemical shift difference between the Pi and PCr resonances [10]. The kinetics of PCr recovery following exercise were quantified by a time constant determined from mono-exponential curve fitting [10] and corrected for muscle acidification (-63s/pH unit) if pH dropped below 6.8 according to Van den Broek et al. [11].
Results
Anthropometric and exercise parameters from the first incremental CPET (outside MRI) are depicted in Table 1. The average peak heart rate and peak respiratory exchange ratio (RER) were in both groups >180 bpm and >1.01, respectively. These latter outcomes indicate that the participants attained maximal performance during the exercise test. Likewise, all patients stated that test termination was due to fatigue of their upper leg muscles. Figure 1 depicts a participant performing the CPET in the exercise laboratory. Details of the bicycle race shoe attachment to the refitted MR ergometer pedals are shown in an insert to Figure 1.
All participants willingly performed the supine in-magnet exercise testing and dynamic in vivo MR datasets were successfully obtained in all but one male participant (control). This single incomplete control test was not associated with technical limitations of the equipment, but rather a lack of willingness to comply with testing procedures within the magnet as a result of mild autism of this participant.
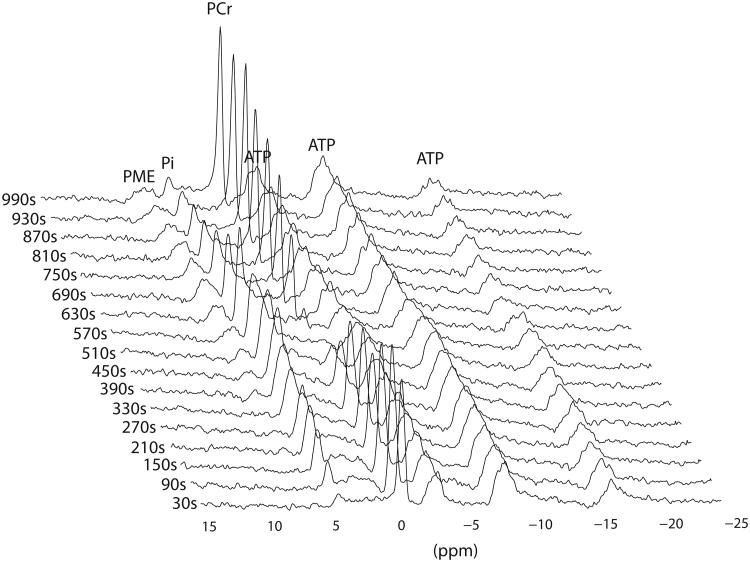
Furthermore, participants attained comparable peak work load during both exercise tests. Figure 2 shows a series of 31P MR spectra obtained from the quadriceps muscle of a patient with JDM, during incremental bicycling exercise. The usual stoichiometric changes in muscle PCr and Pi content during exercise and recovery were observed. Quadriceps muscle pH in this patient dropped from 7.1 at rest to 6.7 at exhaustion.
Figure 2.
Dynamic series of 31P magnetic resonance spectra obtained from quadriceps muscle of a child with juvenile Dermatomyositis in remission (PAT #4) during incremental bicycling exercise. For clarity of presentation one spectrum per 60 seconds is shown. Each spectrum was obtained by averaging all FIDs recorded during the corresponding 60 second period (total: 20 FIDS) and processed with 10Hz line broadening. Resonances of phosphomono-esters (PME), Pi, PCr and ATP are indicated.
Figure 3 shows the time course of quadriceps PCr content (relative to resting level; left panel) and quadriceps pH (right panel) during progressive exercise and recovery in three participants: a patient with JDM (patient #4; blue trace), a gender- and age-matched healthy control subject (black trace), and a female patient with JDM (of the same age) with idiopathic pain complaints following exercise (patient #2; red trace). The observed PCr and pH time courses in patient #4 and its control were qualitatively and quantitatively similar and representative for each group, with the sole exception of patient #2. Specifically, three out of the four patients with JDM and all healthy controls showed a first phase of rapid depletion of quadriceps PCr content upon initiation of exercise (t = 60-120 s; Figure 3A), followed by a second phase of relative stabilization of PCr content (t= 120-300 s; Figure 3A) before a final phase of progressive PCr depletion down to 20% of resting content at the point of exhaustion (t = 300 - 450 s; Figure 3A). Gradual quadriceps acidification in these subjects generally started after about two minutes into the exercise test and continued into recovery (Figure 3B). End-exercise pH values ranged between 6.8 and 6.5. The kinetics of PCr resynthesis following exercise in JDM patients #1, #3 and #4 were not different from healthy controls (time constants 50 ± 13 (n=3) versus 43 ± 7 (n=3) s, respectively; mean ± SD). In patient #2, however, strikingly different quadriceps PCr and pH dynamics were observed. Specifically, both quadriceps PCr depletion and acidification during the first few minutes of the exercise test were much steeper and extensive than other JDM patients or controls (Figure 3A and 3B, respectively; t=60-240 s). During the remainder of the test, the rate of fall of quadriceps PCr and pH leveled off significantly despite continuous increment of workload. Quadriceps depletion at exhaustion in patient #2 was not different from the other study subjects. Strikingly, however, post-exercise PCr resynthesis was approximately twofold faster than control (Figure 3A; time constant 23 versus 43±7 (n=3) s, respectively).
Figure 3.
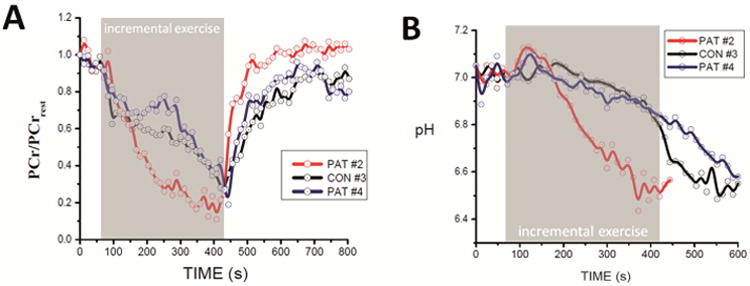
Quadriceps PCr content (scaled to resting content; A) and quadriceps pH (B) time courses during the exercise test and recovery recorded in two JDM patients and their age-matched control. Blue trace: JDM patient #4; Red trace: JDM patient with idiopathic pain complaints following exercise (Pat#2). Black trace: age-matched control. Quadriceps PCr content and pH were determined from 31P Magnetic Resonance spectra as described in Methods. For Clarity of presentation, data points were averaged over 12 seconds. The trend in the data visualized by solid lines was computed using the B spline function in the Origin Software.
Discussion
In the past decennia, diagnostic assessment and knowledge of muscle pathology has been augmented by 31P MRS examination of in vivo energy metabolism. Among its many applications, this non-invasive technology has facilitated in vivo determination of myositis-related deviations in muscle tissue metabolic parameters. To date, only a relative small number of 31P MRS investigations of muscle energy metabolism in juvenile and adult DM have been reported with the majority of patients assessed in an active inflammatory state [12-19]. For example, Park et al. performed static 31P MRS measurement of Pi, PCr and ATP levels in quadriceps muscle of ten patients with active JDM and two patients in remission in two states of metabolic activity (i.e., at rest, during 2 levels of voluntary muscle contraction, and after several minutes of recovery) [14]. Normal Pi/ATP and PCr/ ATP ratios were found compared to healthy controls in all tested conditions [14]. Two 31P MRS studies in adults with active DM equally found no abnormalities in resting leg muscle [12, 16]. The recovery times for Pi and PCr following exercise were, however, significantly prolonged compared with controls [12, 16]. Cea et al. attributed this finding of impaired oxidative metabolism during the active phase of the disease to impaired blood supply to the muscle [12], which may be secondary to a number of pathophysiological factors including: increased concentration of intramuscular cytokines, the systemic inflammation, muscle capillary inflammation, hypoactivity, and increased body mass and protein breakdown secondary to glucocorticoid treatment [20].
The current pilot study was successful in measuring dynamic in vivo MR datasets from the quadriceps muscle during in-magnet maximal bicycle exercise from all pediatric patients with JDM in remission and in all but one healthy control. Objective in vivo data were obtained on the integrated homeostatic performance of intramuscular metabolic and transport networks and the muscle-cardiovascular-pulmonary network with respect to MEB and MPB during maximal in-magnet exercise testing. Our findings suggest that the normal-responders (patient 1, 3 and 4) and their healthy peers present with no metabolic abnormalities to incremental exercise by showing normative stoichiometric changes in muscle PCr and Pi content and normative recovery data. However, the same cannot be said of the female patient (patient #2) presenting with idiopathic pain complaints following strenuous exercise. This patient demonstrated markedly anomalous PCr and pH time courses in response to exercise, a finding that further highlights the potential role of this technology in obtaining differential information with respect to muscle energy metabolism.
The current observation of impaired MEB and MPB during exercise in the quadriceps muscle of patient #2 in remission (Figure 3A and 3B, respectively) fits well with previously reported findings in active adult DM. However, the apparent mismatch between quadriceps muscle oxygen demand and supply during exercise in this pediatric patient was only transient since post-exercise metabolic recovery was approximately twofold faster than in controls. No abnormalities in MEB and MPB during exercise or in PCr resynthesis kinetics during recovery were found in any other patients. Given the similarities with findings in adults with active DM, it could be speculated that this patient is experiencing lingering muscle tissue oxygenation abnormalities from the active phase of the disease, which may manifest as chronic post-exercise idiopathic pain complaints. While this may suggest that the immunosuppressive treatment prescribed for these patients was generally successful in combating any functional limitations of leg muscle oxidative metabolism, an alternative explanation may be that the increased leg blood flow during exercise in the current study was much larger than in past studies, which all employed sub-maximal single-leg exercise regimens as opposed to maximal whole-body exercise. Regardless of these differences, the results of the current study suggest that exercise and exercise training might metabolically suitable for the majority of patients with JDM in remission, however this has to be validated in a larger cohort. Of interest is the potential impact of exercise and exercise training on the abnormal MEB and MPB exercise response seen in patient #2 as this patient might benefit from additional exercise training due to its potential benefits (like increased angiogenesis and type 1 fiber proportion) which might improve the affected oxygenation and thereby the metabolic state of the muscle. Although results of the current pilot study highlight the potential clinical utility of in magnet bicycle exercise testing in combination with 31P MRS assessment of quadriceps MEB and MPB during incremental bipedal cycling, the high costs, low accessibility and the requirement of specialized knowledge/ personnel shall limit the utilization of this application to expert groups as a research tool.
Future 31P MRS studies should focus on (a larger cohort of) the subpopulation of children with JDM who experience idiopathic pain complaints following strenuous exercise in order to make general statements regarding the indicated muscle tissue oxygenation abnormalities and the effects of exercise and exercise training on possible improvements of muscle oxygenation, as the current study is limited by a small sample size.
Conclusion
The results of the current pilot study highlight the potential of dynamic maximal exercise testing inside an MR scanner with concomitant 31P MRS assessments of quadriceps energy metabolism as a non-invasive screening platform for metabolic (contra)indications to exercise interventions in exercise intolerant patients with various (inflammatory) myopathies.
Acknowledgments
Authors thank all participants and their parents for their cooperation and Joyce Obeid for editing of the manuscript. The present work was funded in part by the National Institutes of Health, Bethesda, MD, USA through a subcontract to grants HL-072011 and R01-HL095122(to J.A.L. Jeneson).
No sources of support in the form of grants of industrial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wasserman K, Hanssen JE, Sue DY, Casaburi R, Whipp BJ. Principles of exercise testing and interpretation. 4th. Pa: Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- 2.Constatin-Teodosiu D, Casey A, Short AH, Hultman E, Greenhaff PL. The effect of repeated muscle biopsy sampling on ATP and glycogen resynthesis following exercise in man. Eur J App Physiol Occup Physiol. 1996;73:186–190. doi: 10.1007/BF00262830. [DOI] [PubMed] [Google Scholar]
- 3.Chance B, Im J, Nioka S, Kushmerick M. Skeletal muscle energetics with PNMR: personal views and historic perspectives. NMR in biomedicine. 2006;19:904–926. doi: 10.1002/nbm.1109. [DOI] [PubMed] [Google Scholar]
- 4.Jeneson JA, Bruggeman FJ. Robust homeostatic control of quadriceps pH during natural locomotor activity in man. FASEB J. 2004;18:1010–1012. doi: 10.1096/fj.03-0762fje. [DOI] [PubMed] [Google Scholar]
- 5.Taylor DJ, Bore PJ, Styles P, Gadian DG, Radda GK. Bioenergetics of intact human muscle 31P nuclear magnetic resonance study. A Mol Biol Med. 1983;1:77–94. [PubMed] [Google Scholar]
- 6.Jeneson JA, Schmitz JP, Hilbers PA, Nicolay K. An MR-compatible bicycle ergometer for in-magnet whole-body human exercise testing. Magn Reson Med. 2010;63:257–261. doi: 10.1002/mrm.22179. [DOI] [PubMed] [Google Scholar]
- 7.Rider LG, Miller FW. Classification and treatment of the juvenile idiopathic inflammatory myopathies. Rheum Dis Clin North Am. 1997;23:619–655. doi: 10.1016/s0889-857x(05)70350-1. [DOI] [PubMed] [Google Scholar]
- 8.Feldman BM, Rider LG, Reed AM, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371:2201–2212. doi: 10.1016/S0140-6736(08)60955-1. [DOI] [PubMed] [Google Scholar]
- 9.Takken T, van der Net J, Engelbert RH, Pater S, Helders PJ. Responsiveness of exercise parameters in children with inflammatory myositis. Arthritis Rheum. 2008;59:59–64. doi: 10.1002/art.23250. [DOI] [PubMed] [Google Scholar]
- 10.Prompers JJ, Jeneson JA, Drost MR, Oomens CC, Strijkers GJ, Nicolay K. Dynamic MRS and MRI of skeletal muscle function and biomechanics. NMR Biomed. 2006;19:927–953. doi: 10.1002/nbm.1095. Review. [DOI] [PubMed] [Google Scholar]
- 11.Van den Broek NAM, de Feyter HM, de Graaf L, Nicolay K, Prompers JP. Intersubject differences in the effect of acidosis on phosphocreatine recovery in exercise are due to differences in proton efflux rates. Am J Physiol (Cell Physiol) 2007;293:C228–237. doi: 10.1152/ajpcell.00023.2007. [DOI] [PubMed] [Google Scholar]
- 12.Cea G, Bendahan D, Manners D, Hilton-Jones D, Lodi R, Styles P, et al. Reduced oxidative phosphorylation and proton efflux suggest reduced capillary blood supply in skeletal muscle of patients with dermatomyositis and polymyositis: a quantitive 31P-magnetic resonance spectroscopy and MRI study. Brain. 2002;125:1635–1645. doi: 10.1093/brain/awf163. [DOI] [PubMed] [Google Scholar]
- 13.Park JH, Vansant JP, Kumar NG, Gibbs SJ, Curvin MS, Price RR, et al. Dermatomyositis: correlative MR imaging and P-31 MR spectroscopy for quantitative characterization of inflammatory disease. Radiology. 1990;177:473–479. doi: 10.1148/radiology.177.2.2217788. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Niermann KJ, Ryder NM, Nelson AE, Das A, Lawton AR, et al. Muscle abnormailties in Juvenile dermatomyositis patients: P-31 Magnetic Resonance Spectroscopy studies. Arthritis & Rheum. 2000;43:2359–2367. doi: 10.1002/1529-0131(200010)43:10<2359::AID-ANR25>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 15.Park JH, Olsen NJ. Utility of magnetic resonance imaging in the evaluation of patiens with inflammatory myopathies. Curr Rheumatol Rep. 2001;3:334–345. doi: 10.1007/s11926-001-0038-x. [DOI] [PubMed] [Google Scholar]
- 16.Pfleiderer B, Lange J, Loske KD, Sunderkötter C. Metabolic disturbances during short exercises in dermatomyositis revealed by real-time functional 31P magnetic resonance spectroscopy. Rheumatology. 2004;43:696–703. doi: 10.1093/rheumatology/keh182. [DOI] [PubMed] [Google Scholar]
- 17.Niermann KJ, Olsen NJ, Park JH. Magnesium abnormalities of skeletal muscle in dermatomyositis and juvenile dermatomyositis. Arthritis Rheum. 2002;46:475–88. doi: 10.1002/art.10109. [DOI] [PubMed] [Google Scholar]
- 18.Park JH, Kari S, King LE, Jr, Olsen NJ. Analysis of 31P MR spectroscopy data using artificial neural networks for longitudinal evaluation of muscle diseases: dermatomyositis. NMRBiomed. 1998;11:245–56. doi: 10.1002/(sici)1099-1492(199806/08)11:4/5<245::aid-nbm513>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 19.Slopis JM, Jackson EF, Narayana PA, Papasozomenos SC, Butler IJ. Proton magnetic resonance imaging and spectroscopic studies of the pathogenesis and treatment of juvenile dermatomyositis. J Child Neurol. 1993;8:242–9. doi: 10.1177/088307389300800307. [DOI] [PubMed] [Google Scholar]
- 20.Takken T, Elst EF, van der Net J. Pathophysiological factors which determine the exercise intolerance in patients with juvenile dermatomyositis. Current Rheumatology Reviews. 2005 [Google Scholar]