Abstract
In addition to recurrent pain, fatigue, and increased rates of physical disability, individuals with rheumatoid arthritis (RA) show an increased prevalence of some mental health disorders, particularly those involving mood disturbances. This narrative Review provides an overview of mental health comorbidities in RA, and discusses how these comorbidities interact with disease processes, including dysregulation of inflammatory responses, prolonged difficulties with pain and fatigue, and the development of cognitive and behavioural responses that could exacerbate the physical and psychological difficulties associated with RA. This article describes how the social context of individuals with RA affects both their coping strategies and psychological responses to the disease, and can also impair responses to treatment through disruption of therapeutic alliance and treatment adherence. Evidence from the literature on chronic pain suggests that the resulting alterations in neural pathways of reward processing could yield new insights into the connections between disease processes in RA and psychological distress. Finally, the role of psychological interventions in the effective and comprehensive treatment of RA is discussed.
In comparison to the general population, individuals with rheumatoid arthritis (RA) have an increased prevalence of both physical disability and psychiatric comorbidity.1, 2 The relationship between RA and psychological factors is evident from both the altered prevalence rates of some psychiatric disorders in patients with RA versus the general population, and from the close links between physiological and psychological mechanisms that underlie these comorbidities. In this Review, we examine the extant literature on psychiatric comorbidities in RA and explore the relationships between characteristics of RA and psychosocial, physiological, and neural factors. We discuss these data in the context of effective adaptation to RA, delineating processes that could protect affected individuals against the declines in physical and psychological functioning that commonly occur with progression of RA. We also describe the importance of social context in the experience of living with RA, and the role of social factors in the effective treatment of RA.
Psychiatric comorbidities
In community-based adults under the age of 45 years, having arthritis is a risk factor for the development of a mental health disorder in later life.2 Although the converse is not true (having a mental health condition does not reliably predict the later development of arthritis)2, mental health disorders are associated with adverse health-related outcomes such as pain, disability, and impaired quality of life in patients with RA.3, 4 Compared to individuals without recurrent pain or physical illness, patients with RA show worse outcomes in several domains, including increased psychological distress, decreased quality of sleep, and increased use of passive pain-coping strategies.5 Although individuals with RA do not show consistently elevated prevalence rates for all psychiatric disorders (for example evidence of an increased prevalence of substance abuse or eating disorders is absent), mood disorders seem to be considerably more common in individuals with RA.6, 7
Depression
The psychiatric condition most commonly associated with RA is depression.6 A meta-analysis of studies on depression in individuals with RA found a prevalence of roughly 16.8% for major depressive disorder,7 although the substantial variation between studies was attributed to differences in measurement methodologies.8 Prevalence estimates can also be affected by differences in the course and recurrence of depressive symptoms, which vary greatly from person to person.8, 9 Furthermore, many individuals with RA experience clinically significant depressive symptoms (results from one systematic review suggest that prevalence estimates range from 14% to 48%), but do not meet the full diagnostic criteria for major depressive disorder.7
From a clinical standpoint, comorbid depression is associated with increases in several adverse outcomes in patients with RA, including pain, inflammation, and disability.10 Depression seems to decrease patients’ adherence to medical treatment11 and is associated with increased disability,4, 12 impaired quality of life,13, 14 and reduced rates of clinically significant remission of RA symptoms and treatment response.15–17 Patients with moderate or severe depression are less likely than those with mild or no depression to adhere to medical recommendations across a variety of medical conditions,11 including RA. This association might be due to feelings of hopelessness or to a positive feedback loop in which non-adherence leads to a worsening of physical symptoms, thereby further increasing depression.11 Patients with RA who have chronic depression may be more likely than those with milder or subclinical levels of depression to discontinue DMARDs against the recommendations of their health-care providers.17 Depression-related reductions in treatment adherence could also be partially attributable to social withdrawal, a common correlate of depression that can compromise the efforts of supportive family members and friends to encourage patients to engage in self-care.11 Furthermore, depression in RA is often coincident with other physical health problems,18 and is associated with increased mortality19 and an increased risk of specific adverse outcomes, such as myocardial infarction20 and suicide.21
Anxiety
Anxiety disorders are common in patients with RA,22 and some estimates suggest that anxiety symptoms occur even more frequently than depression in individuals with arthritis.23 One study found a 16% lifetime prevalence rate of anxiety disorders in individuals with RA,24 and in an analysis of survey data, an estimated 11.2% of patients with arthritis reported panic attacks and 5.6% reported receiving a diagnosis of generalized anxiety disorder, though this latter study did not delineate between patients with RA and patients with other forms of arthritis.25 Symptoms of anxiety are more common in individuals with RA who have comorbid major depression than in either the general population of patients with RA or age-matched healthy controls.26 This pattern of findings is unsurprising, given the overlap between depression and anxiety in clinical and empirical studies.6 As with depression, symptoms of anxiety also correlate with impaired health-related quality of life27 and poor responses to medical treatment in patients with RA.15
Post-traumatic stress disorder
Post-traumatic stress disorder (PTSD) has been proposed as a risk factor for developing RA in adulthood above and beyond the effects of familial and genetic factors.28 Notably, PTSD can exacerbate RA, as comorbid PTSD in patients with RA is associated with poor patient-reported outcomes and an increased number of tender joints.29 However, only a few studies have demonstrated a relationship between PTSD and RA, and these have been conducted almost exclusively in veterans28, 29 (among whom the incidence of PTSD is decidedly more common than in the general population30). Consequently, whether this evidence reflects a true interaction between RA and PTSD, or whether the reported association is due to characteristics specific to individuals with a history of military service (such as combat exposure) remains unclear.30
Schizophrenia
In contrast to their increased rates of mood disorders, individuals with RA seem to show decreased rates of schizophrenia compared to the general population.31, 32 A prevailing explanation for this phenomenon concerns the aberrant inflammatory cytokine profile of individuals with schizophrenia,33, 34 which features increased levels of markers of immune cell activation, including soluble IL-2 receptor33 and increased circulating levels of proinflammatory cytokines, such as IL-6.34 Notably, high blood concentrations of IL-1 receptor antagonist,33 an anti-inflammatory cytokine that could have protective effects against RA35, are also found in individuals with schizophrenia. These differences in immune function might reflect factors only peripherally related to RA or schizophrenia (such as the presence of specific genetic variants,31 long-term use of medications that alter glutamatergic signalling,31 or an elevated reactivity to stress33). However, current evidence nevertheless suggests that these profiles of altered immune responses in RA and schizophrenia contribute to a degree of mutual exclusion in these disorders, that these inflammatory processes are complex and mutually influential, and finally that understanding their interactions requires increased knowledge of their underlying processes and consequences.
Mechanisms of affective disturbance
Several mechanisms are thought to link RA with overall psychological functioning, including cognitive, emotional, and behavioural reactions to having RA,10 and psychological reactions to RA-related physical symptoms, including pain intensity, fatigue and altered inflammatory responses.36 Of note, the mechanisms connecting RA and mental health are likely to mutually influence each other, making the outcome of these mechanisms inconsistent across patients and situations. For example, emotional distress could be accompanied by an underlying physiological stressor, such as inflammation, which, if sustained, could compromise physical function, thereby exacerbating emotional distress (Figure 1).
Figure 1. Mental health and disease activity are interconnected in rheumatoid arthritis.
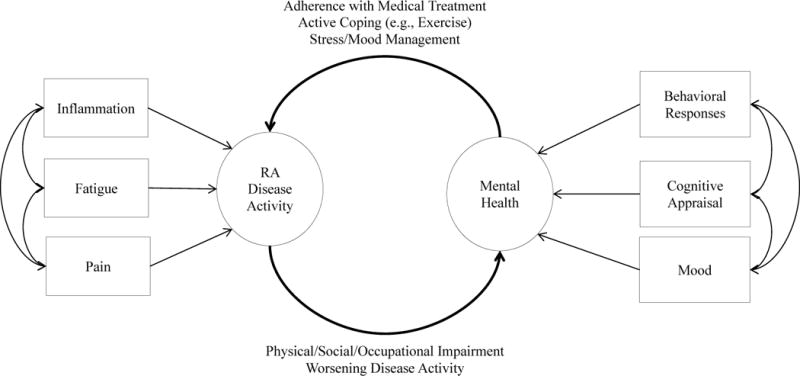
Manifestations of rheumatoid arthritis (RA) disease activity (recurrent pain, fatigue, and prolonged inflammation) act as stressors and influence mental health via changes in cognitive appraisal, affective states, and behavioural coping responses. Cognitive appraisal (the patient’s interpretation of their RA disease status) has major implications for their current mood and subsequent behavioural coping responses. Patients who view RA as a catastrophic personal event (catastrophizing) or have a low degree of belief in their ability to function in the presence of the disease might lack effective behavioural coping strategies such as adherence to medical recommendations or active coping responses such as exercise. By contrast, patients with RA who have strong beliefs in their ability to function and manage their disease are likely to adopt healthy behavioural coping approaches. The extent to which patients adopt healthy and active coping responses to RA affects their subsequent attitudes to medical intervention and their ability to self-manage RA, which can then feed back into the severity of the disease itself. Clustering of physical and psychological factors is also evident, such as coincident depression and fatigue states and inflammatory episodes concurrent with periods of high stress levels and negative emotion, suggesting that these processes interact in complex ways.
Physical symptoms
The physical symptoms of RA, including pain, inflammation, and fatigue, have important implications for affected patients’ mental health. Chronically impaired physical states can change neural processing and thereby adversely affect mental health (discussed later in this Review). A substantial body of literature also suggests that patients with RA demonstrate abnormalities in hypothalamic–pituitary–adrenal (HPA) axis responsivity.37 Patients with RA have altered levels of circulating hormones (such as testosterone), dampened cortisol release in response to stressors, desynchronization of cooperative signalling between the HPA axis and autonomic nervous system, and increased levels of circulating proinflammatory cytokines,38 which collectively contribute to differences in immune function that, in turn, affect psychological states.37
Stress
Stress is a key link between HPA axis abnormalities and mental health in RA. Some evidence suggests that a decline in cooperative signalling between the HPA axis and sympathetic nervous system, resulting from chronic activation of the sympathetic nervous system and impaired regulation of acute physiological responses to stress, might make patients with RA who experience stress particularly susceptible to chronic inflammatory states.38 Thus, stress itself has been identified as an aggravating factor for RA.39 As the results of two meta-analyses have identified increased circulating levels of proinflammatory cytokines in patients with depression,40, 41 we might reasonably expect some of the mood dysregulation seen in patients with RA to be directly attributable to chronic inflammation. However, relationships between stress and inflammation in RA have been observed primarily in cross-sectional or short-term studies, and additional longitudinal studies are needed to verify whether chronic inflammation has implications for long-term mental health in patients with RA.38
Rheumatic disease has itself been characterized as a stressor. A patient’s reaction to having a rheumatic disease can, therefore, have substantial implications for their mood and continued physical and psychosocial function.42 For example, individual differences in stress reactivity and pain intensity can predict the severity of depressive symptoms in patients with RA.43 Likewise, the degree of functional impairment due to RA-related pain and disease processes is positively correlated with rates of depression and anxiety.44, 45 As a related point, one of the key determinants of long-term quality of life in patients with RA seems to be ongoing pain.46 Recurrent pain is a key barrier to function for patients with RA, and how this pain is cognitively interpreted by a given individual can have major implications for their mental health. Consequently, processes driving stress responses in individuals with RA might be insidious and self-sustaining; as stress levels associated with living with RA mount, the dysregulation of physical processes already inherent in RA can persist or worsen.
Fatigue
Another key determinant of RA-related outcomes is fatigue; up to 80% of individuals with RA are considered to experience clinically significant fatigue,47 which greatly impairs both their quality of life27, 48, 49 and their physical functioning.48, 50 Furthermore, fatigue seems to have a complex aetiology involving pain intensity,47 RA disease activity,51 inflammatory processes (which could result in enhanced pain processing),52 and concurrent affective mood disorders, such as distress53 or depression.15, 54 However, considerable debate is ongoing about the mechanisms leading to fatigue in RA; the results of some studies suggest that fatigue occurs independently of RA disease activity,7, 50 whereas other observations suggest that fatigue might be mediated by inflammation51 and could, therefore, be treated with DMARDs (which also reduce pain and disease activity).47 By contrast, several studies have clearly shown close relationships between fatigue, psychological distress55 and depression.15, 36, 53, 56, 57 The link between depression and fatigue is unsurprising, as fatigue is a common correlate of depression, even among individuals without a rheumatic disorder.58 Notably, fatigue and mood might mutually influence each other; on days of high fatigue levels, individuals with RA report having higher levels of negative mood and lower levels of positive mood.59 Conversely, the deleterious effects of fatigue on mood could be attenuated by behavioural responses, as individuals who report high levels of physical activity have a more positive mood on days of high fatigue.59 The relationship between emotion and fatigue is complex, however, and individuals with RA who report frequent positive interpersonal events might still experience increased fatigue at a later time.60 The concurrence of both positive and negative interpersonal events might be the most meaningful factor in determining subsequent fatigue states.61
Considerable debate is also ongoing about whether symptoms traditionally considered to be medically unexplained, for example those related to suspected central nervous system sensitization (such as fibromyalgia or chronic fatigue syndrome, which co-occur with RA), should be classified as mental disorders. The results of a meta-analysis suggest that such symptoms cannot be fully explained by psychological factors.62 Nonetheless, patients with RA and co-morbid chronic fatigue syndrome are more likely than patients with RA alone to attribute their somatic symptoms to unknown disease processes, and to have poorer overall views of their health, which might increase their likelihood of future disability.63
Emotional status
Emotional health is a key contributor to RA adaptation. Ongoing mood problems in patients with RA, such as those noted in the sections on depression and anxiety, predict increased pain intensity64 and decreased function9. Conversely, positive emotions seem to buffer the relationship between pain and negative emotions,65 and are associated with decreased pain intensity, measured over subsequent days or weeks.66 However, positive emotional systems might be impaired in depressed individuals with RA, in part owing to decreased engagement in activities that promote positive emotions.43
To some extent, the emotional consequences of RA are attributable to the reductions in function caused by RA-related symptoms. Individuals with RA and co-morbid depression report reduced quality of life, and these parameters more closely reflect these individuals’ degree of impaired physical function than do their typical level of pain.45 Furthermore, the presence of both RA and depression might contribute to increased affective dysregulation. Studies of daily diaries suggest that patients with RA and a history of depression (who are in most respects otherwise indistinguishable from individuals with RA but no history of depression) demonstrate a greater degree of pain-contingent emotional wellbeing at the daily level.67 Specifically, individuals with RA and a history of depression demonstrate less-effective coping strategies, show larger decreases in wellbeing during times of increased pain, and report feeling less in control of their pain when emotionally distressed, than do patients with RA that have no history of depression.67 These findings suggest that depression could contribute to these individuals’ increased emotional distress related to their RA symptoms, in part because of passive behavioural and cognitive responses that prioritize avoidance or short-term amelioration of pain and distress over personally-meaningful functional goals, which can compromise long-term health.
Cognitive responses
The patient’s appraisal of their disease-related difficulties (for example increased pain or reduced physical function) is a crucial determinant of concurrent emotional states.68 Pain catastrophizing, a pattern of negative cognitive appraisal characterized by feelings of helplessness, ruminative thoughts, and a tendency to magnify the negative consequences of pain, is an important predictor of poor physical and psychological outcomes in patients with RA.69 Pain catastrophizing is more common in individuals with RA than in healthy controls, and predicts increases in pain severity, inflammatory activity, and levels of disability.10 Appraisals of overall health and functional ability also have implications for mood: individuals with RA who perceive their health to be poor and view themselves as disabled tend to have elevated levels of depression70 and reduced health-related quality of life.71 Conversely, some cognitive styles [G] are protective against the deleterious consequences of RA. For example, individuals who report being optimistic or accepting of their pain show increased resilience to pain, and are less likely (than pessimistic and non-accepting patients) to have high levels of physical dysfunction in the early stages of RA.72, 73 Similarly, feelings of self-efficacy and confidence in one’s ability to manage ongoing physical symptoms are predictive of reduced pain, increased goal achievement, and improved quality of life.74 Individuals who engage in healthy coping strategies, such as reappraisal of unrealistic or distressing negative thoughts, also show good overall coping and report increased quality of life and decreased levels of helplessness related to their RA symptoms.71
Behavioural responses
Different trajectories of adjustment to living with RA also contribute to the degree to which RA-related symptoms compromise patients’ efforts at effective coping styles and healthy behaviour.10 Passive coping styles, such as isolating oneself or adjusting behaviour to avoid the experience of pain, increase the likelihood of future functional impairment in patients with RA.75 Conversely, active coping styles, such as using effective problem-solving strategies or increasing physical activity, seem to confer psychological health benefits.71 Patients’ behavioural coping responses to RA are closely related to how they view their illness; as noted previously, individuals who view their pain or their disease as uncontrollable or catastrophic are less likely than patients with positive views to cope effectively with their physical symptoms.10, 70, 71 As a result, the affective, cognitive, and behavioural responses of people with RA are closely linked.
Reward circuitry and neural processing
In the search for mechanisms to explain interindividual variability in RA-related pain and mental health, one worthwhile option to consider is the biobehavioural view that adaptation to pain is governed by the mesolimbic dopaminergic reward system. Given the paucity of data from patients with RA on this topic, we draw upon research in other chronic pain disorders. Dopamine neurotransmission is altered in patients with fibromyalgia76–78, a disorder characterized by chronic widespread pain, fatigue, sleep disturbance, and dysregulation of emotion. Specifically, these patients have reduced levels of dopamine metabolites in the cerebrospinal fluid, presumably reflecting a reduced rate of postsynaptic phasic dopamine burst firing.79 Furthermore, PET imaging studies indicate that patients with fibromyalgia have lower basal levels of presynaptic dopamine77, and release less dopamine in the basal ganglia in response to a tonic pain challenge,78 than do healthy individuals. In a separate analysis of PET data from the same sample of patients, an index of presynaptic dopamine metabolism, estimated through a region of interest (ROI) analysis of nuclei within the ventral tegmental area, correlated positively with reduced cortical grey matter volume.76 Positive emotions are strongly linked to increased dopamine neurotransmission and other activity within the mesolimbic reward system,80, 81 making these findings particularly striking against the backdrop of studies demonstrating that patients with fibromyalgia demonstrate a pattern of affective dysregulation characterized by deficits in positive emotional responses.82, 83
Chronic back pain has also been associated with reward system alterations. The nucleus accumbens, which is the primary afferent terminal of dopamine within the mesolimbic region, is deactivated during a pain challenge in patients with chronic back pain compared to healthy controls.84 Furthermore, PET studies suggest that activation of dopamine receptors D2 and D3 in the nucleus accumbens during a pain challenge is reduced among patients with chronic back pain compared with healthy controls.85 Interestingly, reductions in dopamine neurotransmission in the ventral striatum are correlated with positive mood [G].85 Taken together, these data suggest that chronic pain is associated with abnormalities in dopamine neurotransmission that seem to be involved in processing both nociceptive signalling and the affective component of pain.
Pain in RA is distinct from chronic back pain and fibromyalgia in several respects, perhaps most notably in the prominent contribution of proinflammatory cytokines to RA-related pain. Thus, attempts to understand potential interactions between the brain reward system and pain in RA require an appreciation of how inflammatory processes might contribute to that association. Indeed, the inflammatory milieu inherent in RA might influence both the functioning of the reward system and accompanying behaviours. Proinflammatory cytokines broadly modulate the function of neurotransmitters (including dopamine) at multiple levels, via the activation of metabolic and epigenetic processes that alter neurotransmitter synthesis, reuptake and release.86 Additionally, endotoxin-induced inflammation seems to promote both depressed mood and decreased ventral striatal responses to monetary reward cues,87 extending previous evidence suggesting that proinflammatory cytokines worsen depressed mood.88 Similarly, typhoid vaccination of healthy individuals resulted in an increase in IL-6 levels that attenuated the reduction in activation of the substantia nigra usually seen during a cognitive attention task.89 A candidate gene analysis in patients with cancer revealed that a variant in the gene encoding IL-4, another proinflammatory cytokine, predicts a cluster of pain, fatigue, sleep disturbance, and depressive symptoms,90 a complex of symptoms also commonly observed in patients with RA91, 92 and individuals with dopamine signalling abnormalities.93
The existence of pathways linking inflammation to the functioning of the reward system provides a compelling framework for understanding adaptation to RA. For example, patients with chronic inflammatory conditions (which are partly due to the HPA axis alterations discussed earlier) can experience alterations in dopaminergic neurotransmission that have the potential to impair behaviour (Figure 2). Because dopamine critically regulates positive emotions and motivation,80, 81 aberrant functioning of the mesolimbic dopaminergic reward system could lead to patterns of behavioural responses that increase a patient’s long-term risk of physical or psychological dysfunction by constricting their range of positive coping resources.
Figure 2. Chronic inflammation and altered dopaminergic signalling are connected to coping responses through pain perception and decreased positive affect.
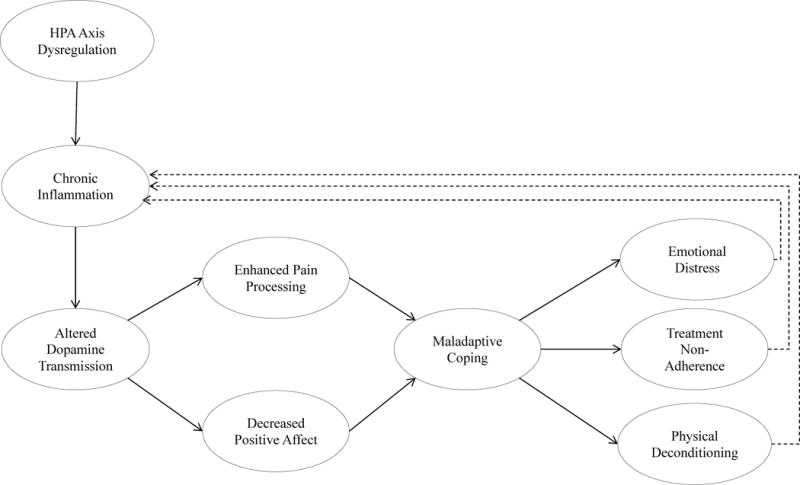
Rheumatoid arthritis (RA) contributes to chronic inflammatory states in part through dysregulation of the hypothalamic–pituitary–adrenal (HPA) axis, which manifests in altered physiological responses to stress, such as downregulation of normal anti-inflammatory responses after stress, and increased sympathetic tone at rest but decreased sympathetic response during stress. Chronic inflammatory states can alter dopamine transmission in the brain (resulting in lower basal levels of presynaptic dopamine, decreased dopamine release from basal ganglia in response to pain states, and decreased D2 and D3 receptor activation in the nucleus accumbens). In turn, these changes enhance pain processing and dampen positive affective responses. Downregulation of positive emotional states can manifest as both increased depression and decreased motivation, thereby impairing otherwise healthy coping responses and potentially affecting disease-relevant behaviour, such as exercise, treatment adherence, and social withdrawal or conflict. These factors can adversely affect RA disease processes in the future. Additionally, the presumed downstream increases in emotional distress that can occur with declines in physical health and function subsequently contribute to chronic inflammatory states via the aforementioned changes in physiological stress processes.
Social factors
Social factors could add valuable detail to models of physiological and psychological processes in RA, but have been underemphasized in traditional views of stress and pain.94 The context in which pain occurs might alter how it is experienced95. Context might also interact with cognitive and neural states to alter a patient’s response to medical intervention.95, 60 Interpersonal stressors, such as daily negative interpersonal events, contribute to acute increases in inflammatory activity,96, 97 which could have implications for fatigue states.97 Similarly, frequent positive or enjoyable interpersonal events are predictive of reduced levels of fatigue and high concurrent levels of positive emotion.60, 98 The frequency of negative daily interpersonal events is positively correlated with levels of RA disease activity,99 as well as with concurrent emotional distress and fatigue.60 Thus, the frequency with which individuals engage with others in their social environment could have implications for overall physical and psychological health. However, the presence of RA could substantially alter these patterns of social engagement; women with RA show evidence of decreased social activity over time compared to healthy women.100 Conversely, interventions that increase patterns of social engagement are efficacious in increasing wellbeing as well as reducing disease activity, although as yet evidence that these effects are sustained across time is lacking.100
Social engagement patterns are shaped throughout a person’s life, beginning in childhood long before inflammatory processes associated with chronic pain emerge.101 Hypervigilance and other fear-related motivational states have been associated with high levels on the anxiety dimension [G] of insecure attachment. Differential responsiveness to social reward and high reactivity to social stressors have been identified among individuals with a history of abuse,103 and a reduction in ventral striatum activation following social rewards has been found in those with insecure attachment characterized by avoidance.104 A history of child abuse and attachment insecurity, as well as contemporaneous social factors, could set the stage for future disability and impair both physiological and psychological processes associated with resilient adaptation to chronic pain.97, 105, 106
The quality of extant relationships also seems to have important implications for psychological adjustment to RA (Figure 3). A high frequency of negative marital interactions related to the disease seems to impair the adjustment processes of both members of a romantic relationship in which one partner has RA,107 and to worsen both the perceived impact of RA and the patient’s concurrent psychiatric symptoms.108 Conversely, supportive relationships bolster the patient’s long-term resilience against stress and other challenges.109 High levels of perceived support from their spouse predict increased use of active coping responses in patients with RA110 and seem to protect against psychiatric comorbidity111 and support the long-term retention of physical function.112 Emotional support might also buffer against the emotional impact of disability due to RA.113
Figure 3. Social factors interact with psychological reactions to rheumatoid arthritis.
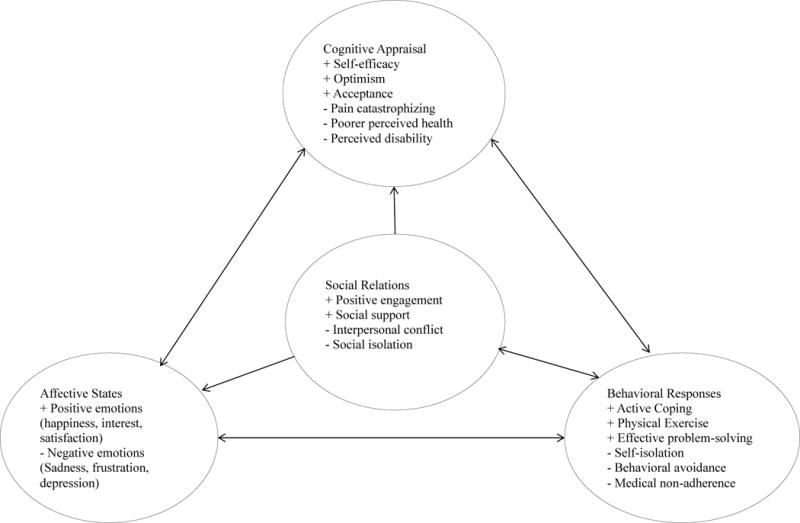
Cognitive, affective, and behavioural factors are closely related and show a high degree of mutual influence. These factors can have both positive and negative effects on an individual’s adaptation to having rheumatoid arthritis (RA). Individuals who maintain optimistic perspectives or high levels of self-efficacy are more likely (than individuals with pessimistic perspectives or low self-efficacy) to sustain healthy repertoires for coping with RA and are less likely to show pronounced adverse psychological reactions to the physical symptoms caused by RA. Additionally, the broader social environment can impact a patient’s psychological reactions to RA; patients in supportive environments seem to be more resilient against RA-related declines in physical and psychological function, whereas those in stressful environments show elevated physical symptoms, reduced function, and poor psychological adjustment to RA. Individuals with RA can also adversely influence their immediate social environments via behavioural coping responses such as self-imposed isolation or initiating conflict with others when pain or frustration levels are high, which could then have consequences for future emotional states.
Relationships with care providers
Patient–care provider relationships are another important predictor of well-being in RA. Optimal care is reliant upon physician–patient relationships that are built on trust, and actively encourage patient participation in treatment and health-care decisions.114 High levels of patient engagement in care are associated with superior health outcomes and health-related quality of life,115, 116 high levels of treatment satisfaction,115 and increased treatment adherence.116 In patients with RA, the decision to take anti-rheumatic medications is associated with trust in the physician,117 suggesting a direct link between therapeutic alliance [G] and adherence to medical recommendations.
The complexity of psychological factors in RA might adversely affect the working relationships of patients with their physicians. One key factor is concurrent psychological distress, most notably symptoms of depression, which can substantially worsen treatment outcomes.118 Detection of depression in RA is difficult, however, for several reasons: the fact that RA symptoms mimic depression, the reluctance of rheumatologists to assess depression, and systematic constraints such as a lack of time in clinic visits.119 Other contributory factors include the dearth of research on how to recognize and manage depression in rheumatology clinics, and inadequate dissemination into care practices of efficacious treatments for patients with RA who have depression.119 Rheumatologists also seem to discuss depression infrequently with their patients; in a sample of 200 patients with RA, 21 (11%) had moderate or severe symptoms of depression, but only four (19% of the depressed patients) discussed depression during their rheumatologist visit.120 Similar issues have been noted with other related symptoms; the results of one study showed that rheumatologists do not adequately address patient concerns about fatigue.121
A disconnect between the patient and rheumatologist could have major implications for therapeutic alliance and the effectiveness of future interventions; rheumatologists who lack an awareness of patients’ experiences of psychological distress, pain, and fatigue are unlikely to have a shared understanding with their patients about disease status.122 Consequently, optimal clinical care involves treating both RA disease processes and the psychological factors that can modulate those processes, both directly (through shared mechanisms) and indirectly (through behaviours that facilitate or impair treatment adherence).123
Implications for treatment
Given the importance of psychological factors in RA, a clear need exists to complement existing medical treatments with psychological interventions (Box 1). Among the most prominent interventions is cognitive-behavioural therapy (CBT), which utilizes behavioural and cognitive principles to improve function and manage stress and negative emotions. This approach has demonstrated efficacy in improving several aspects of patient functioning, including depression, fatigue, medication adherence, and perceptions of social support.124 However, disease stage seems to be a key factor in response to CBT, such that individuals in advanced stages of RA might not benefit to the same extent as do those in early stages of the disease.125
Box 1. Psychological interventions for rheumatoid arthritis.
Cognitive-behavioural therapy (CBT)
Combines principles of cognitive restructuring (identification and reframing of distorted patterns of thinking into more-adaptive and less-distressing thoughts) and behavioural techniques designed to manage stress, increase physical activity, and increase active coping (for example relaxation techniques, activity pacing approaches, and goal setting).
Mindfulness-based stress reduction (MBSR)
Meditation-based approach, designed to treat chronic pain using Buddhist principles by reducing reactivity to emotions, fostering acceptance of physical and psychological states and engaging in mindful and purposeful action. Typically taught as a 10-week course involving several types of meditation, including training of attentional processes (by focusing on the breath and bodily sensations), and being mindful of activities in daily life such as eating or walking. Daily meditation practice is emphasized as a key component of treatment.
Self-management approaches
Typically utilizes trained laypeople or Internet-based platforms to deliver a 6-week course, covering some principles included in CBT (relaxation, effective problem-solving, goal setting), as well as education about the nature of the disease
A nascent, but promising, body of evidence suggests that meditation-based approaches, such as mindfulness-based stress reduction (MBSR) might also bolster psychological health in RA. MBSR is an intervention based on Buddhist principles of non-reactivity, promotion of awareness and purposeful action that has demonstrated promise in the effective treatment of depression and anxiety disorders,126 chronic health conditions,127 and chronic pain disorders.128 Although only a few studies of MBSR have been conducted in patients with RA, their results suggest that MBSR is effective in improving patients’ well-being,129 reducing their psychological distress129 and decreasing their pain intensity.130 However, current evidence suggests that the benefits of MBSR do not directly impact RA disease activity.129
Unfortunately, not all patients with RA have access to therapists trained in these psychological approaches (such as clinical psychologists). Consequently, alternative approaches, such as self-management programmes, have become increasingly important in the treatment of RA.131 One such approach, the Arthritis Self-Management Program,132–134 is effective in improving several clinical variables, including self-efficacy, health behaviours and decreased medical utilization (specifically, a reduced frequency of physician visits). The Arthritis Self-Management Program also seems to reduce the financial burden of RA care.133 Given the apparent cost-effectiveness, low financial burden and efficacy of these interventions, they seem to be a suitable option for many patients with RA for whom consultation with a trained mental health care provider is either undesired or unfeasible.
Future directions for research
As noted previously, reducing the feelings of social disconnection and stigmatization that can accompany a diagnosis of RA, and fostering good treatment alliances between patients, their families, and their health-care providers could yield improved outcomes in patients with RA. Interventions that attend to the bidirectional interplay between these social factors and physical pain,135 and that enhance awareness of the roots of patients’ perceptions of disregard on the part of their medical providers and mindsets that promote stereotyping, distrust and social isolation136 are especially worthwhile, and fill a gap in the current behavioural approaches. Similarly, interventions combining pharmacotherapy and psychotherapy have shown promising preliminary results in treating depression in patients with arthritis137, but remain a relatively poorly studied area overall. We urge further research attention in this area as potentially valuable to future outcomes in rheumatological care. Additionally, despite compelling evidence of reward deficiencies in several chronic pain conditions,84, 85 this area has not been given adequate attention in patients with RA. The examination of reward processing abnormalities in patients with RA is expected to be instrumental in fostering improved understanding of the interplay between physical disease processes and mental health difficulties in this disease.
One potential avenue for future research is the utilization of experimental designs that manipulate inflammatory states in patients with RA. Experimental sleep deprivation, for example, reliably increases levels of proinflammatory cytokines in healthy individuals,138, 139 and also increases pain, fatigue, depressive symptoms, and disease activity in patients with RA.140 In the light of evidence that both insomnia141, 142 and experimental sleep deprivation143 attenuate positive emotions, an intriguing next step might be to examine proinflammatory cytokines as potential mediators of the effects of sleep deprivation on behavioural and neural markers of reward system function.
Conclusions
Effective discussion of the interplay between physical and psychological processes in RA requires an understanding of the many levels at which this interaction could occur. Thus, a patient’s adaptation to RA must be understood within their overall social context, as the presence of interpersonal stressors and support can have short-term and long-term implications for physical health, coping strategies, and treatment responses. Future models that integrate different levels of analysis into a holistic approach could yield useful empirical and clinical insights. For example, consideration of not only physical examination findings and laboratory results, but also the psychosocial factors unique to each patient, could yield improvements in the efficacy of current treatments, slowing disease activity and promoting long-term psychological health and physical function.
Key Points.
Individuals with rheumatoid arthritis (RA) demonstrate an increased prevalence of mood disorders compared to the general population, but a decreased prevalence of schizophrenia, probably due to alterations in inflammatory processes
Several mechanisms underlie the relationship between RA and mental health comorbidities: cognitive, behavioural and affective responses, inflammatory processes, and fatigue
Social factors have important implications in psychological reactions to RA, and could also be involved in pain processing and RA disease activity
Neural reward-processing deficiencies are an emerging area of inquiry in understanding the relationship between pain and psychological states that warrants additional attention in patients with RA
Psychological treatments that increase active coping strategies, ameliorate affective distress, bolster self-efficacy, and increase supportive social relationships could improve treatment outcomes in patients with RA
Acknowledgments
Alex J. Zautra deceased June 2016. Dr Zautra was an accomplished researcher and academic, and a devoted mentor. The authors acknowledge funding from the NIH: grant numbers NIDA 3T32DA035165-02S1 to J.A.S.; NIH K23 DA035915 and NIH P30 NR014131 to P.H.F.; and NIA R01 AG 026006 to A.J.Z.
Glossary of Terms
- Anxious/insecure attachment
A stable cognitive and behavioral style of forming and maintaining meaningful social relationships, characterized by high levels of anxiety and hypervigilance to cues about whether an individual is loved by significant others to whom he or she is attached
- Cognitive style
Relatively stable patterns of cognitive interpretation of events, which have implications for behavior and mood (e.g., an increased tendency towards catastrophic appraisal of pain or health; optimistic views of oneself, health, or future function)
- Therapeutic alliance
The relationship between a patient and his or her clinical provider (e.g., physician, psychologist), which has implications for treatment adherence and treatment outcomes and may evolve with time
Biographies
John Sturgeon, PhD, is a licensed clinical psychologist at the Stanford Pain Management Center and a postdoctoral research fellow at the Stanford Systems Neuroscience and Pain Laboratory working under Sean Mackey, MD, PhD. He completed his PhD in Clinical Psychology under Dr. Alex Zautra at Arizona State University, completed a clinical internship at the VA Ann Arbor Healthcare System, and is currently funded under a T32 training fellowship in chronic pain and substance abuse from the National Institute on Drug Abuse. Dr. Sturgeon’s research emphasizes advanced modelling of factors promoting resilience and vulnerability in individuals with chronic pain, with a particular focus on the role of social relationships and positive emotion in effective adaptation to chronic pain.
Patrick Finan is an Assistant Professor in the Department of Psychiatry and Behavioral Sciences at the Johns Hopkins University School of Medicine. Patrick received his Ph.D. in Clinical Psychology from Arizona State University and completed his clinical psychology internship at VA Connecticut in West Haven with an emphasis on the assessment and treatment of chronic pain. Patrick completed a postdoctoral fellowship at Johns Hopkins on the T32 Biobehavioral Pain Research Fellowship prior to joining the faculty. Since then, he has received funding from the National Institute of Nursing Research and the National Institute of Drug Abuse. Broadly, his research is focused on affective and reward system mechanisms of the association of sleep and pain.
Alex J. Zautra, Ph.D., is a Foundation Professor at Arizona State University & Chairman of the Board of the Social Intelligence Institute. His training has been guided by prominent scholars in their respective disciplines: Professor Bruce Dohrenwend, at Columbia University in public health, Professor Fredrick Herzberg at the University of Utah in Management, and Professor Ernst Beier, in Clinical Psychology at Utah. This foundation across key disciples have informed his interdisciplinary research for 40 years. His current work focuses on the development of public health interventions that further resilience through the humanization of social relations across the life span. Dr. Zautra passed away in June 2016.
Footnotes
Author contributions
All authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and undertook review and/or editing of the manuscript before submission.
Competing interests
The authors declare no competing interests
References
- 1.Sokka T, Krishnan E, Häkkinen A, Hannonen P. Functional disability in rheumatoid arthritis patients compared with a community population in Finland. Arthritis Rheum. 2003;48:59–63. doi: 10.1002/art.10731. [DOI] [PubMed] [Google Scholar]
- 2.van’t Land H, et al. The association between arthritis and psychiatric disorders; results from a longitudinal population-based study. J Psychosom Res. 2010;68:187–193. doi: 10.1016/j.jpsychores.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Rupp I, et al. Poor and good health outcomes in rheumatoid arthritis: the role of comorbidity. J Rheumatol. 2006;33:1488–1495. [PubMed] [Google Scholar]
- 4.Löwe B, et al. Psychiatric comorbidity and work disability in patients with inflammatory rheumatic diseases. Psychosom Med. 2004;66:395–402. [PubMed] [Google Scholar]
- 5.Lee YC, et al. The role of sleep problems in central pain processing in rheumatoid arthritis. Arthritis Rheum. 2013;65:59–68. doi: 10.1002/art.37733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Covic T, et al. Depression and anxiety in patients with rheumatoid arthritis: prevalence rates based on a comparison of the Depression, Anxiety and Stress Scale (DASS) and the hospital, Anxiety and Depression Scale (HADS) BMC Psychiatry. 2012;12:6. doi: 10.1186/1471-244X-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology. 2013;52:2136–2148. doi: 10.1093/rheumatology/ket169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Härter M, et al. A descriptive study of psychiatric disorders and psychosocial burden in rehabilitation patients with musculoskeletal diseases. Arch Phys Med Rehabil. 2002;83:461–468. doi: 10.1053/apmr.2002.30924. [DOI] [PubMed] [Google Scholar]
- 9.Morris A, Yelin EH, Panopalis P, Julian L, Katz PP. Long-term patterns of depression and associations with health and function in a panel study of rheumatoid arthritis. J Health Psychol. 2011;16:667–77. doi: 10.1177/1359105310386635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards RR, Cahalan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011;7:216–224. doi: 10.1038/nrrheum.2011.2. [DOI] [PubMed] [Google Scholar]
- 11.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 12.Margaretten M, et al. Socioeconomic determinants of disability and depression in patients with rheumatoid arthritis. Arthritis Care Res. 2011;63:240–246. doi: 10.1002/acr.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyphantis T, et al. The relationship between depressive symptoms, illness perceptions and quality of life in ankylosing spondylitis in comparison to rheumatoid arthritis. Clin Rheumatol. 2013;32:635–644. doi: 10.1007/s10067-012-2162-6. [DOI] [PubMed] [Google Scholar]
- 14.Piccinni A, et al. Clinical significance of lifetime mood and panic-agoraphobic spectrum symptoms on quality of life of patients with rheumatoid arthritis. Compr Psychiatry. 2006;47:201–208. doi: 10.1016/j.comppsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Kekow J, et al. Improvements in patient-reported outcomes, symptoms of depression and anxiety, and their association with clinical remission among patients with moderate-to-severe active early rheumatoid arthritis. Rheumatology. 2011;50:401–409. doi: 10.1093/rheumatology/keq327. [DOI] [PubMed] [Google Scholar]
- 16.Hider SL, Tanveer W, Brownfield A, Mattey DL, Packham JC. Depression in RA patients treated with anti-TNF is common and under-recognized in the rheumatology clinic. Rheumatology. 2009;48:1152–1154. doi: 10.1093/rheumatology/kep170. [DOI] [PubMed] [Google Scholar]
- 17.Rathbun AM, Reed GW, Harrold LR. The temporal relationship between depression and rheumatoid arthritis disease activity, treatment persistence and response: a systematic review. Rheumatology. 2013;52:1785–1794. doi: 10.1093/rheumatology/kes356. [DOI] [PubMed] [Google Scholar]
- 18.Michaud K, Wolfe F. Comorbidities in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2007;21:885–906. doi: 10.1016/j.berh.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Ang DC, Choi H, Kroenke K, Wolfe F. Comorbid depression is an independent risk factor for mortality in patients with rheumatoid arthritis. J Rheumatol. 2005;32:1013–1019. [PubMed] [Google Scholar]
- 20.Scherrer JF, et al. Depression increases risk of incident myocardial infarction among Veterans Administration patients with rheumatoid arthritis. Gen Hosp Psychiatry. 2009;31:353–359. doi: 10.1016/j.genhosppsych.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Timonen M, et al. Suicides in persons suffering from rheumatoid arthritis. Rheumatology. 2003;42:287–291. doi: 10.1093/rheumatology/keg082. [DOI] [PubMed] [Google Scholar]
- 22.Shih M, Hootman JM, Strine TW, Chapman DP, Brady TJ. Serious psychological distress in US adults with arthritis. J Gen Intern Med. 2006;21:1160–1166. doi: 10.1111/j.1525-1497.2006.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy LB, Sacks JJ, Brady TJ, Hootman JM, Chapman DP. Anxiety and depression among US adults with arthritis: prevalence and correlates. Arthritis Care Res. 2012;64:968–976. doi: 10.1002/acr.21685. [DOI] [PubMed] [Google Scholar]
- 24.Lok EYC, Mok CC, Cheng CW, Cheung EFC. Prevalence and determinants of psychiatric disorders in patients with rheumatoid arthritis. Psychosomatics. 2010;51:338–338.e8. doi: 10.1176/appi.psy.51.4.338. [DOI] [PubMed] [Google Scholar]
- 25.McWilliams LA, Goodwin RD, Cox BJ. Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain. 2004;111:77–83. doi: 10.1016/j.pain.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 26.VanDyke MM, et al. Anxiety in rheumatoid arthritis. Arthritis Care Res. 2004;51:408–412. doi: 10.1002/art.20474. [DOI] [PubMed] [Google Scholar]
- 27.Mok C, Lok E, Cheung E. Concurrent psychiatric disorders are associated with significantly poorer quality of life in patients with rheumatoid arthritis. Scand J Rheumatol. 2012;41:253–259. doi: 10.3109/03009742.2012.664648. [DOI] [PubMed] [Google Scholar]
- 28.Boscarino JA, Forsberg CW, Goldberg J. A twin study of the association between PTSD symptoms and rheumatoid arthritis. Psychosom Med. 2010;72:481–486. doi: 10.1097/PSY.0b013e3181d9a80c. [DOI] [PubMed] [Google Scholar]
- 29.Mikuls TR, et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res. 2013;65:227–234. doi: 10.1002/acr.21778. [DOI] [PubMed] [Google Scholar]
- 30.Helzer JE, Robins LN, McEvoy L. Post-traumatic stress disorder in the general population. N Engl J Med. 1987;317:1630–1634. doi: 10.1056/NEJM198712243172604. [DOI] [PubMed] [Google Scholar]
- 31.Oken RJ, Schulzer M. At issue: schizophrenia and rheumatoid arthritis: the negative association revisited. Schizophr Bull. 1999;25:625. doi: 10.1093/oxfordjournals.schbul.a033407. [DOI] [PubMed] [Google Scholar]
- 32.Jeste DV, Gladsjo JA, Lindamer LA, Lacro JP. Medical comorbidity in schizophrenia. Schizophr Bull. 1996;22:413–430. doi: 10.1093/schbul/22.3.413. [DOI] [PubMed] [Google Scholar]
- 33.Potvin S, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–808. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Gorwood P, et al. Rheumatoid arthritis and schizophrenia: a negative association at a dimensional level. Schizophr Res. 2004;66:21–29. doi: 10.1016/s0920-9964(03)00017-3. [DOI] [PubMed] [Google Scholar]
- 35.Dinarello CA. The many worlds of reducing interleukin-1. Arthritis Rheum. 2005;52:1960–1967. doi: 10.1002/art.21107. [DOI] [PubMed] [Google Scholar]
- 36.Bruce TO. Comorbid depression in rheumatoid arthritis: pathophysiology and clinical implications. Curr Psychiatry Rep. 2008;10:258–264. doi: 10.1007/s11920-008-0042-1. [DOI] [PubMed] [Google Scholar]
- 37.Straub RH, Cutolo M. Involvement of the hypothalamic–pituitary–adrenal/gonadal axis and the peripheral nervous system in rheumatoid arthritis: viewpoint based on a systemic pathogenetic role. Arthritis Rheum. 2001;44:493–507. doi: 10.1002/1529-0131(200103)44:3<493::AID-ANR95>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 38.Straub RH. Rheumatoid arthritis: Stress in RA: a trigger of proinflammatory pathways? Nat Rev Rheumatol. 2014;10:516–518. doi: 10.1038/nrrheum.2014.110. [DOI] [PubMed] [Google Scholar]
- 39.Straub RH, Dhabhar FS, Bijlsma JW, Cutolo M. How psychological stress via hormones and nerve fibers may exacerbate rheumatoid arthritis. Arthritis Rheum. 2005;52:16–26. doi: 10.1002/art.20747. [DOI] [PubMed] [Google Scholar]
- 40.Dowlati Y, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 41.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evers AW, Zautra A, Thieme K. Stress and resilience in rheumatic diseases: a review and glimpse into the future. Nat Rev Rheumatol. 2011;7:409–415. doi: 10.1038/nrrheum.2011.80. [DOI] [PubMed] [Google Scholar]
- 43.Zautra AJ, Smith BW. Depression and reactivity to stress in older women with rheumatoid arthritis and osteoarthritis. Psychosom Med. 2001;63:687–696. doi: 10.1097/00006842-200107000-00022. [DOI] [PubMed] [Google Scholar]
- 44.Söderlin MK, Hakala M, Nieminen P. Anxiety and depression in a community-based rheumatoid arthritis population. Scand J Rheumatol. 2000;29:177–183. doi: 10.1080/030097400750002067. [DOI] [PubMed] [Google Scholar]
- 45.Bazzichi L, et al. Quality of life in rheumatoid arthritis: impact of disability and lifetime depressive spectrum symptomatology. Clin Exp Rheumatol. 2005;23:783. [PubMed] [Google Scholar]
- 46.Courvoisier DS, et al. Pain as an important predictor of psychosocial health in patients with rheumatoid arthritis. Arthritis Care Res. 2012;64:190–196. doi: 10.1002/acr.20652. [DOI] [PubMed] [Google Scholar]
- 47.Pollard L, Choy E, Gonzalez J, Khoshaba B, Scott D. Fatigue in rheumatoid arthritis reflects pain, not disease activity. Rheumatology. 2006;45:885–889. doi: 10.1093/rheumatology/kel021. [DOI] [PubMed] [Google Scholar]
- 48.Öncü J, Başoğlu F, Kuran B. A comparison of impact of fatigue on cognitive, physical, and psychosocial status in patients with fibromyalgia and rheumatoid arthritis. Rheumatol Int. 2013;33:3031–3037. doi: 10.1007/s00296-013-2825-x. [DOI] [PubMed] [Google Scholar]
- 49.Rupp I, Boshuizen HC, Jacobi CE, Dinant HJ, van den Bos GA. Impact of fatigue on health‐related quality of life in rheumatoid arthritis. Arthritis Care Res. 2004;51:578–585. doi: 10.1002/art.20539. [DOI] [PubMed] [Google Scholar]
- 50.Nikolaus S, Bode C, Taal E, de Laar MA. Fatigue and factors related to fatigue in rheumatoid arthritis: a systematic review. Arthritis Care Res. 2013;65:1128–1146. doi: 10.1002/acr.21949. [DOI] [PubMed] [Google Scholar]
- 51.Cutolo M, Kitas GD, van Riel PL. Burden of disease in treated rheumatoid arthritis patients: Going beyond the joint. Semin Arthritis Rheum. 2014;43:479–488. doi: 10.1016/j.semarthrit.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Joharatnam N, et al. A cross-sectional study of pain sensitivity, disease-activity assessment, mental health, and fibromyalgia status in rheumatoid arthritis. Arthritis Res Ther. 2015;17:11. doi: 10.1186/s13075-015-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fifield J, et al. History of affective disorder and the temporal trajectory of fatigue in rheumatoid arthritis. Ann Behav Med. 2001;23:34–41. doi: 10.1207/S15324796ABM2301_6. [DOI] [PubMed] [Google Scholar]
- 54.Belt N, Kronholm E, Kauppi M. Sleep problems in fibromyalgia and rheumatoid arthritis compared with the general population. Clin Exp Rheumatol. 2009;27:35. [PubMed] [Google Scholar]
- 55.Smedstad L, Mourn T, Vaglum P, Kvien T. The impact of early rheumatoid arthritis on psychological distress. Scand J Rheumatol. 1996;25:377–382. doi: 10.3109/03009749609065649. [DOI] [PubMed] [Google Scholar]
- 56.Wolfe F, Michaud K. Predicting depression in rheumatoid arthritis: the signal importance of pain extent and fatigue, and comorbidity. Arthritis Care Res. 2009;61:667–673. doi: 10.1002/art.24428. [DOI] [PubMed] [Google Scholar]
- 57.Isik A, Koca SS, Ozturk A, Mermi O. Anxiety and depression in patients with rheumatoid arthritis. Clin Rheumatol. 2007;26:872–878. doi: 10.1007/s10067-006-0407-y. [DOI] [PubMed] [Google Scholar]
- 58.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR®. American Psychiatric Pub; 2000. [Google Scholar]
- 59.Hegarty RS, Conner TS, Stebbings S, Treharne GJ. Feel the fatigue and be active anyway: Physical activity on high fatigue days protects adults with arthritis from decrements in same‐day positive mood. Arthritis Care Res. 2015 doi: 10.1002/acr.22582. [DOI] [PubMed] [Google Scholar]
- 60.Parrish BP, Zautra AJ, Davis MC. The role of positive and negative interpersonal events on daily fatigue in women with fibromyalgia, rheumatoid arthritis, and osteoarthritis. Health Psychol. 2008;27:694. doi: 10.1037/0278-6133.27.6.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Finan P, et al. Interplay of concurrent positive and negative interpersonal events in the prediction of daily negative affect and fatigue for rheumatoid arthritis patients. Health Psychol. 2010;29:429. doi: 10.1037/a0020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henningsen P, Zimmermann T, Sattel H. Medically Unexplained Physical Symptoms, Anxiety, and Depression: A Meta‐Analytic Review. Psychosom Med. 2003;65:528–533. doi: 10.1097/01.psy.0000075977.90337.e7. [DOI] [PubMed] [Google Scholar]
- 63.Moss-Morris R, Chalder T. Illness perceptions and levels of disability in patients with chronic fatigue syndrome and rheumatoid arthritis. J Psychosom Res. 2003;55:305–308. doi: 10.1016/s0022-3999(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 64.Smith BW, Zautra AJ. The effects of anxiety and depression on weekly pain in women with arthritis. Pain. 2008;138:354–361. doi: 10.1016/j.pain.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Strand EB, et al. Positive affect as a factor of resilience in the pain—negative affect relationship in patients with rheumatoid arthritis. J Psychosom Res. 2006;60:477–484. doi: 10.1016/j.jpsychores.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 66.Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. J Consult Clin Psychol. 2005;73:212–20. doi: 10.1037/0022-006X.73.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conner TS, et al. Coping with rheumatoid arthritis pain in daily life: within-person analyses reveal hidden vulnerability for the formerly depressed. Pain. 2006;126:198–209. doi: 10.1016/j.pain.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 68.Zautra AJ, et al. Arthritis and perceptions of quality of life: an examination of positive and negative affect in rheumatoid arthritis patients. Health Psychol. 1995;14:399. doi: 10.1037//0278-6133.14.5.399. [DOI] [PubMed] [Google Scholar]
- 69.Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7:524. [Google Scholar]
- 70.Nakajima A, et al. Disability and patient’s appraisal of general health contribute to depressed mood in rheumatoid arthritis in a large clinical study in Japan. Mod Rheumatol. 2006;16:151–157. doi: 10.1007/s10165-006-0475-5. [DOI] [PubMed] [Google Scholar]
- 71.Englbrecht M, et al. The impact of coping strategies on mental and physical well-being in patients with rheumatoid arthritis. Semin Arthritis Rheum. 2012;41:545–555. doi: 10.1016/j.semarthrit.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Pinto-Gouveia J, Costa J, Marôco J. The first 2 years of rheumatoid arthritis: The influence of acceptance on pain, physical limitation and depression. J Health Psychol. 2015;20:102–112. doi: 10.1177/1359105313499807. [DOI] [PubMed] [Google Scholar]
- 73.Treharne GJ, Kitas GD, Lyons AC, Booth DA. Well-being in rheumatoid arthritis: the effects of disease duration and psychosocial factors. J Health Psychol. 2005;10:457–474. doi: 10.1177/1359105305051416. [DOI] [PubMed] [Google Scholar]
- 74.Knittle KP, et al. Effect of self-efficacy and physical activity goal achievement on arthritis pain and quality of life in patients with rheumatoid arthritis. Arthritis Care Res. 2011;63:1613–1619. doi: 10.1002/acr.20587. [DOI] [PubMed] [Google Scholar]
- 75.Scharloo M, et al. Illness perceptions, coping and functioning in patients with rheumatoid arthritis, chronic obstructive pulmonary disease and psoriasis. J Psychosom Res. 1998;44:573–585. doi: 10.1016/s0022-3999(97)00254-7. [DOI] [PubMed] [Google Scholar]
- 76.Wood PB, Glabus MF, Simpson R, Patterson JC. Changes in gray matter density in fibromyalgia: correlation with dopamine metabolism. J Pain. 2009;10:609–618. doi: 10.1016/j.jpain.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 77.Wood PB, et al. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study. J Pain. 2007;8:51–58. doi: 10.1016/j.jpain.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 78.Wood PB, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576–3582. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- 79.Russell IJ, Vaeroy H, Javors M, Nyberg F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550–556. doi: 10.1002/art.1780350509. [DOI] [PubMed] [Google Scholar]
- 80.Ashby FG, Isen AM. A neuropsychological theory of positive affect and its influence on cognition. Psychol Rev. 1999;106:529. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- 81.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 82.Zautra AJ, et al. Fibromyalgia: evidence for deficits in positive affect regulation. Psychosom Med. 2005;67:147. doi: 10.1097/01.psy.0000146328.52009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Finan PH, Zautra AJ, Davis MC. Daily affect relations in fibromyalgia patients reveal positive affective disturbance. Psychosom Med. 2009;71:474–82. doi: 10.1097/PSY.0b013e31819e0a8b. [DOI] [PubMed] [Google Scholar]
- 84.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Martikainen IK, et al. Chronic back pain is associated with alterations in dopamine neurotransmission in the ventral striatum. J Neurosci. 2015;35:9957–9965. doi: 10.1523/JNEUROSCI.4605-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: an inflammatory challenge induces feelings of social disconnection in addition to depressed mood. Brain Behav Immun. 2010;24:558–63. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reichenberg A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 89.Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63:1022–1029. doi: 10.1016/j.biopsych.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Illi J, et al. Association between pro-and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58:437–447. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ulus Y, et al. Sleep quality in fibromyalgia and rheumatoid arthritis: associations with pain, fatigue, depression, and disease activity. Clin Exp Rheumatol. 2010;29:S92–6. [PubMed] [Google Scholar]
- 92.Lee YC, et al. Subgrouping of patients with rheumatoid arthritis based on pain, fatigue, inflammation, and psychosocial factors. Arthritis Rheum. 2014;66:2006–2014. doi: 10.1002/art.38682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Finan PH, Smith MT. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev. 2013;17:173–183. doi: 10.1016/j.smrv.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Finan PH, Zautra AJ. Rheumatoid arthritis: Stress affects rheumatoid arthritis, but via what mechanisms? Nat Rev Rheumatol. 2013;9:569–570. doi: 10.1038/nrrheum.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Carlino E, Frisaldi E, Benedetti F. Pain and the context. Nat Rev Rheumatol. 2014;10:348–355. doi: 10.1038/nrrheum.2014.17. [DOI] [PubMed] [Google Scholar]
- 96.Zautra AJ, et al. Examination of changes in interpersonal stress as a factor in disease exacerbations among women with rheumatoid arthritis. Ann Behav Med. 1997;19:279–286. doi: 10.1007/BF02892292. [DOI] [PubMed] [Google Scholar]
- 97.Davis MC, et al. Chronic stress and regulation of cellular markers of inflammation in rheumatoid arthritis: implications for fatigue. Brain Behav Immun. 2008;22:24–32. doi: 10.1016/j.bbi.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith TW, Christensen AJ. Positive and negative affect in rheumatoid arthritis: Increased specificity in the assessment of emotional adjustment. Ann Behav Med. 1996;18:75–78. doi: 10.1007/BF02909578. [DOI] [PubMed] [Google Scholar]
- 99.Potter P, Zautra A. Effects of major and minor stressors on disease activity in rheumatoid arthritis patients. J Consult Clin Psychol. 1997;65:319–323. doi: 10.1037//0022-006x.65.2.319. [DOI] [PubMed] [Google Scholar]
- 100.Zautra AJ, Hamilton N, Yocum D. Patterns of positive social engagement among women with rheumatoid arthritis. OTJR (Thorofare N J) 2000;20:21S–40S. [Google Scholar]
- 101.Bowlby J. Attachment and Loss. I. Pimlico; 1969. Attachment. [Google Scholar]
- 102.Fraley CR, Niedenthal PM, Marks M, Brumbaugh C, Vicary A. Adult attachment and the perception of emotional expressions: Probing the hyperactivating strategies underlying anxious attachment. J Pers. 2006;74:1163–1190. doi: 10.1111/j.1467-6494.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 103.Infurna FJ, Rivers CT, Reich J, Zautra AJ. Childhood Trauma and Personal Mastery: Their influence on emotional reactivity to everyday events in a community sample of middle-aged adults. PLoS one. 2015;10:e0121840. doi: 10.1371/journal.pone.0121840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vrtička P, Andersson F, Grandjean D, Sander D, Vuilleumier P. Individual attachment style modulates human amygdala and striatum activation during social appraisal. PLoS One. 2008;3:e2868–e2868. doi: 10.1371/journal.pone.0002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Davis DA, Luecken LJ, Zautra AJ. Are reports of childhood abuse related to the experience of chronic pain in adulthood? A meta-analytic review of the literature. Clin J Pain. 2005;21:398–405. doi: 10.1097/01.ajp.0000149795.08746.31. [DOI] [PubMed] [Google Scholar]
- 106.Weber S, Jud A, Landolt M. Quality of life in maltreated children and adult survivors of child maltreatment: a systematic review. Qual Life Res. 2016;25:237–255. doi: 10.1007/s11136-015-1085-5. [DOI] [PubMed] [Google Scholar]
- 107.Manne SL, Zautra AJ. Couples coping with chronic illness: Women with rheumatoid arthritis and their healthy husbands. J Behav Med. 1990;13:327–342. doi: 10.1007/BF00844882. [DOI] [PubMed] [Google Scholar]
- 108.Kasle S, Wilhelm MS, Zautra AJ. Rheumatoid arthritis patients’ perceptions of mutuality in conversations with spouses/partners and their links with psychological and physical health. Arthritis Care Res. 2008;59:921–928. doi: 10.1002/art.23821. [DOI] [PubMed] [Google Scholar]
- 109.Arewasikporn A, Davis MC, Zautra A. In: Health and social relationships: The good, the bad, and the complicated. Newman ML, Roberts NA, editors. American Psychological Association; 2013. [Google Scholar]
- 110.Manne SL, Zautra AJ. Spouse criticism and support: their association with coping and psychological adjustment among women with rheumatoid arthritis. J Pers Soc Psychol. 1989;56:608. doi: 10.1037//0022-3514.56.4.608. [DOI] [PubMed] [Google Scholar]
- 111.Zyrianova Y, et al. Depression and anxiety in rheumatoid arthritis: the role of perceived social support. Ir J Med Sci. 2006;175:32–36. doi: 10.1007/BF03167946. [DOI] [PubMed] [Google Scholar]
- 112.Evers AWM, Kraaimaat FW, Geenen R, Jacobs JWG, Bijlsma JWJ. Pain coping and social support as predictors of long-term functional disability and pain in early rheumatoid arthritis. Behav Res Ther. 2003;41:1295–1310. doi: 10.1016/s0005-7967(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 113.Benka J, et al. Social support as a moderator of functional disability’s effect on depressive feelings in early rheumatoid arthritis: A four-year prospective study. Rehabil Psychol. 2014;59:19. doi: 10.1037/a0035115. [DOI] [PubMed] [Google Scholar]
- 114.Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60:301–312. doi: 10.1016/j.pec.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 115.Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff. 2013;32:207–214. doi: 10.1377/hlthaff.2012.1061. [DOI] [PubMed] [Google Scholar]
- 116.Bennett JK, Fuertes JN, Keitel M, Phillips R. The role of patient attachment and working alliance on patient adherence, satisfaction, and health-related quality of life in lupus treatment. Patient Educ Couns. 2011;85:53–59. doi: 10.1016/j.pec.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 117.Martin RW, et al. Patient decision-making related to antirheumatic drugs in rheumatoid arthritis: the importance of patient trust of physician. J Rheumatol. 2008;35:618–624. [PubMed] [Google Scholar]
- 118.Sheehy C, Murphy E, Barry M. Depression in rheumatoid arthritis—underscoring the problem. Rheumatology. 2006;45:1325–1327. doi: 10.1093/rheumatology/kel231. [DOI] [PubMed] [Google Scholar]
- 119.Nicassio PM. The problem of detecting and managing depression in the rheumatology clinic. Arthritis Care Res. 2008;59:155–158. doi: 10.1002/art.23348. [DOI] [PubMed] [Google Scholar]
- 120.Sleath B, et al. Communication about depression during rheumatoid arthritis patient visits. Arthritis Care Res. 2008;59:186–191. doi: 10.1002/art.23347. [DOI] [PubMed] [Google Scholar]
- 121.Repping-Wuts H, Repping T, van Riel P, van Achterberg T. Fatigue communication at the out-patient clinic of Rheumatology. Patient Educ Couns. 2009;76:57–62. doi: 10.1016/j.pec.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 122.Khan NA, et al. Determinants of discordance in patients’ and physicians’ rating of rheumatoid arthritis disease activity. Arthritis Care Res. 2012;64:206–214. doi: 10.1002/acr.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tander B, et al. A comparative evaluation of health related quality of life and depression in patients with fibromyalgia syndrome and rheumatoid arthritis. Rheumatol Int. 2008;28:859–65. doi: 10.1007/s00296-008-0551-6. [DOI] [PubMed] [Google Scholar]
- 124.Evers AW, Kraaimaat FW, van Riel PL, de Jong AJ. Tailored cognitive-behavioral therapy in early rheumatoid arthritis for patients at risk: a randomized controlled trial. Pain. 2002;100:141–153. doi: 10.1016/s0304-3959(02)00274-9. [DOI] [PubMed] [Google Scholar]
- 125.Kraaimaat F, Brons M, Geenen R, Bijlsma J. The effect of cognitive behavior therapy in patients with rheumatoid arthritis. Behav Res Ther. 1995;33:487–495. doi: 10.1016/0005-7967(94)00094-z. [DOI] [PubMed] [Google Scholar]
- 126.Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: A meta-analytic review. J Consult Clin Psychol. 2010;78:169. doi: 10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bohlmeijer E, Prenger R, Taal E, Cuijpers P. The effects of mindfulness-based stress reduction therapy on mental health of adults with a chronic medical disease: a meta-analysis. J Psychosom Res. 2010;68:539–544. doi: 10.1016/j.jpsychores.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 128.Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance-based interventions for the treatment of chronic pain: a systematic review and meta-analysis. Pain. 2011;152:533–542. doi: 10.1016/j.pain.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 129.Pradhan EK, et al. Effect of Mindfulness-Based stress reduction in rheumatoid arthritis patients. Arthritis Care Res. 2007;57:1134–1142. doi: 10.1002/art.23010. [DOI] [PubMed] [Google Scholar]
- 130.Zautra AJ, et al. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. J Consult Clin Psychol. 2008;76:408. doi: 10.1037/0022-006X.76.3.408. [DOI] [PubMed] [Google Scholar]
- 131.Dures E, Hewlett S. Cognitive–behavioural approaches to self-management in rheumatic disease. Nat Rev Rheumatol. 2012;8:553–559. doi: 10.1038/nrrheum.2012.108. [DOI] [PubMed] [Google Scholar]
- 132.Lorig K, Lubeck D, Kraines RG, Seleznick M, Holman HR. Outcomes of self-help education for patients with arthritis. Arthritis Rheum. 1985;28:680–685. doi: 10.1002/art.1780280612. [DOI] [PubMed] [Google Scholar]
- 133.Lorig KR, Mazonson PD, Holman HR. Evidence suggesting that health education for self-management in patients with chronic arthritis has sustained health benefits while reducing health care costs. Arthritis Rheum. 1993;36:439–446. doi: 10.1002/art.1780360403. [DOI] [PubMed] [Google Scholar]
- 134.Lorig KR, Ritter PL, Laurent DD, Plant K. The internet-based arthritis self-management program: A one-year randomized trial for patients with arthritis or fibromyalgia. Arthritis Care Res. 2008;59:1009–1017. doi: 10.1002/art.23817. [DOI] [PubMed] [Google Scholar]
- 135.Sturgeon JA, Zautra AJ. Social pain and physical pain: shared paths to resilience. Pain Management. 2016;6:63–74. doi: 10.2217/pmt.15.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zautra EK, Zautra AJ, Gallardo CE, Velasco L. Can We Learn to Treat One Another Better? A Test of a Social Intelligence Curriculum. PLoS One. 2015;10:e0128638. doi: 10.1371/journal.pone.0128638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lin EH, et al. Effect of improving depression care on pain and functional outcomes among older adults with arthritis: a randomized controlled trial. JAMA. 2003;290:2428–2429. doi: 10.1001/jama.290.18.2428. [DOI] [PubMed] [Google Scholar]
- 138.Haack M, Sanchez E, Mullington JM. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep. 2007;30:1145. doi: 10.1093/sleep/30.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24:775–784. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Irwin MR, et al. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. 2012;35:537. doi: 10.5665/sleep.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.de Wild-Hartmann JA, et al. Day-to-day associations between subjective sleep and affect in regard to future depression in a female population-based sample. Br J Psychiatry. 2013;202:407–412. doi: 10.1192/bjp.bp.112.123794. [DOI] [PubMed] [Google Scholar]
- 142.Kalmbach DA, Pillai V, Roth T, Drake CL. The interplay between daily affect and sleep: a 2‐week study of young women. J Sleep Res. 2014;23:636–645. doi: 10.1111/jsr.12190. [DOI] [PubMed] [Google Scholar]
- 143.Finan P, Quartana P, Smith M. The effects of sleep continuity disruption on positive mood and sleep architecture in healthy adults. Sleep. 2015;38:1735–1742. doi: 10.5665/sleep.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]


