Abstract
The development of multiple organ failure in patients with hemorrhagic shock is significantly influenced by patient age. Adenosine monophosphate–activated protein kinase (AMPK) is a crucial regulator of energy homeostasis, which coordinates metabolic repair during cellular stress. We investigated whether AMPK-regulated signaling pathways are age-dependent in hemorrhage-induced lung injury and whether AMPK activation by 5-amino-4-imidazole carboxamide riboside (AICAR) affords lung protective effects. Male C57/BL6 young mice (3–5 mo), mature adult mice (9–12 mo), and young AMPKα1 knockout mice (3–5 mo) were subjected to hemorrhagic shock by blood withdrawing, followed by resuscitation with shed blood and lactated Ringer’s solution. Plasma proinflammatory cytokines were similarly elevated in C57/BL6 young and mature adult mice after hemorrhagic shock. However, mature adult mice exhibited more severe lung edema and neutrophil infiltration, and higher mitochondrial damage in alveolar epithelial type II cells, than did young mice. No change in autophagy was observed. At molecular analysis, the phosphorylation of the catalytic subunit AMPKα1 was associated with nuclear translocation of peroxisome proliferator-activated receptor γ co-activator-α in young, but not mature, adult mice. Treatment with AICAR ameliorated the disruption of lung architecture in mice of both ages; however, effects in mature adult mice were different than young mice and also involved inhibition of nuclear factor-κB. In young AMPKα1 knockout mice, AICAR failed to improve hypotension and lung neutrophil infiltration. Our data demonstrate that during hemorrhagic shock, AMPK-dependent metabolic repair mechanisms are important for mitigating lung injury. However, these mechanisms are less competent with age.
Keywords: hemorrhagic shock, AMPK, AICAR, mitochondria, autophagy
Clinical Relevance
Acute lung injury is a significant cause of morbidity and mortality in trauma victims and appears significantly influenced by the age of the patient. Despite this clinical evidence, the molecular mechanisms that link age to enhanced susceptibility to lung injury and diminished capability to recovery are not known. We demonstrate that AMP-activated kinase is a crucial regulator of the metabolic recovery of the lung after hemorrhagic shock by favoring mitochondrial quality control in mice. However, this recovery capability is impaired with aging. We also demonstrate that, despite diverse age-dependent cellular and molecular mechanisms, pharmacological activation of AMP-activated kinase may afford lung protective effects and may represent a novel treatment in the management of trauma and hemorrhagic shock.
Hemorrhagic shock is associated with high rates of acute lung injury and is a predictor of poor outcome in trauma victims (1). Clinical evidence suggests that the clinical course of organ failure and the risk of mortality are influenced significantly by the age of the patient (2–4). However, the underlying molecular mechanisms that link age to enhanced susceptibility to organ failure and diminished capability to recovery are not known.
Dysfunction of the mitochondria has been suggested as a pathophysiological event of organ injury during resuscitation despite early goal-oriented maximization of oxygen delivery (5). The AMP-activated protein kinase (AMPK) is a crucial sensor of energy status and contributes to many important metabolic processes. In this context, AMPK regulates mitochondrial biogenesis through activation of the transcription coactivator peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1-α) (6). AMPK is also a positive regulator of autophagy, a highly conserved cellular process that is responsible for the degradation of long-lived proteins and damaged organelles to maintain energy production during stress (7). Aging-associated loss in AMPK activity has been reported to contribute to mitochondrial dysfunction in skeletal muscles and myocardium in advanced-aged rodents and to correlate with dysfunction of autophagy (8, 9).
In this study, by using a model of hemorrhagic shock in C57/BL6 young mice (3–5 mo) and mature adult mice (9–12 mo), we investigated whether the AMPK-mediated metabolic pathways of mitochondrial recovery and autophagy were altered during acute lung injury and whether they were age dependent. Furthermore, we tested whether pharmacological activation of AMPK by the AMP analog, 5-amino-4-imidazole carboxamide riboside (AICAR) (10) would maintain mitochondrial quality control and ameliorate hemorrhage-induced lung injury. To verify the specificity of AICAR on the activation of AMPK, we performed additional studies in young mice genetically deficient of the catalytic α1-subunit of AMPK, which is the predominant form expressed in the lung (11, 12). Some of the results of these studies have been reported previously in the form of an abstract (13).
Materials and Methods
Detailed methods are described in the online supplement.
Hemorrhagic Shock
The experiments conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (eighth edition, 2011) and had the approval of the Institutional Animal Care and Use Committee of the Cincinnati Children’s Hospital. Male C57/BL6 wild-type young mice (3–5 mo) and mature adult mice (9–12 mo) and young AMPKα1 knockout mice in C57/BL6 background (3–5 mo) were anesthetized with pentobarbital (80 mg/kg) intraperitoneally. Hemorrhagic shock was induced by blood removal until mean arterial blood pressure (MABP) reached 30 ± 5 mm Hg (14). The mice were kept in this MABP range for 90 minutes and then resuscitated with their shed blood and lactated Ringer’s solution (2×). At the time of transfusion, all mice were randomly assigned to two treatment groups: a vehicle group received distilled water (200 μl intraperitoneally), and an AICAR group received AICAR (100 mg/kg intraperitoneally). Sham mice underwent surgical preparation without blood withdrawal. At 3 hours after resuscitation, mice were killed. Bronchoalveolar lavage fluid (BALF) was collected to determine total cell count, protein, and cytokine levels. Blood and lungs were collected for other biochemical assays.
Cytokine Analysis
Plasma levels of IL-1β, IL-10, IL-6, IL-17, TNF-α, and INF-γ, and BALF levels of IL-6 were evaluated by a multiplex array system.
Lung Injury
The ratio of lung wet weight to dry weight was used as an indicator of lung edema. Lung injury was estimated in hematoxylin and eosin–stained sections by a score that was based on histopathologic features of parenchymal damage and cellular infiltration (15). Myeloperoxidase (MPO) activity was determined as an index of neutrophil accumulation (16) in lung homogenates. Transmission electron microscopy (TEM) was used to determine the total number of healthy and damaged mitochondria, autophagosomes, and multivesicular bodies by National Institutes of Health ImageJ analysis (17).
Western Blot Analysis and NF-κB Activity
Lung cytosol and nuclear extracts were used to determine the contents of AMPKα1 and its phosphorylated active form (pAMPKα1), PGC1-α, sirtuin 1 (SIRT1), and light-chain 3B (LC3B)-I and LC3B-II proteins by immunoblot analyses. Nuclear activity of the NF-κB p65 subunit was analyzed by a TransAM Transcription Factor assay kit.
Statistical Analysis
Statistical analysis was performed using SigmaPlot (Systat Software, San Jose, CA). Data are presented as mean ± SD or SEM, or medians with interquartile range of n = 3–13 animals for each group. For multiple group analysis, one-way analysis of variance (ANOVA) at a single time point or two-way ANOVA at different time points was used, followed by the Student-Newman-Keuls correction test. If data failed to follow a normal distribution, a Mann–Whitney rank sum test or an ANOVA on ranks test was performed. P values <0.05 were considered significant.
Results
Age-Dependent Effect of AICAR on Hemorrhage-Induced Hypotension
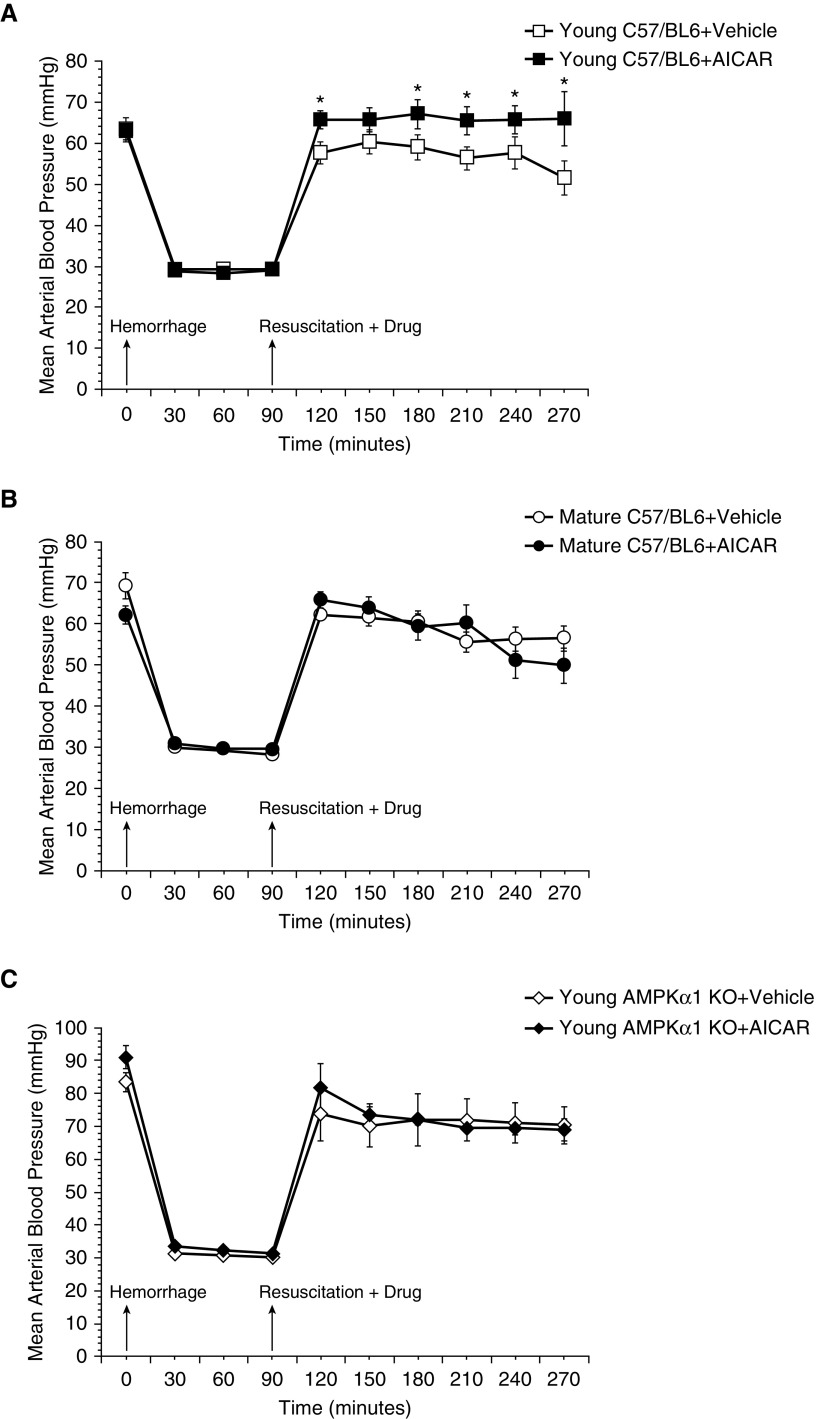
To obtain a similar degree of hypoperfusion, C57/BL6 wild-type mice of both ages and young AMPKα1 knockout mice were hemorrhaged to an MABP of 30 ± 5 mm Hg and were resuscitated at 90 minutes after the initial hemorrhage. After an early increase soon after transfusion, MABP declined progressively in both vehicle-treated wild-type young and mature adult animals. AICAR treatment of young mice ameliorated MABP significantly throughout the resuscitation phase when compared with vehicle treatment. In contrast, AICAR treatment did not affect MABP in mature adult mice. In additional experiments, AICAR treatment did not affect MABP in young AMPKα1 knockout mice, which experienced a hypotension after resuscitation similar to that of the vehicle-treated group (Figure 1).
Figure 1.
Effect of in vivo treatment with 5-amino-4-imidazole carboxamide riboside (AICAR) on mean arterial blood pressure in (A) C57BL/6 wild-type young, (B) C57BL/6 wild-type mature, and (C) young AMP-activated protein kinase (AMPK) α1 knockout (KO) mice subjected to hemorrhage and resuscitation. Data represent the mean ± SD of 3 to 10 mice in each group. Vehicle (distilled water) or AICAR (100 mg/kg) was administered intraperitoneally at the time of resuscitation with shed blood and lactated Ringer’s solution (×2). Arrows indicate time of induction of hemorrhage and initiation of resuscitation and AICAR or vehicle administration. *P < 0.05 versus vehicle-treated group.
Effect of AICAR on Plasma Cytokines
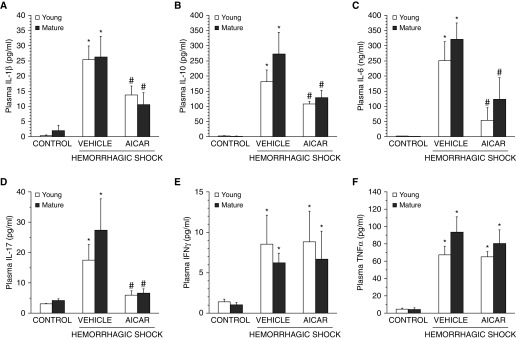
To evaluate the hemorrhage-induced systemic inflammatory response, a panel of Th1/Th2/Th17 cytokines was measured. At 3 hours after resuscitation, plasma levels of IL-1β, IL-6, IL-10, IL-17, TNF-α, and INF-γ were increased significantly in both C57/BL6 wild-type vehicle-treated young and mature adult mice compared with age-matched control mice. AICAR treatment significantly decreased levels of IL-1β, IL-6, IL-10, and IL-17 in both age groups, whereas it did not affect plasma levels of TNF-α and INF-γ (Figure 2). In additional experiments, young AMPKα1 knockout mice showed a higher magnitude of elevation of IL-1β and TNF-α when compared with young C57/BL6 wild-type mice. AICAR treatment did not affect plasma elevation of IL-1β or TNF-α in young AMPKα1 knockout mice, which experienced an exaggerated inflammatory response after resuscitation similar to that of the vehicle-treated group (Table 1).
Figure 2.
Effect of in vivo treatment with AICAR on plasma levels of (A) IL-1β, (B) IL-10, (C) IL-6, (D) IL-17, (E) IFNγ, and (F) TNF-α in C57BL/6 wild-type young and mature mice after hemorrhagic shock. Each data point represents the mean ± SEM of four to six animals for each group. *P < 0.05 versus age-matched control mice; #P < 0.05 versus vehicle-treated group of the same age.
Table 1.
Plasma Levels of Cytokines in Young C57/BL6 Wild-Type and AMPKα1 Knockout Mice (3–5 mo) Subjected to Hemorrhagic Shock
| Cytokine (pg/ml) | Young C57/BL6 Wild-Type Mice |
Young AMPKα1 Knockout Mice |
||||
|---|---|---|---|---|---|---|
| Basal | At 3 h after Transfusion |
Basal | At 3 h after Transfusion |
|||
| Vehicle | AICAR | Vehicle | AICAR | |||
| IL-1β | 0.2 ± 0.2 | 25.4 ± 4.5* | 13.7 ± 2.9† | 1.6 ± 1.6 | 123.8 ± 32.0*‡ | 78.3 ± 27.2*‡ |
| TNF-α | 4.8 ± 1.2 | 67.3 ± 9.9* | 65.2 ± 6.0* | 0.2 ± 0.2 | 137.2 ± 45.7*‡ | 113.6 ± 21.1*‡ |
Definition of abbreviations: AICAR, 5-amino-4-imidazole carboxamide riboside; AMPK, AMP-activated protein kinase.
Data represent the mean ± SEM of 8–10 mice in wild-type group and 3–4 mice in knockout group. Vehicle or AICAR (100 mg/kg) was administered intraperitoneally at the time of resuscitation.
P < 0.05 versus baseline at time 0 before hemorrhage of mice of the same genotype.
P < 0.05 versus vehicle treatment of mice of the same genotype.
P < 0.05 versus wild-type mice.
Age-Dependent Lung Histological Changes and Neutrophil Infiltration
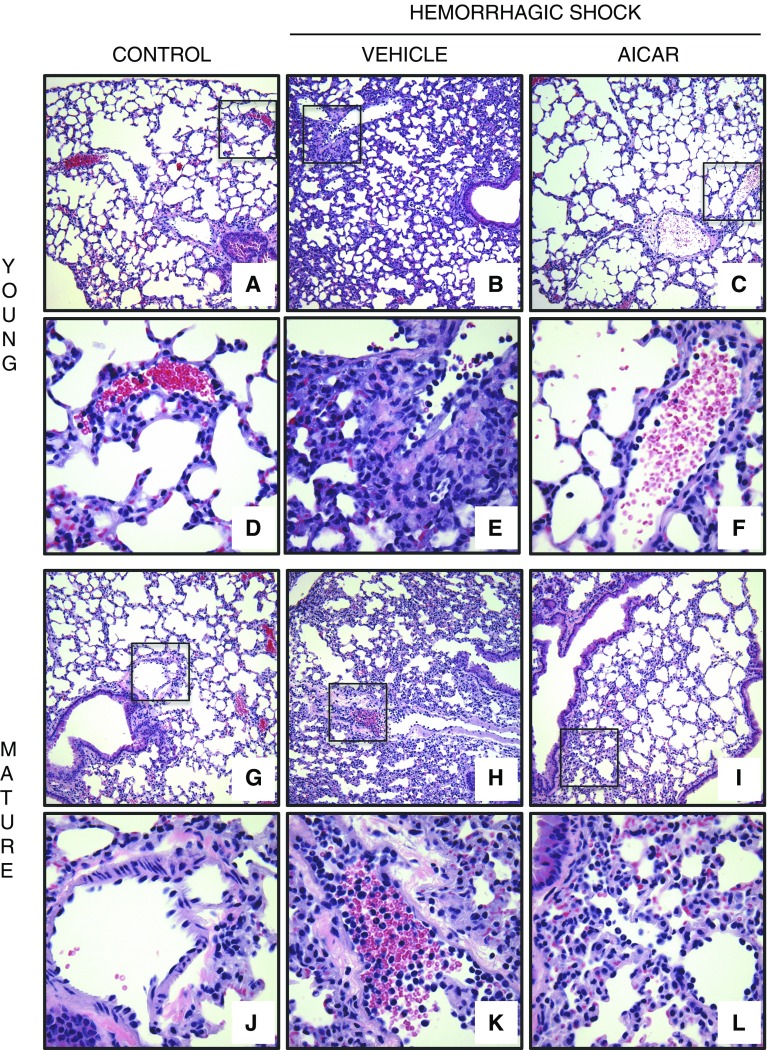
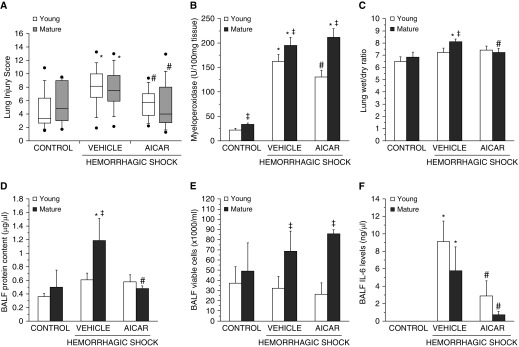
At 3 hours after resuscitation, histological analysis revealed that vehicle-treated C57/BL6 wild-type young and mature adult mice had a similar degree of total lung injury because >75% of the lung sections were involved (Figures 3 and 4A). However, the pathological characteristics of cellular infiltration were different between the young and mature adult mice; the older group exhibited marked neutrophil margination along vessel walls (Figure 3). AICAR treatment, significantly decreased the lung injury score in both young and mature adult mice. Interestingly, although AICAR treatment appeared to improve the alveolar space (Figure 3) and to reduce the total damage score in the mature adult mice (Figure 4A), there was still a persistent neutrophil infiltration at histological analysis of vessels and parenchyma (Figure 3). To confirm the degree of neutrophil infiltration, we measured the activity of MPO, a lysosomal enzyme specific to neutrophils. C57/BL6 mature adult mice had higher MPO activity than that of young mice, both at basal control conditions and after hemorrhagic shock. AICAR treatment significantly decreased MPO activity compared with vehicle treatment in young mice, but not in mature adult mice, thus confirming the histological findings (Figure 4B). In additional experiments, young, vehicle-treated AMPKα1 knockout mice exhibited a remarkable increase in lung MPO compared with their sham mice (517.2 ± 16.5 versus 152.3 ± 6.7 U/100 mg tissue, P < 0.01). AICAR treatment did not affect the MPO increase in AMPKα1 knockout mice (553.16 ± 50.1 U/100 mg tissue).
Figure 3.
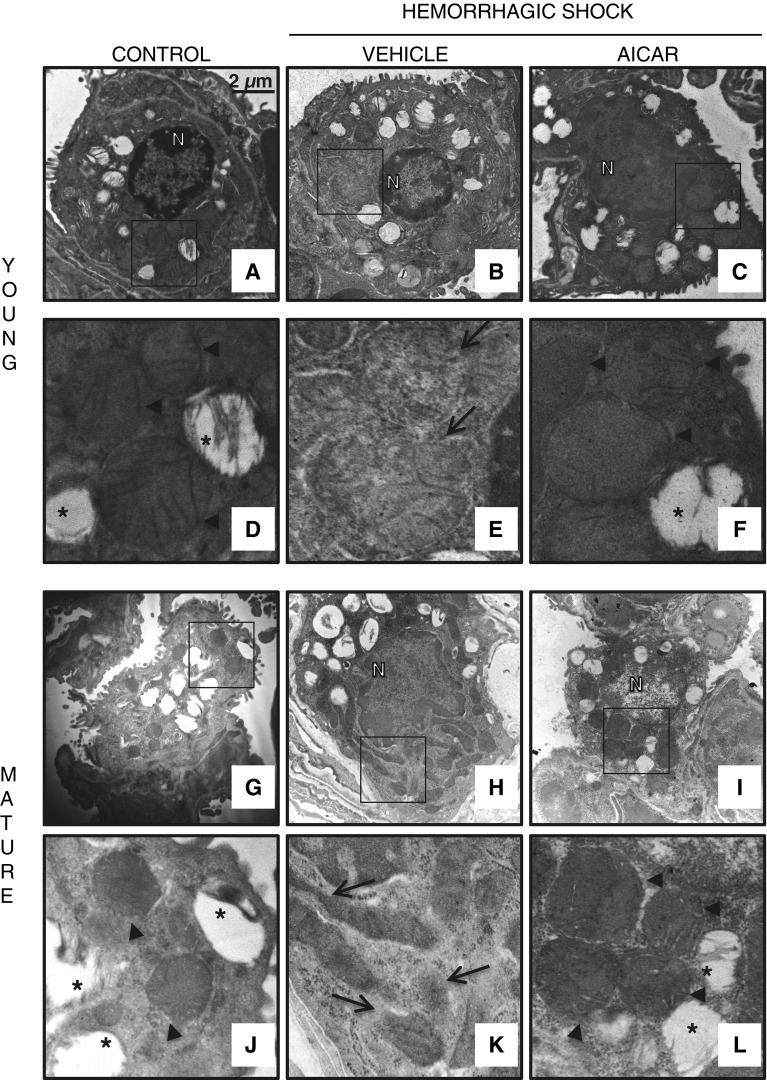
Representative histology photomicrographs of lung sections. Normal lung architecture in control C57BL/6 wild-type (A) young and (G) mature mice presenting patent alveoli, and vessels with few or no adhering neutrophils (insets shown in D and J). Lung damage in vehicle-treated (B) young and (H) mature mice after hemorrhagic shock, with reduction of alveolar space and neutrophil adhesion along vascular wall and infiltration of inflammatory cells into parenchyma (insets shown in E and K). Improvement in lung architecture in AICAR-treated (C) young and (I) mature mice after hemorrhagic shock, with reduction of neutrophil infiltration in young mice (inset shown in F) but persistence of high neutrophil adhesion along vascular wall and parenchymal infiltration of inflammatory cell in mature mice (inset shown in L). Magnification × 100 for A, B, C, G, H, and I; magnification × 400 for D, E, F, J, K, and L. A similar pattern was seen in n = 4–6 different tissue sections in each experimental group.
Figure 4.
Histopathologic scores of lung sections of C57BL/6 wild-type young and mature mice (n = 4–10 mice for each group). Lung injury was scored from 0 (no damage) to 16 (maximal damage). (A) Box plots represent 25th percentile, median, and 75th percentile; error bars define 10th and 90th percentiles; whiskers define minimal and maximal values. (B) Lung myeloperoxidase activity, (C) lung wet to dry weight ratio, and BAL fluid (BALF) analysis of (D) total protein content, (E) total count of viable inflammatory cells, and (F) IL-6 levels in C57BL/6 wild-type young and mature mice. Data represent the mean ± SEM of 4–10 animals per group. *P < 0.05 versus age-matched control mice; #P < 0.05 versus vehicle-treated group of the same age; ‡P < 0.05 versus young group.
Effect of AICAR on Lung Edema and IL-6 Levels in BALF
C57/BL6 vehicle-treated mature adult mice, but not young mice, developed pulmonary edema after hemorrhagic shock, as determined by elevated lung wet to dry weight ratio and protein content in BALF when compared with sham age-matched mice. Vehicle-treated mature adult mice also had a higher count of viable BALF inflammatory cells when compared with young mice. AICAR treatment significantly attenuated pulmonary edema in mature adult mice but did not affect total cell number in the BALF (Figures 4C–4E). Similar high levels of IL-6 were detected in the BALF of vehicle-treated mice of both age groups after hemorrhagic shock, whereas they were significantly reduced in the AICAR-treated mice of both age groups when compared with vehicle-treated mice (Figure 4F).
Age-Dependent Changes in Lung Mitochondrial Ultrastructure
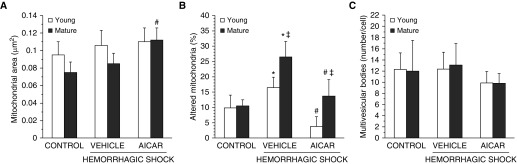
Because of the metabolic function of alveolar epithelial type II cells, we evaluated the mitochondrial changes in these cells by TEM (Figures 5 and 6). After hemorrhagic shock, mild mitochondria damage was found in C57/BL6 vehicle-treated young mice and was characterized by the presence of organelles with distorted cristae, translucent matrix and/or disrupted membrane, and a modest number of elongated mitochondria. In vehicle-treated mature adult mice, hemorrhagic shock induced a higher number of thin and elongated mitochondria with poorly arranged cristae compared with the younger group. AICAR treatment ameliorated mitochondrial structure in both age groups; however, AICAR-treated mature adult mice still exhibited a higher degree of mitochondrial damage when compared with the AICAR-treated young group. AICAR treatment also increased the average volume density of the organelles in mature adult animals when compared with vehicle treatment. No differences were observed in the number of lamellar or other multivesicular bodies in all treatment groups (Figures 5 and 6).
Figure 5.
Transmission electronic microscopy of alveolar epithelial type II cells. Normal cellular structure of alveolar type II cells in (A) control young and (G) mature mice with normal electron dense mitochondria presenting organized cristae (arrowheads) and multivesicular bodies (*) (insets shown in D and J). Structural changes in vehicle-treated (B) young and (H) mature mice after hemorrhagic shock with mitochondria presenting translucent matrix, disrupted membrane and cristae and elongated shape (arrows; insets shown in E and K). Amelioration of cell structure in AICAR-treated (C) young and (I) mature mice after hemorrhagic shock with normal electron dense mitochondria (arrowheads; insets shown in F and L). Scale bar, 2 μm. N, nucleus.
Figure 6.
Quantification of (A) average mitochondrial area, (B) altered mitochondria, and (C) multivesicular bodies in alveolar type II cells as determined by using the National Institutes of Health ImageJ software. Altered mitochondria were determined as percentage of total number of mitochondria; multivesicular bodies were determined as total number in a single cell. Data are expressed as mean ± SEM. *P < 0.05 versus age-matched control mice; #P < 0.05 versus vehicle-treated group of the same age; ‡P < 0.05 versus young group.
Age-Dependent Cellular Localization and Activation of AMPKα1
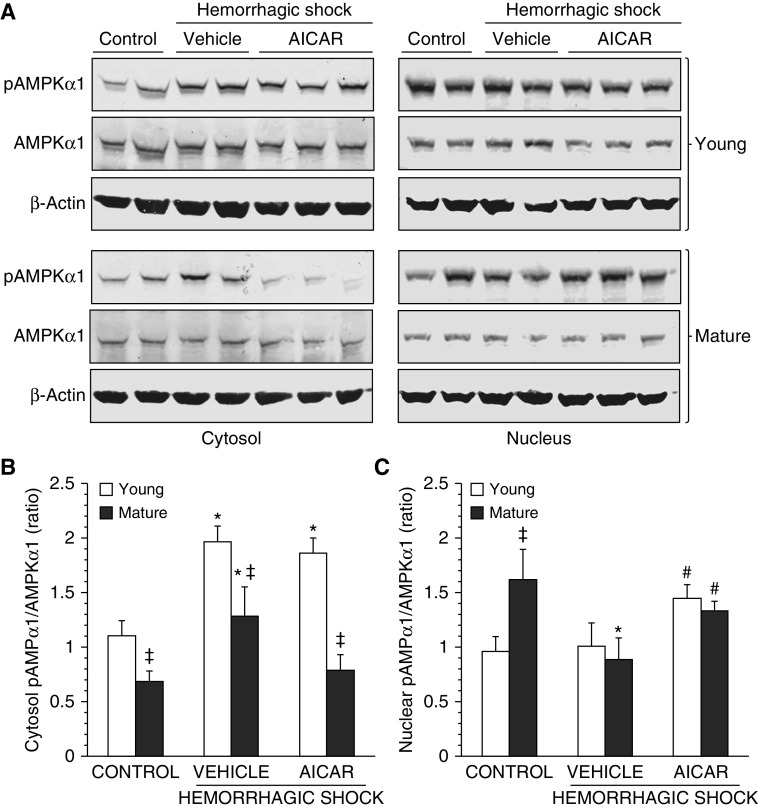
Because AMPKα1 is the predominant catalytic subunit in the lung (12), we measured the expression of this subunit and its active phosphorylated form, pAMPKα1. At basal conditions the pAMPKα1/AMPKα1 ratio of C57/BL6 mature adult control mice was significantly lower in the cytosol but higher in the nucleus when compared with control young mice, thus suggesting a distinct age-dependent cellular localization of the active pAMPKα1. After hemorrhagic shock, pAMPKα1 increased significantly in the cytosol in vehicle-treated mice of both ages when compared with basal levels of age-matched control mice, whereas it decreased markedly in the nucleus of mature adult, but not young, mice. The degree of cytosolic AMPKα1 activation was significantly higher in the vehicle-treated young group when compared with mature adult mice. Interestingly, AICAR treatment in mature adult animals markedly down-regulated levels of pAMPKα1 in the cytosol, allowing instead its nuclear translocation. AICAR treatment also increased the nuclear pAMPKα1 in young mice when compared with vehicle treatment (Figure 7).
Figure 7.
(A) Western blot analysis of active phosphorylated AMPK (pAMPK) α1, AMPKα1, and β-actin (used as loading control protein) in lung cytosol and nuclear extracts. Image analyses of pAMPKα1/AMPKα1 ratio as determined by densitometry (B) in the cytosol and (C) in the nucleus. Data are expressed as mean ± SEM of four to six animals for each group and are expressed as ratio of relative intensity units. *P < 0.05 versus age-matched control mice; #P < 0.05 versus vehicle-treated group of the same age; ‡P < 0.05 versus young group.
Age-Dependent Nuclear Translocation of PGC-1α
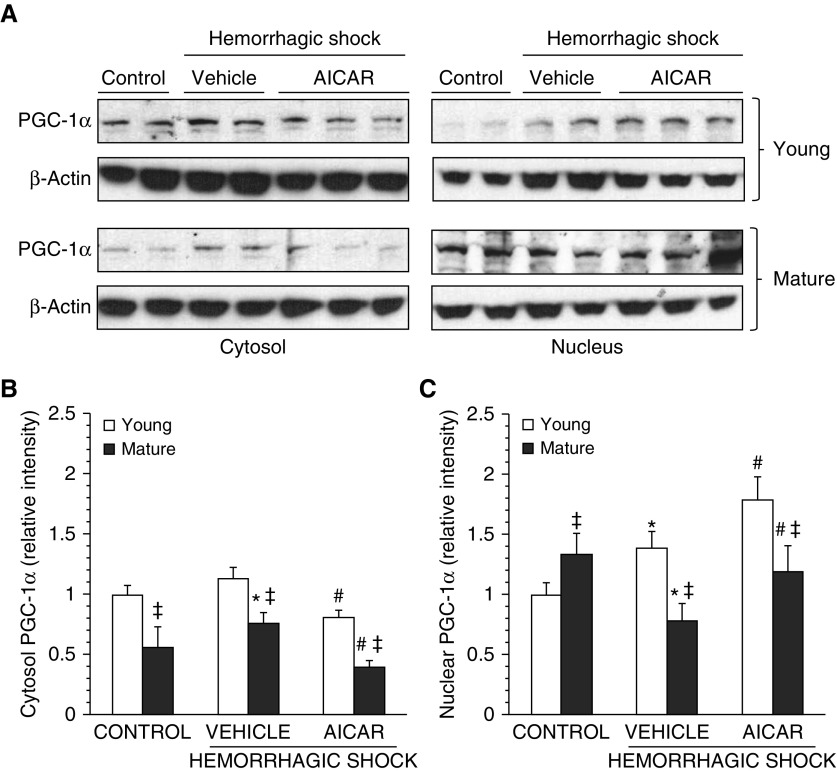
We then evaluated the lung expression of PGC-1α, the main orchestrator of mitochondrial biogenesis (18). C57/BL6 vehicle-treated young mice exhibited a significant increase in the nuclear content of PGC-1α at 3 hours after resuscitation when compared with basal levels of age-matched control mice. On the contrary, in vehicle-treated mature adult animals, PGC-1α was significantly increased in the cytosol but down-regulated in the nucleus when compared with age-matched control mice, thus suggesting an impairment of its nuclear translocation. AICAR treatment increased PGC-1α nuclear translocation while decreasing cytosolic levels in both C57/BL6 young and mature adult mice. However, levels of PGC1α in AICAR-treated mature adult mice were significantly lower than those in AICAR-treated young mice (Figure 8).
Figure 8.
(A) Western blot analysis of proliferator-activated receptor-γ coactivator 1α (PGC1-α) and β-actin (used as loading control protein) in lung cytosol and nuclear extracts. Image analyses of PGC-1α expression as determined by densitometry (B) in the cytosol and (C) in the nucleus. Data are expressed as mean ± SEM of four to six animals for each group and are expressed as ratio of relative intensity units. *P < 0.05 versus age-matched control mice; #P < 0.05 versus vehicle-treated group of the same age; ‡P < 0.05 versus young group.
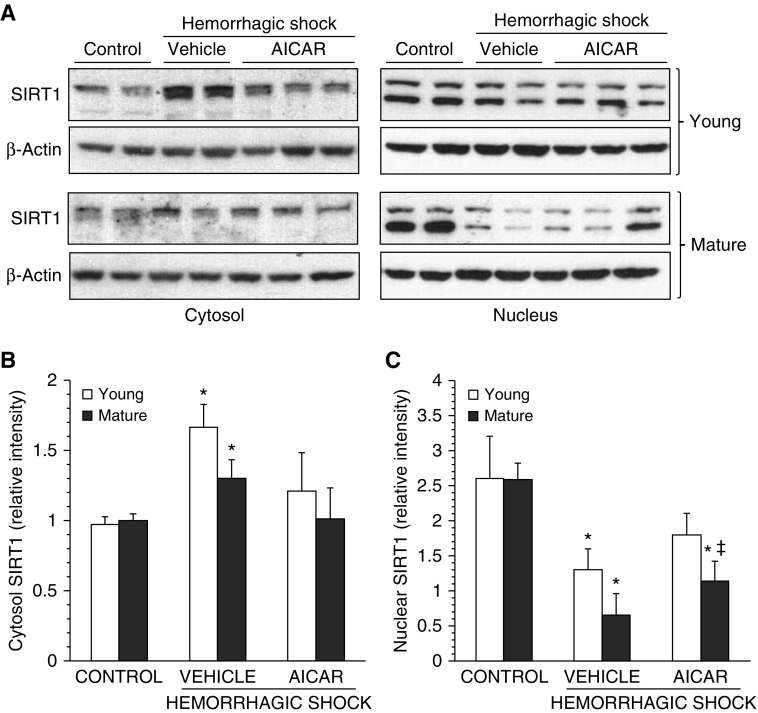
Age-Dependent Effect of AICAR on SIRT1 Nuclear Translocation
Because SIRT1 may participate in activating PGC-1α (19), we evaluated the cellular localization of this deacetylase (Figure 9). Cytosolic SIRT1 levels were elevated similarly in C57/BL6 vehicle-treated mice of both ages at 3 hours after resuscitation. In the nucleus, two SIRT1 bands were detected, a finding that is consistent with post-translational events such as glycosylation, phosphorylation, or ubiquitination (20). These bands were markedly down-regulated in both age groups at 3 hours after resuscitation when compared with basal levels of age-matched control mice, thus suggesting an impairment of nuclear translocation. AICAR treatment reestablished cytosolic and nuclear SIRT1 in young mice to levels similar to those of age-matched control mice. AICAR treatment also reestablished cytosolic SIRT1 in mature adult mice to basal levels similar to those of age-matched control mice. However, nuclear SIRT1 levels of AICAR-treated mature adult mice were significantly down-regulated when compared with levels of age-matched control mice or to levels of AICAR-treated young mice.
Figure 9.
(A) Western blot analysis of sirtuin 1 (SIRT1) and β-actin (used as loading control protein) in lung cytosol and nuclear extracts. Image analyses of SIRT1 expression as determined by densitometry (B) in the cytosol and (C) in the nucleus. Data are expressed as mean ± SEM of four to six animals for each group and are expressed as ratio of relative intensity units. *P < 0.05 versus age-matched control mice; ‡P < 0.05 versus young group.
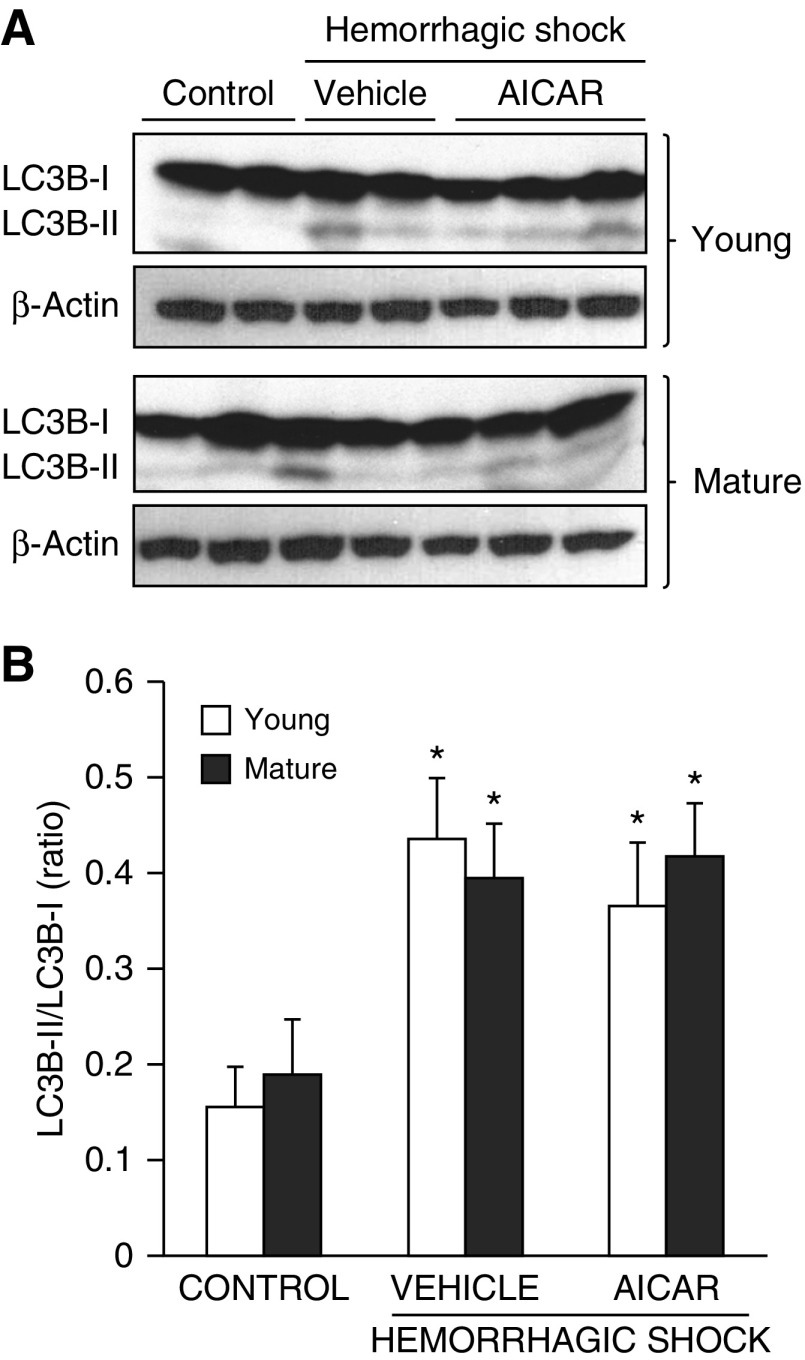
Age-Independent Increase of Autophagy in the Lung
Because AMPK is an upstream regulator of autophagy, we quantified the process of autophagy by evaluating the expression of the LC3B. The protein is converted from a cytosolic LC3B-I form to a conjugated LC3B-II form in the autophagosomal membrane and correlates with autophagic vesicle formation (7, 21). In both vehicle-treated young and mature adult mice, the LC3B-II/LC3B-I ratio increased after hemorrhagic shock when compared with age-matched control mice, thus suggesting the capability of autophagy. AICAR treatment did not affect the levels of autophagy in either age group (Figure 10).
Figure 10.
(A) Western blot analysis of light-chain 3B (LC3B)-I and LC3B-II and β-actin (used as loading control protein) in lung cytosol extracts. (B) Image analyses of LC3B-II/LC3B-I ratio as determined by densitometry. Data are expressed as mean ± SEM of four to six animals for each group and are expressed as ratio of relative intensity units. *P < 0.05 versus age-matched control mice.
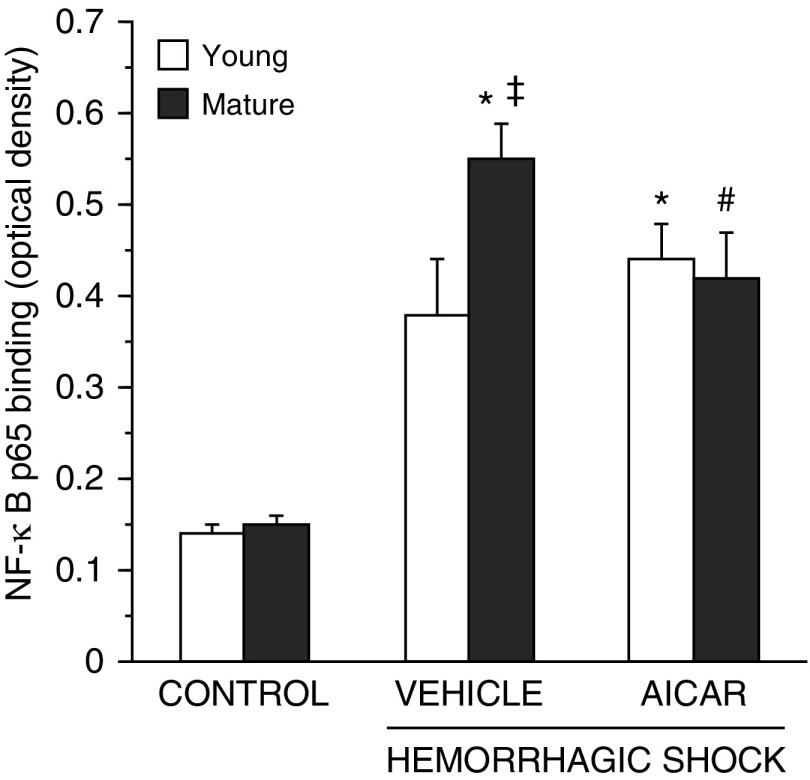
Age-Dependent Effect of AICAR on Lung NF-κB Activation
Previous studies have suggested that AMPK can inhibit NF-κB activation (22). Therefore, we evaluated the activity of this transcription factor. NF-κB activation significantly increased in C57/BL6 vehicle-treated mice of both ages after hemorrhagic shock when compared with age-matched control mice. However, the degree of activation was significantly higher in vehicle-treated mature adult mice than young mice. AICAR treatment decreased NF-κB activation in the mature adult mice but not in the young group (Figure 11).
Figure 11.
Activity of the p65 subunit of nuclear factor (NF)-κB in lung nuclear extracts at 3 hours after resuscitation. Data are expressed as mean ± SEM of 5–13 animals for each group and are expressed as optical density units. *P < 0.05 versus age-matched control mice; #P < 0.05 versus vehicle-treated group of the same age; ‡P < 0.05 versus young group.
Discussion
Age is a significant risk factor for the development of multiple organ failure and mortality in adult patients suffering trauma (1–4). Even with the application of standard procedures of resuscitation and mechanical ventilation, lung injury remains a treatment challenge in trauma centers (23). Our study provides evidence of age-related features of acute lung injury after hemorrhagic shock and identifies AMPKα1 as a crucial age-dependent regulator of lung mitochondrial homeostasis and a potential therapeutic target to improve pulmonary function. We demonstrated that during hemorrhagic shock, despite the presence of a similar systemic inflammatory response, the lung pathological characteristics were different between C57/BL6 wild-type young and mature adult mice treated with vehicle; the mature adult mice exhibited an exuberant neutrophil infiltration and pulmonary edema associated with a higher mitochondrial structural derangement in alveolar epithelial type II cells. We also demonstrated that treatment with the AMPK activator AICAR provided beneficial effects and improved the lung architecture in both young and mature adult mice. However, AICAR was less effective in reversing mitochondrial damage and did not affect systemic hypotension or lung neutrophil infiltration in mature adult mice when compared with vehicle treatment.
Although AMPK is expressed ubiquitously, different catalytic isoforms (α1 and α2) display tissue-specific distribution, and their phosphorylation by upstream kinases is essential for AMPK activation. In the lung, the catalytic α1 isoform is expressed abundantly (12, 24). We demonstrate, we believe for the first time, that phosphorylation of AMPKα1 had a distinct age-dependent cellular localization during hemorrhagic shock. In young C57/BL6 wild-type mice, activation of AMPKα1 increased in the cytosol, whereas the nuclear pAMPKα1 remained stable. This event paralleled the nuclear translocation of PGC-1α, raising the possibility that AMPK regulates gene expression in response to cellular stresses. However, the most intriguing finding in our study was that with mature age, the cytosolic activation of AMPKα1 paralleled a decrease in the nuclear pAMPKα1, suggesting a nucleocytoplasmatic shuttling in response to the cellular stress of hemorrhagic shock. This shuttling was associated with down-regulation of nuclear PGC-1α expression. This might well explain why vehicle-treated mature adult animals also exhibited a higher number of damaged mitochondria in type II alveolar epithelial cells when compared with young mice. Our study, therefore, suggests that during ischemic stresses, AMPK activation may increase in the lung as a mechanism to maintain metabolic demands. Subcellular localization of AMPK could also have important functional consequences by affecting nuclear substrates such as the PGC-1α, the master regulator of mitochondrial biogenesis. However, these compensatory mechanisms may be lacking in aging animals.
In agreement with these age-dependent changes in mitochondrial structure and the dynamics of the AMPK-mediated signaling pathways, the molecular mechanisms of AICAR lung protective effects were also age dependent. In C57/BL6 wild-type young mice, AICAR provided a significant improvement in lung injury, a reduction in circulating proinflammatory cytokines, and hemodynamic stability. To prove the role of AMPK in the protective effects of AICAR, we also demonstrated that genetic deficiency of the α1 isoform of AMPK in young knockout mice completely abolished the beneficial effects of AICAR on blood pressure, lung neutrophil infiltration, and plasma cytokine elevation. Furthermore, AMPKα1 knockout mice exhibited an exaggerated inflammatory response of higher magnitude when compared with age-matched C57/BL6 wild-type mice. Our studies are in agreement with previous reports demonstrating that AICAR treatment improves survival, cardiovascular parameters, or intestinal barrier function during hemorrhagic shock in young rats or pigs (25–27). Activation of AMPK by metformin has also been shown to improve lung injury induced by severe hemorrhage and intraabdominal infection in young mice (28). However, these beneficial effects of AMPK activation have never been tested in aging models of hemorrhagic shock. In our study, administration of AICAR to a more mature group of mice significantly reduced the plasma levels of proinflammatory cytokines but had no effect on hypotension. These data are consistent with the findings of our previous study demonstrating that an age-dependent dysregulation of AMPK signaling also occurs in the heart of mice subjected to hemorrhagic shock (14).
Neutrophil accumulation along the vascular endothelium and infiltration into the interstitium and alveolar space is a critical pathophysiological event of hemorrhage-induced lung injury in both humans and animals (29–32). In our study, AICAR treatment of C57/BL6 mature adult mice resulted in improvement in lung alveolar architecture after hemorrhage, while surprisingly having no effect on parenchymal neutrophil infiltration. In the young C57/BL6 group, the lung protective effects of AICAR were instead characterized by a significant decrease in neutrophil infiltration. These results suggest that other cellular mechanisms may contribute to the disruption of lung architecture and may not correlate directly with neutrophil sequestration. Interestingly, in a recent study, neutrophil infiltration was not required for acute lung injury after endotoxin administration and mechanical ventilation in mice. On the contrary, inhibition of IL-1β signaling improved oxygen saturation despite the persistence of lung neutrophil engulfment (33). In our study, AICAR treatment reduced the elevation of several inflammatory cytokines in the plasma and in the BALF in both age groups, thus providing a further mechanism of lung protection. Our data also suggest that AMPK activation affects the innate immune response during stress, because AICAR treatment significantly decreased the Th2 (IL-6 and IL-10) and the Th17 (IL-17) responses, but not the Th1 response, in both age groups after hemorrhagic shock.
The alveolar type II cells secrete pulmonary surfactant, regulate fluid balance, and serve as progenitor cells for alveolar type I cells. Because of these energy-dependent functions, maintenance of mitochondrial homeostasis provides a crucial stress-resolving mechanism (34, 35). In our study, C57/BL6 vehicle-treated mature adult mice exhibited a high degree of mitochondrial damage and elongation in alveolar type II cells. Of note, elongation has been suggested to account for the occurrence of hyperfused dysfunctional mitochondria in aging muscles (36) and has been associated with increased cellular senescence (37). In our study, AICAR preserved the mitochondrial structure in the airway epithelium in both age groups, but these beneficial effects were more pronounced in the young animals. Our data support other studies demonstrating a prosurvival and antiinflammatory role of AMPK in airway epithelial cells (38, 39). However, our data also suggest that the AMPK cytoprotective effects are age dependent.
AMPK can induce autophagy via the inhibition of mammalian target of rapamycin signaling (6, 7). In addition to maintaining mitochondrial quality control by removing damaged organelles, autophagy participates in the biogenesis of multilamellar bodies for lipid and surfactant storage in the airway epithelium (40). In our study, at examination of the molecular events of LC3B-II conjugation, vehicle-treated C57/BL6 wild-type mice of both ages appeared to be capable of mounting an autophagic event in response to the cellular stress of hemorrhagic shock. However, at TEM examination, the quantity of multivesicular bodies did not change in either animal group after hemorrhagic shock. Furthermore, AICAR treatment did not affect LC3B-II conversion or multivesicular bodies count. These conflicting observations could simply reflect the difficulty in using TEM to distinguish autolysosomes from endocytic compartments (41). Thus, we cannot exclude the possibility of autophagic events controlling turnover of damaged mitochondria during hemorrhagic shock. These important aspects of metabolic recovery require further investigation.
Several studies have reported that AMPK activation can afford cytoprotective effects through indirect inhibition of NF-κB, which is a key regulator of the inflammatory response (22). We have demonstrated previously that age-dependent differences in hemorrhage-induced lung injury in mature rats correlate with age-dependent kinetics of NF-κB DNA binding when compared with young rats (42). Similarly, in the current study, NF-κB activity was higher in the C57/BL6 mature adult mice than in the younger group after hemorrhagic shock. There is also extensive evidence that SIRT1 shares several targets with AMPK and may increase the nuclear PGC-1α activity by deacetylation (19, 20). SIRT1 resides mainly in the nucleus (43) but can shuttle from the nucleus to the cytosol (44). In our study, nuclear translocation of different active forms of SIRT1 was impaired after hemorrhagic shock in C57/BL6 wild-type vehicle-treated mice of both ages. Interestingly, AICAR treatment attenuated NF-κB activity in mature adult mice but not in young animals. On the contrary, AICAR fully restored the subcellular localization of SIRT1 to basal conditions in young mice only. These different effects of AICAR on NF-κB and SIRT1 further imply that AMPK may affect transcriptional factors in an age-dependent manner. Our data also suggest that lung injury in the young group is probably repaired by AMPK activation independently of NF-κB–mediated inflammatory influences and that the metabolic recovery is a key component of the resolution of tissue damage. These findings also confirm recent studies demonstrating that under certain cellular conditions, AICAR may exert antiinflammatory properties without interfering with NF-κB nuclear translocation (45).
Conclusions
In conclusion, our study demonstrates that pharmacologic activation of AMPK by AICAR reverses hemorrhage-induced lung injury and activates metabolic repair mechanisms in the alveolar epithelium. However, the beneficial effects of AICAR appear age dependent because they exhibit different molecular mechanisms in mature adult mice when compared with a younger group of animals. Whether AMPK activation may serve as a novel therapeutic approach to lung injury in a human application warrants further investigation.
Footnotes
This work was supported by National Institutes of Health grants R01 AG-027990, R01 GM-067202, and R01 GM-115973 (B.Z.) and training grant T32 GM08478 to (P.K.)
Author Contributions: Conception and design: L.R.K. and B.Z.; acquisition of data: L.R.K., P.K., G.P., M.O’C., P.W.H., and V.W.; analysis and interpretation of data: L.R.K., P.K., G.P. and B.Z.; and writing and preparation of manuscript and figures: L.R.K. and B.Z.
This article has an online data supplement, which is accessible from this issue's table of content online at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0118OC on January 13, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann R, Beekley A, Casey L, Salim A, Martin M. The impact of advanced age on trauma triage decisions and outcomes: a statewide analysis. Am J Surg. 2009;197:571–574, discussion 574–575. doi: 10.1016/j.amjsurg.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 3.Dewar DC, Tarrant SM, King KL, Balogh ZJ. Changes in the epidemiology and prediction of multiple-organ failure after injury. J Trauma Acute Care Surg. 2013;74:774–779. doi: 10.1097/TA.0b013e31827a6e69. [DOI] [PubMed] [Google Scholar]
- 4.Vanzant EL, Hilton RE, Lopez CM, Zhang J, Ungaro RF, Gentile LF, Szpila BE, Maier RV, Cuschieri J, Bihorac A, et al. Inflammation and Host Response to Injury Investigators. Advanced age is associated with worsened outcomes and a unique genomic response in severely injured patients with hemorrhagic shock. Crit Care. 2015;19:77. doi: 10.1186/s13054-015-0788-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairns CB, Moore FA, Haenel JB, Gallea BL, Ortner JP, Rose SJ, Moore EE. Evidence for early supply independent mitochondrial dysfunction in patients developing multiple organ failure after trauma. J Trauma. 1997;42:532–536. doi: 10.1097/00005373-199703000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Carling D, Viollet B. Beyond energy homeostasis: the expanding role of AMP-activated protein kinase in regulating metabolism. Cell Metab. 2015;21:799–804. doi: 10.1016/j.cmet.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:1845–1846. doi: 10.1056/NEJMc1303158. [DOI] [PubMed] [Google Scholar]
- 8.Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, et al. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turdi S, Fan X, Li J, Zhao J, Huff AF, Du M, Ren J. AMP-activated protein kinase deficiency exacerbates aging-induced myocardial contractile dysfunction. Aging Cell. 2010;9:592–606. doi: 10.1111/j.1474-9726.2010.00586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 11.Jørgensen SB, Viollet B, Andreelli F, Frøsig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the α2 but not α1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- 12.Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, et al. Mammalian AMP-activated protein kinase subfamily. J Biol Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- 13.Klingbeil LR, Piraino G, Hake P, Ledford JR, Zingarelli B.The effects of AICAR on metabolic pathways in the lung following murine hemorrhagic shock Shock 201543Suppl 127. [Google Scholar]
- 14.Matsiukevich D, Piraino G, Klingbeil LR, Hake PW, Wolfe V, O’Connor M, Zingarelli B. The AMPK activator AICAR ameliorates age-dependent myocardial injury in murine hemorrhagic shock. Shock. 2016;47:70–78. doi: 10.1097/SHK.0000000000000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolthuis EK, Vlaar AP, Choi G, Roelofs JJ, Juffermans NP, Schultz MJ. Mechanical ventilation using non-injurious ventilation settings causes lung injury in the absence of pre-existing lung injury in healthy mice. Crit Care. 2009;13:R1. doi: 10.1186/cc7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullane KM, Kraemer R, Smith B. Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J Pharmacol Methods. 1985;14:157–167. doi: 10.1016/0160-5402(85)90029-4. [DOI] [PubMed] [Google Scholar]
- 17.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki T, Maier B, Bartke A, Scrable H. Progressive loss of SIRT1 with cell cycle withdrawal. Aging Cell. 2006;5:413–422. doi: 10.1111/j.1474-9726.2006.00235.x. [DOI] [PubMed] [Google Scholar]
- 21.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. J Mol Med (Berl) 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sauaia A, Moore EE, Johnson JL, Chin TL, Banerjee A, Sperry JL, Maier RV, Burlew CC. Temporal trends of postinjury multiple-organ failure: still resource intensive, morbid, and lethal. J Trauma Acute Care Surg. 2014;76:582–592, discussion 592–593. doi: 10.1097/TA.0000000000000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardie DG. The AMP-activated protein kinase pathway--new players upstream and downstream. J Cell Sci. 2004;117:5479–5487. doi: 10.1242/jcs.01540. [DOI] [PubMed] [Google Scholar]
- 25.Smith Sonneborn J, Rutten M. Acute therapeutic use of 5-aminoimidazole-4-carboxamide ribonucleoside extends survival interval in response to severe hemorrhagic shock. Shock. 2011;36:191–195. doi: 10.1097/SHK.0b013e3182205d7d. [DOI] [PubMed] [Google Scholar]
- 26.Spiers JP, Fabian TC, Kudsk KA, Proctor KG. Resuscitation of hemorrhagic shock with hypertonic saline/dextran or lactated Ringer’s supplemented with AICA riboside. Circ Shock. 1993;40:29–36. [PubMed] [Google Scholar]
- 27.Ragsdale DN, Proctor KG. Acadesine and intestinal barrier function after hemorrhagic shock and resuscitation. Crit Care Med. 2000;28:3876–3884. doi: 10.1097/00003246-200012000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Bone N, Jiang S, Park DW, Tadie JM, Deshane J, Rodriguez CA, Pittet JF, Abraham E, Zmijewski JW.AMP-activated protein kinase and glycogen synthase kinase 3β modulate the severity of sepsis-induced lung injury Mol Med 201521937–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chollet-Martin S, Jourdain B, Gibert C, Elbim C, Chastre J, Gougerot-Pocidalo MA. Interactions between neutrophils and cytokines in blood and alveolar spaces during ARDS. Am J Respir Crit Care Med. 1996;154:594–601. doi: 10.1164/ajrccm.154.3.8810592. [DOI] [PubMed] [Google Scholar]
- 30.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31:S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 31.Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage- or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1137–L1145. doi: 10.1152/ajplung.2000.279.6.L1137. [DOI] [PubMed] [Google Scholar]
- 32.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones HD, Crother TR, Gonzalez-Villalobos RA, Jupelli M, Chen S, Dagvadorj J, Arditi M, Shimada K. The NLRP3 inflammasome is required for the development of hypoxemia in LPS/mechanical ventilation acute lung injury. Am J Respir Cell Mol Biol. 2014;50:270–280. doi: 10.1165/rcmb.2013-0087OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mason RJ. Biology of alveolar type II cells. Respirology. 2006;11:S12–S15. doi: 10.1111/j.1440-1843.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 35.Lottes RG, Newton DA, Spyropoulos DD, Baatz JE. Alveolar type II cells maintain bioenergetic homeostasis in hypoxia through metabolic and molecular adaptation. Am J Physiol Lung Cell Mol Physiol. 2014;306:L947–L955. doi: 10.1152/ajplung.00298.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marzetti E, Calvani R, Cesari M, Buford TW, Lorenzi M, Behnke BJ, Leeuwenburgh C. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol. 2013;45:2288–2301. doi: 10.1016/j.biocel.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S, Jeong S-Y, Lim W-C, Kim S, Park Y-Y, Sun X, Youle RJ, Cho H. Mitochondrial fission and fusion mediators, hFis1 and OPA1, modulate cellular senescence. J Biol Chem. 2007;282:22977–22983. doi: 10.1074/jbc.M700679200. [DOI] [PubMed] [Google Scholar]
- 38.Myerburg MM, King JD, Jr, Oyster NM, Fitch AC, Magill A, Baty CJ, Watkins SC, Kolls JK, Pilewski JM, Hallows KR. AMPK agonists ameliorate sodium and fluid transport and inflammation in cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol. 2010;42:676–684. doi: 10.1165/rcmb.2009-0147OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goncharova EA, Goncharov DA, James ML, Atochina-Vasserman EN, Stepanova V, Hong SB, Li H, Gonzales L, Baba M, Linehan WM, et al. Folliculin controls lung alveolar enlargement and epithelial cell survival through E-cadherin, LKB1, and AMPK. Cell Reports. 2014;7:412–423. doi: 10.1016/j.celrep.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hariri M, Millane G, Guimond MP, Guay G, Dennis JW, Nabi IR. Biogenesis of multilamellar bodies via autophagy. Mol Biol Cell. 2000;11:255–268. doi: 10.1091/mbc.11.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. J Pathol. 2010;221:117–124. doi: 10.1002/path.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zingarelli B, Hake PW, O’Connor M, Burroughs TJ, Wong HR, Solomkin JS, Lentsch AB. Lung injury after hemorrhage is age dependent: role of peroxisome proliferator-activated receptor γ. Crit Care Med. 2009;37:1978–1987. doi: 10.1097/CCM.0b013e31819feb4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanno M, Sakamoto J, Miura T, Shimamoto K, Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–6832. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- 45.Peairs A, Radjavi A, Davis S, Li L, Ahmed A, Giri S, Reilly CM. Activation of AMPK inhibits inflammation in MRL/lpr mouse mesangial cells. Clin Exp Immunol. 2009;156:542–551. doi: 10.1111/j.1365-2249.2009.03924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]