Abstract
Well-differentiated primary human bronchial epithelial (HBE) cell cultures are vital for cystic fibrosis (CF) research, particularly for the development of cystic fibrosis transmembrane conductance regulator (CFTR) modulator drugs. Culturing of epithelial cells with irradiated 3T3 fibroblast feeder cells plus the RhoA kinase inhibitor Y-27632 (Y), termed conditionally reprogrammed cell (CRC) technology, enhances cell growth and lifespan while preserving cell-of-origin functionality. We initially determined the electrophysiological and morphological characteristics of conventional versus CRC-expanded non-CF HBE cells. On the basis of these findings, we then created six CF cell CRC populations, three from sequentially obtained CF lungs and three from F508 del homozygous donors previously obtained and cryopreserved using conventional culture methods. Growth curves were plotted, and cells were subcultured, without irradiated feeders plus Y, into air–liquid interface conditions in nonproprietary and proprietary Ultroser G–containing media and were allowed to differentiate. Ussing chamber studies were performed after treatment of F508 del homozygous CF cells with the CFTR modulator VX-809. Bronchial epithelial cells grew exponentially in feeders plus Y, dramatically surpassing the numbers of conventionally grown cells. Passage 5 and 10 CRC HBE cells formed confluent mucociliary air–liquid interface cultures. There were differences in cell morphology and current magnitude as a function of extended passage, but the effect of VX-809 in increasing CFTR function was significant in CRC-expanded F508 del HBE cells. Thus, CRC technology expands the supply of functional primary CF HBE cells for testing CFTR modulators in Ussing chambers.
Keywords: cystic fibrosis, electrophysiology, human bronchial epithelial cells, in vitro models
Clinical Relevance
Primary cystic fibrosis human airway epithelial cells are vital for development of cystic fibrosis transmembrane conductance regulator modulator drugs, but their supply is limited. These studies demonstrate that coculture with irradiated feeder cells and a Rho kinase inhibitor enables massive expansion of cells useful for testing cystic fibrosis transmembrane conductance regulator modulators in Ussing chambers.
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which encodes an anion channel vital for normal transepithelial electrolyte and fluid transport in multiple organs. CFTR is synthesized at the endoplasmic reticulum and is trafficked to the apical epithelial cell membrane where it regulates luminal surface properties. Loss of functional CFTR in the airways results in thick, viscous mucus, impaired mucociliary clearance, chronic infection, inflammation, and tissue damage.
The ∼2,000 known CFTR mutations have variable effects on RNA production and protein synthesis, folding, stability, cellular trafficking, and channel function (1). CFTR mutations are classified according to their disruptive mechanisms, and therapies targeting the underlying cause are directed by mutation class (2, 3). The class III G551D CFTR mutation results in a properly localized but dysfunctional channel. The drug ivacaftor (Kalydeco; Vertex Pharmaceuticals, Boston, MA) potentiates G551D CFTR function, lowering sweat Cl− levels, improving pulmonary function, decreasing exacerbation rates, and improving quality of life (4). Approximately 4% of individuals with CF have G551D CFTR, and ivacaftor has been granted Food and Drug Administration approval in the United States for G551D and nine additional mutations.
Deletion of phenylalanine at position 508 (F508 del) is the most common severe class II mutation, present in 90% of individuals with CF. Misfolded F508 del CFTR protein is directed for degradation rather than trafficking to the apical cell surface. A combination drug (Orkambi; Vertex Pharmaceuticals) consisting of the CFTR corrector lumacaftor (developed as VX-809) and ivacaftor is Food and Drug Administration approved for individuals who are homozygous, but not heterozygous, for F508 del CFTR (5). However, CFTR functional restoration by Orkambi in F508 del homozygous individuals is apparently not as impressive as ivacaftor in G551D heterozygotes. Uncertainty exists about the relationship between the magnitude of CFTR restoration and clinically meaningful changes in organ physiology and function. Thus, there is a strong impetus to find additional, efficacious F508 del CFTR modulator compounds.
CFTR modulator discovery follows a paradigm of screening in heterologous cells expressing mutant CFTR, and then milestone testing of compound efficacy in primary, polarized CF human bronchial epithelial (HBE) cells. Primary CF HBE cells are obtained from lung tissues explanted during transplant, lobectomy, or autopsy. The CF Foundation supports several laboratories for this purpose, but it is likely that many opportunities to harvest CF lungs are missed. Furthermore, multidrug-resistant microbes pose additional challenges that limit the overall supply and availability of primary CF HBE cells.
Coculture of keratinocytes with irradiated 3T3 feeder cells in the presence of the RhoA kinase inhibitor Y-27632 (Y) massively expands the cell number (6). Primary mammary and prostate epithelial cells under these conditions exhibit a baslaoid phenotype termed “conditionally reprogrammed cells” (CRCs), which remain diploid and nontumorigenic and, on removal of Y, differentiate into organ-specific phenotypes (7). Ectocervical keratinocyte CRCs expressed markers of somatic stem cells rather than embryonic or induced pluripotent stem cells, and both keratinocytes and airway epithelial CRCs, in the absence of Y, retained their original organ-specific identity in air–liquid interface (ALI) cultures (8). Enhanced proliferation and lentivirus transduction of human and mouse airway epithelial cells cultured in the presence of Y were observed (9), the CRC technique was useful to study primary ciliary dyskinesia nasal curettage cells (10), and the method has been used to expand nasal respiratory epithelial cells manipulated with CRISPR/Cas9 (11). Recent studies suggest the feasibility of cell expansion and seeding in airway bioprostheses (12).
Our goal was to determine whether F508 del homozygous CF HBE cells expanded with irradiated feeders plus Y retained electrophysiological and morphological properties comparable to cells prepared via conventional methods, and whether the cells would respond similarly to the CFTR modulator lumacaftor (VX-809). Our results indicate that the CRC technique will enhance the world supply of CF HBE cells useful for testing CFTR modulators.
Materials and Methods
3T3J2 Cell Culture
The 3T3J2 Swiss mouse fibroblast cell line was obtained from a stock originally provided by Dr. Howard Green and was verified as homogeneous by DNA fingerprinting. It was grown, irradiated, and cryopreserved as described in the online supplement.
Conventional HBE Cultures
Human lung tissue was procured under the University of North Carolina Office of Research Ethics Biomedical Institutional Review Board–approved protocol No. 03-1396. Primary HBE cells were harvested and cultured as described in detail (13). Briefly, freshly isolated passage (P) 0 HBE cells were cultured in bronchial epithelial growth media (BEGM) on collagen type I/III-coated plastic dishes with supplemental antibiotics. Passaged HBE cells were seeded on Millicell CM porous supports as described below (ALI cultures).
HBE Coculture with 3T3J2 Cells
Irradiated 3T3J2 cells and primary HBE cells were cocultured as described previously (6), with modifications as given in the online supplement and in Table E1 (online supplement). Briefly, irradiated 3T3 cells were plated on culture dishes 2–24 hours before coculture with HBE cells in F media supplemented with 5 μM Y.
Growth Curve
When conventionally cultured HBE cells reached 70–90% confluence, cells were passaged using trypsin-EDTA. HBE and 3T3J2 co-cultures were briefly treated with trypsin-EDTA to remove the majority of fibroblasts, and were re-exposed to trypsin-EDTA to remove epithelial cells. Epithelial cell counts were performed on a hemocytometer in the presence of 50% trypan blue. Population doublings were calculated as the log base 2 of the final cell number divided by the seeding cell number, and growth curves were created by plotting the cumulative population doublings versus time.
ALI Cultures
At 70–90% confluence, HBE cells at specified passages were trypsinized, counted, and transferred to human placental type IV collagen–coated, 0.4 μm pore size Millicell (Millipore PICM01250; Billerica, MA) inserts. The HBE cells were seeded at a density of 1 × 105 cells/cm2 and were cultured in a nonproprietary media termed “UNC ALI” (13) or a media containing Ultroser G (USG) serum substitute (Pall; Port Washington, NY) termed “VALI” (14) in the absence of feeder cells or Y. A comparison of the media components is given in Table E2. On confluence, usually between 5 and 7 days, cells were maintained at an ALI by removing apical media and providing media to the basal compartment only. The apical surface was washed with phosphate-buffered saline (PBS), and the medium was replaced in the basal compartment two to three times per week.
Whole Mount Immunofluorescence
ALI cultures at 28–35 days were washed with PBS and fixed in 4% paraformaldehyde. Fixed cells were permeabilized, blocked, and incubated in primary antibodies against MUC5AC/MUC5B (Thermo 45M1 for MUC5AC, MUC5B Ab kindly provided by Mehmet Kesimer, UNC Chapel Hill), α-tubulin (cilia, Millipore mAb1864), phalloidin (filamentous actin, Invitrogen, Carlsbad, CA), and Hoechst (nuclei). Appropriate fluorescent secondary antibodies (Jackson ImmunoResearch, West Grove, PA) were used, and confocal images were obtained using a Leica SP2-AOBS confocal microscope.
Histology and Morphometry
After 28–35 days, ALI cultures were washed with PBS, fixed in 10% neutral buffered formalin, processed for embedding in paraffin, and sectioned as described previously (15). Hematoxylin and eosin (H&E) and alcian blue, periodic acid Schiff staining was performed. Sections were subjected to morphometric analysis by systematically photographing five portions of each specimen. Digital images were acquired and assessed without knowledge of experimental group. One of four sections of the embedded membrane was selected for analysis using a random number generator (https://www.random.org/). Each section was divided into five subportions, excluding the culture edges, which are typically thicker. A random number again determined which subportion was to be recorded digitally in its center. If the same subportion was picked again, a location midway between the center and edge was recorded. The total number of cells, the epithelial thickness (measured using Image J [http://fiji.sc/Fiji]), the number of alcian blue, periodic acid Schiff–positive goblet cells, and the number of ciliated cells were tabulated, and the averages per section were calculated.
Bioelectric Properties
ALI cultures, 18 to 24 days old, at specified passage numbers were mounted in Ussing chambers (Physiologic Instruments, San Diego, CA). The epithelium was voltage clamped, and the short-circuit current (Isc) and resistance were measured. Data was collected and analyzed using Acquire and Analysis software (Physiologic Instruments). The bilateral solution was 5 ml of 37°C Krebs-bicarbonate-Ringer containing 140 mM Na+, 120 mM Cl-, 5.2 mM K+, 1.2 mM Ca2 + , 1.2 mM Mg2 + , 2.4 mM HPO42-, 0.4 mM H2PO4-, 25 mM HCO3-, and 5 mM glucose, circulated with 95% O2-5% CO2 gas, pH 7.4. Compounds were added, in order, as indicated: amiloride, 100 μM (mucosal) to inhibit the epithelial sodium channel; forskolin, 10 μM (mucosal and serosal) to induce cAMP activation of CFTR; VX-770, 5 μM (mucosal) or genistein 10 μM (mucosal) to further potentiate current; and CFTR inhibitor 172 (CFTRinh-172), (10 μM, mucosal) to inhibit CFTR-specific current. Changes in short-circuit current (ΔIsc) in response to agonists and inhibitors were calculated as the average basal Isc before acute addition minus Isc max or min after acute addition. Results are presented as mean ± SEM.
Data Analysis
Independent variables were modeled using linear mixed model standard least square fit with JMP software (SAS, Cary, NC). Fixed effect factors (such as culture condition, passage, and treatment) and their interactions were analyzed by multiway analysis of variance. When data from multiple donors were analyzed, the donor was modeled as a random effect factor to perform paired analysis among the fixed-effect factors. Random effect and variances were estimated by the restricted maximum likelihood method, and the post hoc comparisons of the least squares means between different groups were performed by using either Student’s t test (between two groups) or the Tukey’s honestly significant difference test (multiple groups). P values <0.05 were considered significant.
Results
Non-CF HBE Cells
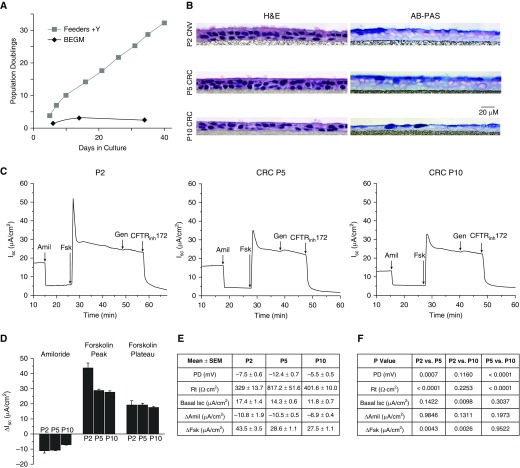
Initial studies were performed using non-CF HBE cells from two donors, with comparable results, as shown in Figure 1 and Figure E1A. HBE cells grown conventionally on plastic in BEGM reached senescence, defined as no increase in cell number in 21 days, before 10 population doublings at P3–P4, whereas cells grown in coculture with irradiated 3T3 cells in F-medium supplemented with Y (CRC conditions) grew exponentially beyond 25 population doublings, typically by P10. This translates into a >30,000-fold increase in cell number between the two methods. ALI cultures, of conventionally grown cells at P2, and CRC-expanded cells at P5 and P10, were created and studied histologically and electrophysiologically. At all passages, the cells formed a pseudostratified mucociliary epithelium that exhibited the expected baseline and agonist/inhibitor–stimulated electrophysiological properties, including baseline Isc, amiloride-sensitive epithelial sodium channel currents, and forskolin-stimulated CFTR currents (Figure 1). Non-CF HBE cells from a second donor (Figures E1B and E1C) confirmed that CFTR responses were maintained under CRC expansion conditions at P5 and P10. In non-CF ALI cultures from both donors, peak forskolin responses were lower in P5 and P10 CRC-expanded cells than in P2 conventional cells, but forskolin plateau and CFTRinh-172 responses were not significantly different.
Figure 1.
Growth, morphology, and electrophysiological properties of conventional versus conditionally reprogrammed cell (CRC)–expanded human bronchial epithelial (HBE) cells. (A) Population doublings of non–cystic fibrosis (CF) HBE cells from a cryopreserved stock. Feeders + Y-27632 (Y) = CRC method as per included protocol. (B) Hematoxylin and eosin (H&E) and alcian blue–periodic acid Schiff (AB-PAS) staining of Day 28–35 HBE cell air–liquid interface (ALI) cell cultures (collagen IV-coated Millicell CM inserts, University of North Carolina [UNC] ALI media). Representative histological cross-sections, all from a single donor. Scale bar: 20 μm. (C) Representative traces of electrophysiological responses in Ussing chambers. Amiloride (100 μM) was added to block the epithelial sodium channel, followed by forskolin (10 μM) to stimulate cystic fibrosis transmembrane conductance regulator (CFTR), and genistein (10 μM) to further activate CFTR. CFTR was then inhibited with CFTR inhibitor 172 (CFTRinh-172) (10 μM). (D) Graph showing mean electrophysiological responses. (E) Mean electrophysiological values. (F) ANOVA was performed, followed by Tukey's multiple comparison test to compare cells at P2, P5, and P10. P values are indicated, mean ± SEM; n = 4–5 inserts/group. Amil, amiloride; BEGM, bronchial epithelial growth media; CNV, conventional method; Fsk, forskolin; Gen, genistein; Isc, short-circuit current; P, passage; PD, potential difference; Rt, transepithelial resistance.
CF HBE Cells
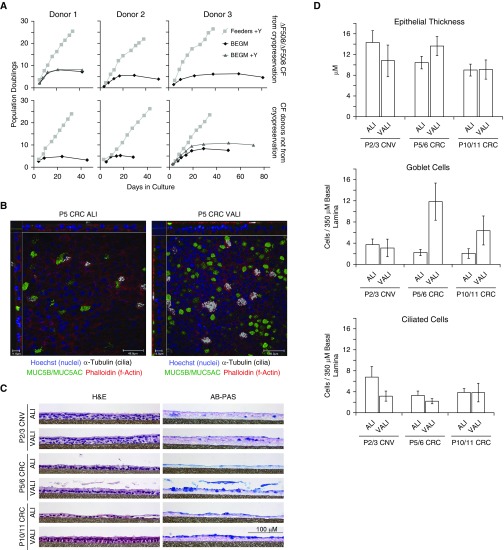
The promising results with non-CF HBE cells stimulated two strategies for CF cells. First, both the conventional and the CRC method were used to culture HBE cells from three sequentially received explanted CF lungs, beginning at P0. Two of the three lungs were from F508 del homozygous donors. Second, we converted cryopreserved P1 cells that were prepared previously by conventional methods to the CRC technique, the so-called “converted CRC” cells. All three were from F508 del homozygous donors. Growth curves and morphological data for the CF cells are shown in Figure 2 and Table E3, and electrophysiology results are given in Figure 3 and Tables E4–E6. The growth of the CF cells was comparable to that of the non-CF cells reported above, with the conventional culture method in BEGM resulting in senescence within three to six passages at fewer than 10 population doublings. The addition of Y-compound to BEGM in two of the six specimens did not greatly increase cell growth. All cells under CRC conditions grew well, with exponential increases exceeding 25 population doublings by P10. CF cells at all passages successfully created confluent ALI cultures that excluded media from the apical culture surface. Morphological differences were present in ALI cultures under different growth conditions and as a function of passage. In general, the conventional method cells at P2 or P3 were thicker than the later-passage CRC-expanded cells.
Figure 2.
Growth and morphology of conventional versus CRC-expanded CF HBE cells. (A) Population doublings of HBE cells from six CF donors, three from cryopreserved stocks initially prepared conventionally and three from sequentially, newly acquired lungs as indicated. Feeders + Y = CRC method as per included protocol. (B) Confocal imaging of whole-mount immunostained HBE cell cultures. ALI is nonproprietary UNC ALI media, and Ultroser G is VALI media with proprietary supplement. Going across from left to right, scale bars: 11, 46, 14, and 46 μm, respectively. (C) Representative histological cross-sections from a single donor. Scale bar: 100 μm. (D) Graphical representation of mean morphometric data from four to eight donors per group, including two non-CF donors and six CF donors. Statistical analysis results are given in Table E3.
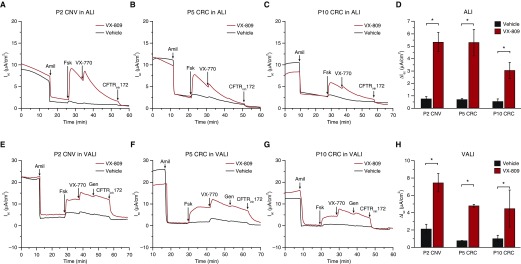
Figure 3.
Electrophysiological properties of conventional versus CRC-expanded CF HBE (∆F508/∆F508) cells. (A–C) Representative Ussing chamber traces of P2 CNV, P5 CRC, and P10 CRC cells grown in UNC ALI media. (D) Forskolin responses (UNC ALI media). (E–G) Representative Ussing chamber traces of cells grown in VALI media. (H) Forskolin responses (VALI media). Forskolin responses in D and H (mean ± SEM, minimum of three replicates) were analyzed by ANOVA, followed by Tukey's multiple-comparison test. *P < 0.0001. Additional data, including mean baseline electrophysologic properties, changes in epithelial sodium channel current in response to amiloride (ΔAmil), and changes in CFTR current in response to forskolin (ΔFsk Peak) of cells cultured in UNC ALI and VALI from three different donors, as well as the results for individual donors, are given in Tables E4–E6.
A USG-containing medium, which we term VALI, is commonly used for preclinical testing of CFTR modulators (14). We studied cells cultured in UNC ALI (13) or VALI media. Morphological differences between the two differentiation media were most apparent in later-passage CRC cells, where the USG-containing VALI media resulted in cultures that appeared more pseudostratified and had considerably more goblet cells. More ciliated cells were present in early-passage conventional P2 or P3 cells in the UNC ALI media than in all other conditions.
Ussing chamber transepithelial electrophysiological studies of P2/3 conventional-method CF cells in UNC ALI and VALI exhibited the expected baseline and drug-stimulated properties, including correction of CFTR function after treatment with VX-809 (Figure 3). Although there were diminutions in currents in the P5/6 and P10/11 CRC-expanded cells compared with the conventional method P2/3 CF cells, treatment with VX-809 significantly increased forskolin-induced CFTR-mediated currents compared with the vehicle control in both media at all passages. Thus, both the UNC ALI and the VALI media enable functional differentiation CF HBE cells in the ALI model, and CRC-expanded CF HBE cells appear suitable to test the efficacy of CFTR corrector compounds such as VX-809 up to P10/11.
Discussion
Primary CF HBE cells are crucial for research, particularly to advance CFTR modulator compound development. However, these cells are not widely available. We examined whether culture of F508 del homozygous CF HBE cells under CRC conditions, namely irradiated feeder cells and the RhoA kinase inhibitor Y, could expand the supply of cells useful for CFTR modulator testing. As expected from previous studies (8, 13), HBE cells cultured under conventional conditions on plastic in LHC9-based nonproprietary BEGM exhibited limited lifespans and underwent senescence within ∼10 population doublings, whereas cells grown in CRC conditions grew exponentially to ∼25 population doublings within 10 passages. When massively expanded F508 del homozygous cells were switched to differentiation conditions on porous supports, they formed confluent ALI cultures. Treatment of the cultures with VX-809 resulted in significant CFTR rescue. Thus, the CRC method enabled dramatic expansion of primary CF HBE cell resources. For example, a single HBE cell grown by the conventional nonproprietary BEGM method will generate 512 cells before senescence at 10 population doublings, whereas by 25 population doublings in the CRC method (∼P10), it will generate >16 million cells.
There were morphological and functional differences between the early-passage conventional cells and the later-passage CRC-expanded cells. Later-passage CRC ALI cultures, especially in the nonproprietary UNC ALI media, were thinner, with fewer ciliated cells, whereas later-passage cells in USG-containing VALI media had abundant goblet cells. Later-passage cells in both media exhibited certain changes in baseline electrophysiological properties and smaller residual and corrected CFTR currents than did early-passage conventional cells. Ussing chamber measurements in the USG-containing VALI media were less stable and exhibited higher variability. However, both media were similar in terms of being able to detect CFTR rescue by VX-809.
Differences in cell morphology between the different media (e.g., a thicker epithelial layer in P5 or P6 ALI cultures in VALI versus UNC ALI media) suggest that further improvements are possible. We speculate that added factors in the proprietary USG media supplement are more supportive of growth of extensively passaged cells. Furthermore, in our studies, late-passage cells were suddenly switched from expansion media (F media + Y) to differentiation media (UNC ALI or VALI). Maintaining the expanded cells on porous supports in F media + Y until confluence, perhaps in concert with a systematically optimized differentiation media, may enhance the functionality of late-passage cells. Further improvement in methods is a clear opportunity for future research. Airway surface layer (ASL) depth is a useful measure of CFTR function. In our hands, ASL changes resulting from VX-809 treatment of P2 conventional F508 del homozygous cells were difficult to detect. Because CFTR currents were reduced after extensive population doublings, we did not attempt ASL measurements in CRC-expanded cells. As more efficacious CFTR correctors are developed, studies of ASL thickness and mucus properties are warranted. In addition, the ability of CRC-expanded cells to generate spheroids and their responses to CFTR modulators should be tested.
Previous studies demonstrate the usefulness of the CRC technique in expanding cells from small, minimally invasive curette or brush samples (10–12). It should be noted that Y-dependent changes in the airway epithelial cell transcriptome include genes involved in basal cell cytoskeleton, cell–cell junctions, and cell-extracellular matrix interactions (16). We furthermore demonstrated morphological and functional changes as a consequence of extended population doublings. Thus, CRC-expanded HBE cells may be different in some respects regarding complex cell biological mechanisms and may not precisely replicate less extensively expanded cells for all types of studies. Future studies to better understand the reasons for the changes and to minimize them may be useful. Nevertheless, expansion of donor-derived cell supplies for preclinical studies of CFTR modulators will be enhanced by the CRC method. Recently, inhibition of SMAD signaling pathways in a feeder-free culture system was used to expand airway basal stem cells (17). However, decreases in CFTR rescue as a function of cell expansion were quite profound in ALI cultures generated with this method.
Numerous CFTR modulators are currently in development in the pharmacological pipeline (https://tools.cff.org/research/drugdevelopmentpipeline/), and testing their efficacy for treatment of CF individuals with common and rare mutations is crucial. If coupled with reliable, high-throughput assays of CFTR function, our studies raise the important prospect of using CRC expansion of cells for personalized, precision medicine in CF.
Acknowledgments
Acknowledgments
The authors thank the University of North Carolina Marsico Lung Institute/CF Research Center Tissue Procurement and Cell Culture Core for provision of cells and media, Kimberlie Burns for histology services, and Diana Lotito for editorial assistance.
Footnotes
This work was supported by Cystic Fibrosis Foundation (CFF) GENTZSCH15XX0, National Institutes of Health P30 DK065988, and CFF BOUCHE15R0.
Author Contributions: Conception and design: M.G., C. Cheluvaraju, X.L., R.S., and S.H.R.; performance of experiments: M.G., S.E.B., C. Cheluvaraju, I.G.C., N.L.Q., C. Cho, and S.H.R.; data analysis and interpretation: M.G., S.E.B., C. Cheluvaraju, I.G.C., N.L.Q., C. Cho, H.D., and S.H.R.; drafting the manuscript for important intellectual content: M.G., C. Cheluvaraju, and S.H.R.; and critical review of the manuscript: all authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0276MA on December 16, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Sosnay PR, Siklosi KR, Van Goor F, Kaniecki K, Yu H, Sharma N, Ramalho AS, Amaral MD, Dorfman R, Zielenski J, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet. 2013;45:1160–1167. doi: 10.1038/ng.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodlie M, Haq IJ, Roberts K, Elborn JS. Targeted therapies to improve CFTR function in cystic fibrosis. Genome Med. 2015;7:101. doi: 10.1186/s13073-015-0223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veit G, Avramescu RG, Chiang AN, Houck SA, Cai Z, Peters KW, Hong JS, Pollard HB, Guggino WB, Balch WE, et al. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell. 2016;27:424–433. doi: 10.1091/mbc.E14-04-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, Colombo C, Davies JC, De Boeck K, Flume PA, et al. TRAFFIC Study Group; TRANSPORT Study Group. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220–231. doi: 10.1056/NEJMoa1409547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman S, Liu X, Meyers C, Schlegel R, McBride AA. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest. 2010;120:2619–2626. doi: 10.1172/JCI42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, et al. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol. 2012;180:599–607. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suprynowicz FA, Upadhyay G, Krawczyk E, Kramer SC, Hebert JD, Liu X, Yuan H, Cheluvaraju C, Clapp PW, Boucher RC, Jr, et al. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc Natl Acad Sci USA. 2012;109:20035–20040. doi: 10.1073/pnas.1213241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horani A, Nath A, Wasserman MG, Huang T, Brody SL. Rho-associated protein kinase inhibition enhances airway epithelial Basal-cell proliferation and lentivirus transduction. Am J Respir Cell Mol Biol. 2013;49:341–347. doi: 10.1165/rcmb.2013-0046TE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knowles MR, Ostrowski LE, Leigh MW, Sears PR, Davis SD, Wolf WE, Hazucha MJ, Carson JL, Olivier KN, Sagel SD, et al. Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype. Am J Respir Crit Care Med. 2014;189:707–717. doi: 10.1164/rccm.201311-2047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu HW, Rios C, Huang C, Wesolowska-Andersen A, Burchard EG, O’Connor BP, Fingerlin TE, Nichols D, Reynolds SD, Seibold MA. CRISPR-Cas9-mediated gene knockout in primary human airway epithelial cells reveals a proinflammatory role for MUC18. Gene Ther. 2015;22:822–829. doi: 10.1038/gt.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler CR, Hynds RE, Gowers KH, Lee DdoH, Brown JM, Crowley C, Teixeira VH, Smith CM, Urbani L, Hamilton NJ, et al. Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am J Respir Crit Care Med. 2016;194:156–168. doi: 10.1164/rccm.201507-1414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fulcher ML, Randell SH. Human nasal and tracheo-bronchial respiratory epithelial cell culture. Methods Mol Biol. 2013;945:109–121. doi: 10.1007/978-1-62703-125-7_8. [DOI] [PubMed] [Google Scholar]
- 14.Neuberger T, Burton B, Clark H, Van Goor F. Use of primary cultures of human bronchial epithelial cells isolated from cystic fibrosis patients for the pre-clinical testing of CFTR modulators. Methods Mol Biol. 2011;741:39–54. doi: 10.1007/978-1-61779-117-8_4. [DOI] [PubMed] [Google Scholar]
- 15.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 16.Reynolds SD, Rios C, Wesolowska-Andersen A, Zhuang Y, Pinter M, Happoldt C, Hill CL, Lallier SW, Cosgrove GP, Solomon GM, et al. Airway progenitor clone formation is enhanced by Y-27632-dependent changes in the transcriptome. Am J Respir Cell Mol Biol. 2016;55:323–336. doi: 10.1165/rcmb.2015-0274MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mou H, Vinarsky V, Tata PR, Brazauskas K, Choi SH, Crooke AK, Zhang B, Solomon GM, Turner B, Bihler H, et al. Dual SMAD signaling inhibition enables long-term expansion of diverse epithelial basal cells. Cell Stem Cell. 2016;19:217–231. doi: 10.1016/j.stem.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]