Abstract
Diverse classes of ligands have recently been discovered that relax airway smooth muscle (ASM) despite a transient increase in intracellular calcium concentrations ([Ca2+]i). However, the cellular mechanisms are not well understood. Gelsolin is a calcium-activated actin-severing and -capping protein found in many cell types, including ASM cells. Gelsolin also binds to phosphatidylinositol 4,5-bisphosphate, making this substrate less available for phospholipase Cβ–mediated hydrolysis to inositol triphosphate and diacylglycerol. We hypothesized that gelsolin plays a critical role in ASM relaxation and mechanistically accounts for relaxation by ligands that transiently increase [Ca2+]i. Isolated tracheal rings from gelsolin knockout (KO) mice showed impaired relaxation to both a β-agonist and chloroquine, a bitter taste receptor agonist, which relaxes ASM, despite inducing transiently increased [Ca2+]i. A single inhalation of methacholine increased lung resistance to a similar extent in wild-type and gelsolin KO mice, but the subsequent spontaneous relaxation was less in gelsolin KO mice. In ASM cells derived from gelsolin KO mice, serotonin-induced Gq-coupled activation increased both [Ca2+]i and inositol triphosphate synthesis to a greater extent compared to cells from wild-type mice, possibly due to the absence of gelsolin binding to phosphatidylinositol 4,5-bisphosphate. Single-cell analysis showed higher filamentous:globular actin ratio at baseline and slower cytoskeletal remodeling dynamics in gelsolin KO cells. Gelsolin KO ASM cells also showed an attenuated decrease in cell stiffness to chloroquine and flufenamic acid. These findings suggest that gelsolin plays a critical role in ASM relaxation and that activation of gelsolin may contribute to relaxation induced by ligands that relax ASM despite a transient increase in [Ca2+]i.
Keywords: actin cytoskeleton, bitter taste receptor agonist, cell stiffness, flexiVent, smooth muscle relaxation
Clinical Relevance
Relaxation of bronchoconstriction is critical in diseases such as asthma, and currently recommended therapies are inadequate in controlling patients with severe asthma. These studies demonstrate that the absence of the actin regulating protein gelsolin results in impaired airway smooth muscle relaxation in response to diverse pro-relaxant ligands. Modifying the expression or function of gelsolin to regulate the actin cytoskeleton of airway smooth muscle cells could be a novel therapeutic approach in bronchoconstrictive diseases.
Actin, a cytoskeletal protein found within all living cells, plays key roles in many developmental, physiological, and pathological processes (1). In the smooth muscle of human bronchi, a reversible polymerization of globular actin (G-actin) monomers to filamentous actin (F-actin) is a necessary prerequisite for myosin-driven airway smooth muscle (ASM) contraction and relaxation (2, 3). In ASM, actin filaments can span the entire length of an individual cell and are anchored to the plasma membrane integrin proteins by macromolecular complexes of actin-associated and cross-linking proteins (3, 4). Within this structural framework, F-actin assembly and disassembly (polymerization/depolymerization) are meticulously regulated in turn by a multitude of cytoskeletal regulatory proteins (4). Most importantly, the cytoskeleton (CSK) of living cells is in a continuous state of remodeling (5), and dysregulation of the actin CSK is now implicated in the pathogenesis of reactive airways in asthma (6–8).
Gelsolin is an actin filament capping protein belonging to the superfamily of gelsolin/villin proteins and is found in many cell types, including ASM. Classically, gelsolin is a calcium-activated protein that undergoes a conformational change that allows it to bind to the barbed end of F-actin and, thereby, prevents further monomer exchange, causing net F-actin depolymerization (9). It is now known that gelsolin can also actively cleave actin filaments (10, 11). Both F-actin–capping and –severing functions are dependent on increased intracellular calcium concentrations ([Ca2+]i) (12). In addition, gelsolin has a binding domain for phosphatidylinositol 4,5-bisphosphate (PIP2), the critical substrate for phospholipase Cβ (PLCβ) (13). As such, it may play a homeostatic role of controlling and/or modulating G protein receptor–mediated generation of inositol triphosphate (IP3) and calcium release from the sarcoplasmic reticulum/endoplasmic reticulum. However, the cognate ligand/receptor signaling and the mechanistic action of gelsolin in ASM contraction and relaxation remain poorly understood.
Recently, a diverse class of ligands has been identified as acute smooth muscle relaxants despite causing an acute transient increase in [Ca2+]i. These include natural flavonoids (14), chloride channel antagonists (15), γ-aminobutyric acid A agonists (16), and bitter taste receptor (TAS2R) agonists (17). For example, similar to type II taste receptor cells on the tongue, activation of TAS2Rs mediates the coupling of the gustducin G protein to PLCβ and evokes an increase in [Ca2+]i (17, 18). Paradoxically, TAS2R-mediated increases in [Ca2+]i cause membrane hyperpolarization, ASM relaxation, and bronchodilation (17). Although bitter tastants are effective in relaxing ASM, and a role for the large-conductance Ca2+-activated K+ channels have been suggested (17), the precise cellular mechanism(s) of TAS2R-mediated relaxation remain to be determined. Here, we show that mice genetically deficient in the gelsolin protein exhibit impaired spontaneous bronchodilation that typically follows a single inhaled dose of methacholine in vivo. In addition, ASM relaxation ex vivo in response to both β-agonist and TAS2R agonist, chloroquine, was impaired. In isolated ASM cells, gelsolin deficiency led to concordant increases in inositol phosphate synthesis, [Ca2+]i and cell stiffness in response to serotonin, while demonstrating a markedly attenuated decrease in cell stiffness in response to chloroquine. Taken together, the correlative biochemical, mechanical, and physiological findings suggest that gelsolin may be involved in TAS2R-mediated intracellular signaling that ultimately leads to ASM relaxation and bronchodilation.
Materials and Methods
A detailed Material and Methods section can be found in the online supplement.
Animals
All animal studies were approved by the Institutional Animal Care and Use Committee at Columbia University (New York, NY). C57BL/6 wild-type male mice at 8–10 weeks of age were purchased from Jackson Laboratory (Bar Harbor, ME). Gelsolin knockout (KO) mice were generated (19) and kindly provided by D. J. Kwiatkowski (Harvard Medical School, Boston, MA, and Division of Pulmonary Medicine, Brigham and Women’s Hospital, Boston, MA).
Mouse Myograph Study
Tracheal rings from wild-type and gelsolin KO mice were mounted in a myograph system (DMT-USA, Ann Arbor, MI) between two pins, with one side attached to a force transducer. Each ring was contracted to its individually calculated EC50 concentration with acetylcholine. When the acetylcholine-induced contraction achieved a plateau in force, relaxing ligands were added to the buffer bathing the tracheal rings.
Mouse ASM Culture
Tracheal rings were isolated from five male mice and the posterior wall enriched for ASM was excised. The tissue was enzymatically digested using the Papain Dissociation System (Worthington Biochemical Corp., Lakewood, NJ) according to the manufacturer’s protocol.
F-Actin:G-Actin Ratio Measurements
ASM cells were plated on eight-well chamber slides and exposed to vehicle or 50 μM acetylcholine for 5 minutes, followed by the addition of vehicle or 100 μM isoproterenol. After 15 minutes, the cells were fixed, permeabilized, and stained with rhodamine-conjugated phalloidin (Molecular Probes R415; ThermoFisher Scientific, Waltham, MA) and Alexa 488–conjugated DNase I (Molecular Probes D12371; ThermoFisher Scientific). Fluorescent intensities were recorded within each microscopic field, quantified using Image J software (National Institutes of Health, Bethesda, MD), and recorded as ratios, as previously described (20).
Spontaneous Nanoscale Tracer Motions
Remodeling dynamics of the CSK network within the living cell were measured by spontaneous nanoscale tracer motion, as we have described in detail elsewhere (21, 22).
Magnetic Twisting Cytometry
Dynamic changes in cell stiffness were measured using magnetic twisting cytometry (MTC) as described previously (17, 23). In brief, the functionalized ferrimagnetic microbeads were magnetized horizontally and then twisted in a vertically aligned magnetic field. This sinusoidal twisting magnetic field caused both a rotation and a pivoting displacement of the bead. The ratio of specific torque to bead displacements was computed and expressed as the cell stiffness.
Intracellular Calcium Assay
Primary cultures of mouse ASM cells were grown to confluence on 96-well plates and loaded with 5 μM of fura-2 AM (intracellular calcium indicator; Life Technologies, Grand Island, NY). Baseline fluorescence was recorded and then 100 μM serotonin or buffer control was injected into each well. The calcium-mediated fluorescence was continuously measured using a FlexStation 3 microplate reader (Molecular Devices, Sunnyvale, CA).
Inositol Phosphate Assay
Inositol phosphate synthesis was measured in confluent cultures of primary (passage 1–4) ASM cells from wild-type and gelsolin KO mice, as previously described (24).
Respiratory System Mechanics Measurements
Wild-type and gelsolin KO male mice were anesthetized and tracheotomized, and mechanically ventilated using a flexiVent FX1 module (SCIREQ, Montreal, QC, Canada). The central airway resistance change in response to methacholine aerosol challenge was recorded by the repeated force oscillatory technique.
Results
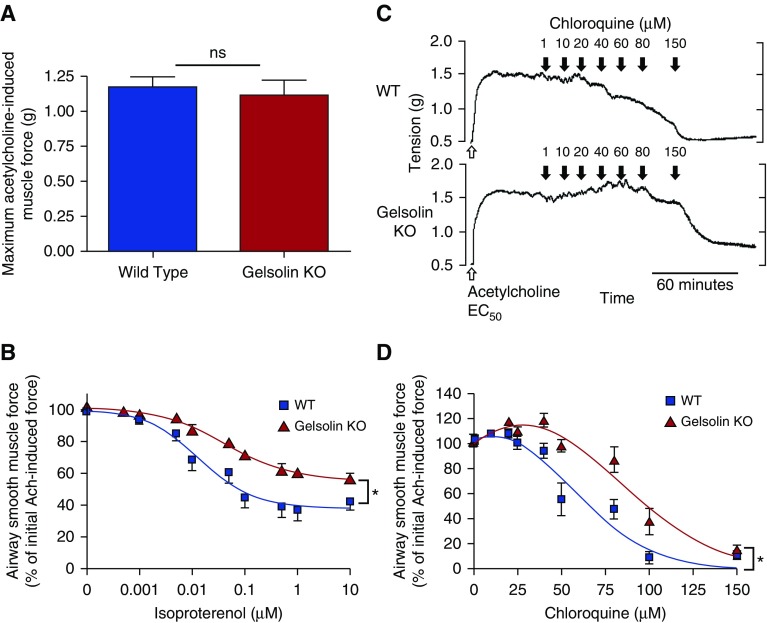
Muscle force from isolated mouse tracheal rings was measured ex vivo in a wire myograph to assess contraction and relaxation of ASM from wild-type and gelsolin KO mice. Tracheal rings from gelsolin KO mice showed a similar magnitude in maximal contractile force in response to 1 mM acetylcholine (n = 22 rings from 11 mice each, P = 0.66; Figure 1A). In contrast, tracheal rings from gelsolin KO mice, precontracted with an EC50 concentration of acetylcholine, showed impaired relaxation in response to cumulatively increasing concentrations of the β-agonist isoproterenol when compared with tracheal rings from wild-type mice (n = 6, P = 0.024; Figure 1B). We next questioned whether another relaxant would also show a similarly impaired response in ASM from gelsolin KO mice. Ligands for TAS2Rs have recently been shown to induce ASM relaxation despite an initial acute increase in [Ca2+]i (17). A representative muscle force tracing of tracheal rings from wild-type and gelsolin KO mice, contracted with an EC50 concentration of acetylcholine and then exposed to increasing concentrations of the bitter tastant, chloroquine, is shown in Figure 1C. Similar to the findings with the β-agonist, isoproterenol, chloroquine was less effective at relaxing acetylcholine-induced contraction in tracheal rings from gelsolin KO mice compared with wild-type mice (n = 7, P = 0.023; Figure 1D).
Figure 1.
Muscle force measurements of isolated tracheal rings from wild-type or gelsolin knockout (KO) mice using wire myography. (A) There was no difference in the maximum acetylcholine-induced force between tracheal rings from wild-type and gelsolin KO mice (n = 22 rings, 11 mice for each group; ns, not significant). (B) Tracheal rings from gelsolin KO mice showed significantly impaired relaxation by isoproterenol from a contraction induced by acetylcholine (EC50; n = 6, *P < 0.05). Isoproterenol EC50 for wild-type and gelsolin KO mice was 14.1 and 44.6 nM, respectively. (C) Representative wire myograph tracing of chloroquine-induced relaxation from wild-type (WT; upper panel) and gelsolin KO (lower panel) mouse trachea. The rings were contracted with an EC50 of acetylcholine, then exposed to cumulative doses of chloroquine (1–150 μM). (D) Tracheal rings from gelsolin KO mice showed significantly impaired relaxation by chloroquine (n = 8–11, *P < 0.05). Chloroquine EC50 for WT and gelsolin KO mice were 59.3 and 89.5 μM, respectively. Data are presented as mean (±SEM).
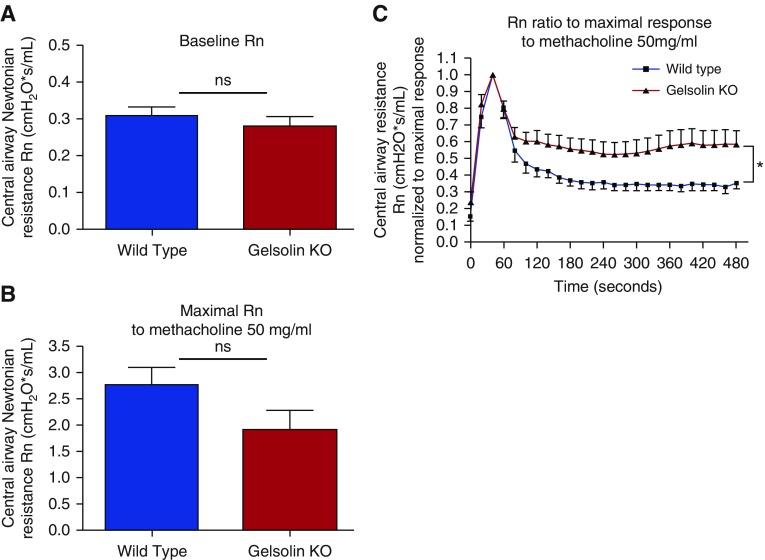
Consistent with ex vivo studies, in vivo central airway resistance at baseline was similar in wild-type and gelsolin KO mice (n = 10 each, P = 0.44; Figure 2A), and the maximal central airway resistance in response to nebulized 50 mg/ml methacholine was not different between the groups (n = 5–6, P = 0.13; Figure 2B), as assessed using the flexiVent system. Typically, a single dose of inhaled methacholine leads to a transient increase in airway resistance followed by a gradual spontaneous decrease. Strikingly, compared with wild-type mice, gelsolin KO mice showed a more sustained maintenance of central airway resistance over time (n = 5 each, P = 0.039; Figure 2C).
Figure 2.
In vivo lung resistance measurements. Gelsolin KO mice exhibit impaired spontaneous reduction in central airway resistance (Rn) after inhalation of a single concentration of methacholine. (A) Baseline Rn was not different between WT and gelsolin KO mice (n = 10; ns). (B) Inhalation of 50 mg/ml methacholine caused a similar degree of acute increase in airway resistance in WT and gelsolin KO mice (n = 5–6; ns). (C) The spontaneous reduction in Rn over time after the single dose of methacholine was less in gelsolin KO mice (n = 5 in each group; *P < 0.05). Data are presented as mean (±SEM).
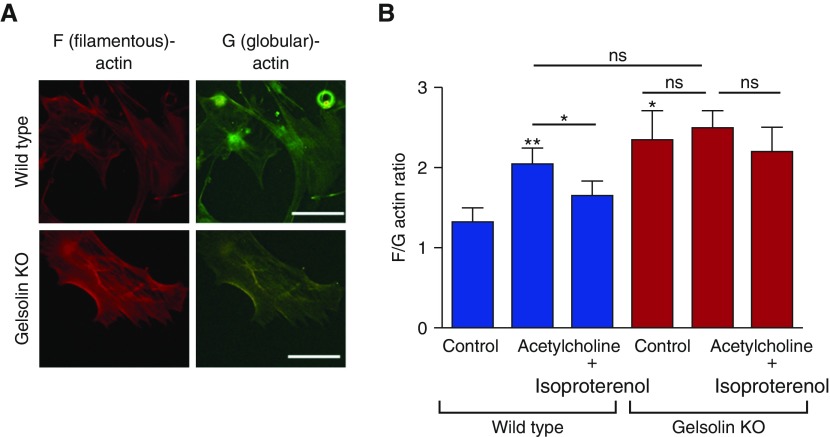
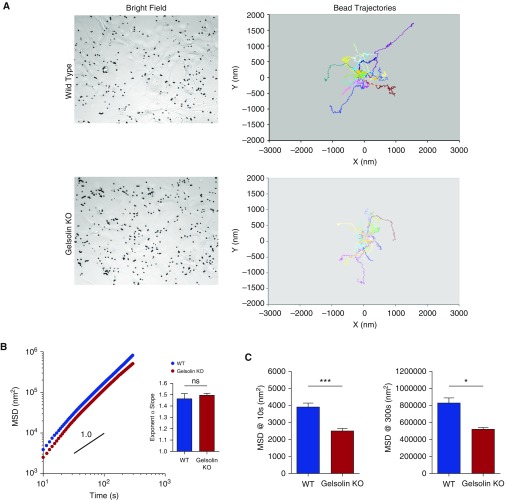
To investigate the mechanism of impaired airway relaxation in gelsolin KO mice, we used cell-based models using primary cultures of ASM cells. We confirmed that these cells exhibited typical morphology and expressed phenotypic markers of ASM cells at passages 1–4 when they were used for experiments (see Figure E1 in the online supplement). Under baseline conditions, ASM cells isolated from gelsolin KO mice were bigger in size (Figure E2), and showed higher F-actin:G-actin ratios, as seen in a representative image (Figure 3A), than those from wild-type mice (Figure 3B, P = 0.016). However, there were no statistical differences in cell traction stress between wild-type and gelsolin KO ASM cells (Figure E2). These results suggest that gelsolin may play a homeostatic role in the regulation of the actin CSK, and that gelsolin deficiency may lead to enhanced F-actin formation. Consistent with this notion, addition of acetylcholine did not further increase the F-actin:G-actin ratio in gelsolin KO ASM cells (Figure 3B, P = 0.94), but did significantly increase the F-actin:G-actin ratio in ASM cells from wild-type mice (P = 0.0078). Moreover, the acetylcholine-induced increase in the F-actin:G-actin ratio was attenuated by the β-agonist, isoproterenol, in ASM cells from wild-type mice (P = 0.043), but not in gelsolin KO ASM cells (P = 0.88; Figure 3B). Similar to the findings in the wire myograph study, a TAS2R agonist, chloroquine, also attenuated the acetylcholine-induced increase in the F-actin:G-actin ratio in ASM cells from wild-type mice (P = 0.049), but not in gelsolin KO ASM cells (P = 0.43; Figure E3). These results indicate an enhanced polymerization of actin CSK and/or a structurally stable polymerized actin network in gelsolin KO ASM cells. Indeed, compared with wild-type ASM cells, gelsolin KO ASM cells exhibited, in real time, slower cytoskeletal remodeling dynamics as assessed by spontaneous tracer motions of microbeads tethered to the living CSK (Figure 4A). In both ASM cells (wild-type and gelsolin KO), the computed mean square displacements of unforced beads increased with time as a power law with an exponent α greater than unity (Figure 4B, inset); there were no statistical differences in the exponent α between wild-type and gelsolin KO ASM cells. As we have defined elsewhere (22), these super-diffusive motions are not characteristics of simple Brownian motions that are thermally driven, but are rather characteristics of an active process attributable to the molecular-level rearrangement (remodeling) of the actin CSK. Compared with wild-type cells, gelsolin KO cells exhibited marked decreases in mean square displacement that became apparent at times greater than 10 seconds, with continual decreases through 300 seconds (P < 0.0001 at 10 s, P = 0.0184 at 300 s, n = 677–827 individual cells from five to six independent measurements; Figure 4C). These results, taken together, implicate a critical homeostatic role for gelsolin in the regulation of actin CSK.
Figure 3.
The filamentous (F) actin to globular (G) actin ratio in cultured airway smooth muscle (ASM) cells from WT and gelsolin KO mice. (A) F- and G-actin were stained with rhodamine-conjugated phalloidin and Alexa 488–conjugated DNase I, respectively. Representative fluorescence microscopy images under baseline conditions are shown. ASM cells from gelsolin KO mice showed increased F-actin staining compared with the WT. Scale bars, 100 μm. (B) In WT ASM cells, acetylcholine markedly increased the F-actin:G-actin (F/G) ratio, indicating increased F-actin polymerization, and the ratio was decreased by adding the β-agonist isoproterenol. Cultured ASM cells from gelsolin KO mice showed a significantly increased F/G ratio at baseline, but the ratio was unaffected by the addition of acetylcholine or acetylcholine followed by isoproterenol (n = 4–7, *P < 0.05, **P < 0.01). Data are presented as mean (±SEM).
Figure 4.
Dynamics of the cytoskeleton network within the living ASM cells of WT and gelsolin KO mice. (A) Representative bright-field images and tracings of spontaneous bead motions on the living cells (showing 20 individual beads per group). (B) The trajectories of bead motions in two dimensions were characterized by computing the mean squared displacement (MSD) of all beads as a function of time t. Data are presented as mean (±SE; WT, n = 677 cells; gelsolin KO, n = 827 cells). Inset: the exponent α of the bead motions were estimated from a least square fit of a power law to the ensemble average of MSD data versus time for each well and averaged (n = 5–6 wells). (C) Computed MSD at 10 and 300 seconds (WT, n = 677 cells; gelsolin KO, n = 827 cells from five to six independent measurements; ***P < 0.0001, *P < 0.05).
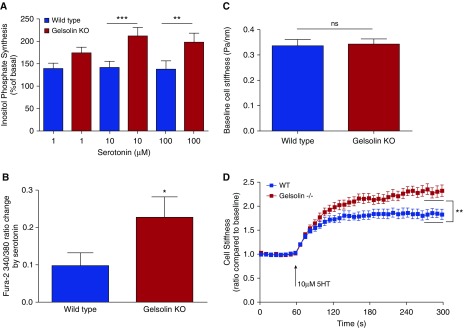
Gq-coupled agonists, such as acetylcholine and serotonin, are known to increase intracellular calcium by activating PLCβ, leading to hydrolysis of PIP2, resulting in increased levels of IP3, causing release of calcium from sarcoplasmic reticulum, facilitating contraction. Gelsolin is known to bind to PIP2, and PIP2-bound gelsolin is in an inactive state (13). If the gelsolin protein is absent from the cell, more PIP2 would be available for hydrolysis, and Gq-coupled agonist-mediated IP3 synthesis and calcium response would be enhanced. To test this hypothesis, we measured serotonin-induced generation of IP3, increases in [Ca2+]i, and single-cell contraction using MTC. ASM cells from gelsolin KO mice demonstrated enhanced serotonin-induced synthesis of inositol phosphates (n = 5, P < 0.001 for 10 μM serotonin; P < 0.01 for 100 μM serotonin; Figure 5A) and augmented increases in [Ca2+]i (n = 6 different sets of ASM culture, P = 0.033; Figure 5B). Corroborating these biochemical results, ASM cells from wild-type and gelsolin KO mice exhibited the same baseline stiffness (Figure 5C), but gelsolin KO ASM cells showed an enhanced cell stiffening response to serotonin (n = 15 wells of cells plated on different wells from four independent experiments, ∼100–200 cells/well measured, P = 0.004; Figure 5D). These results are consistent with the hypothesis that, under normal conditions, gelsolin “anchors” PIP2 and, thereby, makes it a less available substrate for PLCβ-mediated hydrolysis and muscle contraction. In agreement with the above findings with serotonin as a stimulus, ASM cells from gelsolin KO mice also demonstrated enhanced acetylcholine-induced synthesis of inositol phosphates as compared with ASM cells from wild-type mice (n = 6 from different sets of ASM culture, P = 0.0313; Figure E4).
Figure 5.
Gelsolin KO cells showed increased inositol phosphate synthesis, intracellular calcium release and single-cell contraction in response to serotonin. (A) Inositol phosphate synthesis at 20 minutes in response to serotonin in primary cultures of mouse ASM cells. Cells from gelsolin KO mice generated more inositol phosphate in response to the Gq-coupled ligand serotonin, consistent with an increased amount of phosphatidylinositol 4,5-bisphosphate substrate available for inositol phosphate synthesis in the absence of gelsolin (n = 5; **P < 0.01, ***P < 0.001). (B) Intracellular calcium concentrations in response to serotonin treatment were measured using the fura-2 ratiometric fluorescent indicator; 340:380 nm excitation ratio for fura-2 was obtained and normalized to the preinjection ratio. Gelsolin KO ASM cells showed significantly increased intracellular calcium in response to 100 μM serotonin (n = 6 from different sets of ASM cell cultures; *P < 0.05). (C and D) Stiffness of ASM cells from WT and gelsolin KO mice as measured by magnetic twisting cytometry in response to 10 μM serotonin (5HT). Baseline cell stiffness was not different between WT and gelsolin KO ASM cells (n = 15 individual wells of cells each). Cell stiffening in response to serotonin measured with this single-cell technology is a robust index of isolated ASM contraction. For each individual cell, baseline stiffness was measured for the first 60 seconds, and, after drug addition, stiffness was measured continuously for the next 240 seconds. For each cell, stiffness was normalized to its baseline stiffness before the agonist stimulation. The steady-state (last 30 s) maximal cell stiffening values, which are indicated by the black horizontal lines between 270 and 300 seconds, were compared. Gelsolin KO cells showed increased cell stiffening in response to serotonin compared with WT cells (n = 15 wells of cells measured from four independent experimental dates; **P < 0.01). Data are presented as mean (±SEM).
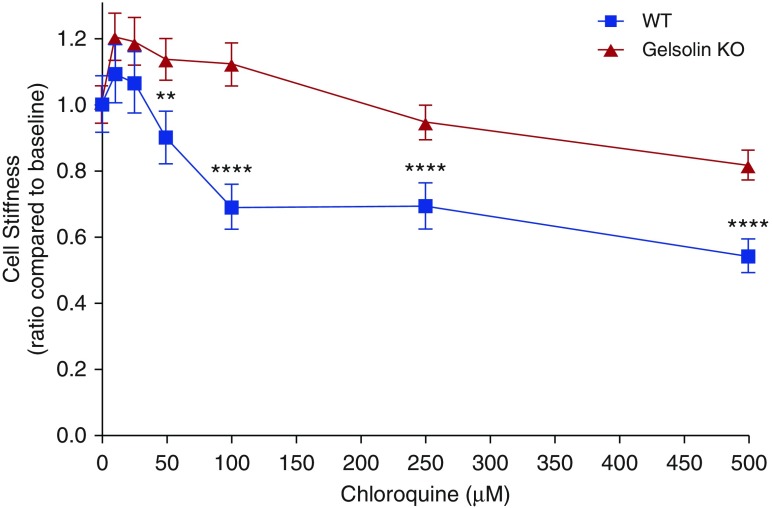
Most interestingly, we found that, consistent with ex vivo and in vivo studies (Figures 1 and 2), gelsolin KO ASM cells showed an attenuated decrease in cell stiffness in response to cumulatively increasing concentrations of the bitter tastant, chloroquine (n = 289–438 cells, P = 0.00148 for 50 μM, P < 0.0001 for 100 μM and 500 μM; Figure 6). Compared with wild-type ASM cells, gelsolin KO ASM cells also showed impaired relaxation to the TAS2R14 subtype–specific agonist, flufenamic acid (Figure E5). As such, despite an initial acute increase in [Ca2+]i, TAS2R ligands caused relaxation of wild-type–derived ASM, and this relaxation is linked to activation of gelsolin.
Figure 6.
Bitter taste receptor agonist chloroquine-induced reduction in cell stiffness of isolated ASM cells was reduced in gelsolin KO mice. Cell stiffness was measured for 30 seconds after each increment of drug addition at 1-minute intervals, which was normalized to baseline stiffness before addition of chloroquine (n = 289–438 cells; ****P < 0.0001, **P < 0.01). EC50 for chloroquine for WT and gelsolin KO cells were 0.171 and 8.43 mM, respectively. Data are presented as mean (±SEM).
Discussion
In the present study, we demonstrate that the actin-capping and -severing protein, gelsolin, plays a role in β-agonist– and bitter tastant–mediated relaxation of ASM. Gelsolin’s function in the regulation of the actin CSK has been well studied in many cell types, and its role in migration of ASM cells has been suggested (25). Our study illustrates the critical role that gelsolin plays in both β-agonist– and bitter tastant–mediated ASM relaxation and inositol phosphate/intracellular calcium signaling in ASM.
This is of particular interest, as relaxation of contracted airway is critical in several bronchoconstrictive diseases, including asthma. The most common current therapy for asthma consists of maintenance therapy with inhaled corticosteroids with or without long-acting β2-adrenceptor agonists and short-acting β2-adrenoceptor agonists as rescue bronchodilators, but this therapy is inadequate in controlling as many as 40% of subjects with asthma (26). Therefore, there is an unmet need for discovering new classes of bronchodilators. Our results suggest that gelsolin is involved in not only β2-adrenceptor agonist–induced relaxation, but also in relaxation mediated by TAS2R ligands. β2-adrenceptor agonists increase cAMP, which reduces the influx of calcium, thereby reducing the frequency of calcium oscillations (27, 28), leading to overall relaxation. In contrast, TAS2R ligands acutely relax smooth muscle, despite causing a transient increase in [Ca2+]i (17, 29). At first glance, this appears paradoxical, as an increase in [Ca2+]i has classically been linked to contraction, not relaxation. However, it is now appreciated that microdomains of calcium regulation and the frequency of intracellular calcium oscillations ultimately dictate contractile tone (30). Gelsolin may provide a unifying mechanism for relaxation induced by diverse agents that cause a transient increase in [Ca2+]i, because gelsolin is acutely activated by increases in [Ca2+]i and then severs and caps actin filaments, thereby disassembling the intracellular contractile framework and leading to ASM relaxation. Thus, gelsolin severing of actin could be a compensatory mechanism that limits the amount of cellular contraction induced by calcium-elevating events, and possibly an additional target to achieve bronchodilation. Future studies in this mouse model using other classes of ligands that increase [Ca2+]i but induce relaxation would further support the hypothesis that agents that acutely increase [Ca2+]i activate gelsolin and lead to relaxation.
The measurement of muscle force in ex vivo tracheal rings demonstrated impaired relaxation of an established acetylcholine contraction by either a β-agonist (isoproterenol) or bitter tastant (chloroquine) in tracheal rings from gelsolin KO mice. These experiments were done using isometric relaxation, which can provide information regarding the ASM tone, but not the actual narrowing of the airway lumen. To overcome this limitation, we performed in vivo measurements of airway resistance and found that gelsolin KO mice maintained an increase in central airway resistance after inhaled methacholine for a longer period of time compared with wild-type mice.
Having demonstrated that ASM from gelsolin KO mice exhibited impaired ex vivo and in vivo relaxation, we next corroborated these findings in isolated primary ASM cells. The enhanced state of actin polymerization (increased F-actin:G-actin ratio) under basal conditions in gelsolin KO ASM cells, and the failure of acetylcholine to increase or isoproterenol and chloroquine to decrease this ratio, suggests that the actin CSK exists in a more polymerized state compared with wild-type cells under basal conditions that can’t be further enhanced by acetylcholine. This is consistent with our ex vivo tracheal ring studies, where acetylcholine did not contract rings from gelsolin KO mice to a greater extent than wild-type mice, suggesting that the gelsolin KO ASM is “stiffer” and unresponsive to further contractile stimuli. This supposition was further supported by MTC experiments in which primary cultured ASM cells from gelsolin KO mice demonstrated an impaired ability to relax to the bitter tastants, chloroquine and flufenamic acid. This cellular impaired relaxation again correlated with impaired tracheal ring relaxation in ex vivo studies in response to isoproterenol or chloroquine, as well as agreeing with delayed spontaneous reductions in airway resistance in vivo. On the other hand, gelsolin KO ASM cells exhibited increased stiffness in response to serotonin, which does not agree with the previously mentioned observation that their airway contraction is not different from wild-type both ex vivo and in vivo. This could be due to sensitivity of the assay methods, where MTC was able to detect smaller changes in cell stiffness.
Using human ASM cells, it was recently discovered that increases in [Ca2+]i induced by G protein–coupled receptors was attenuated by some, but not all, TAS2Rs (29). In this study, chloroquine reduced histamine-induced increase in intracellular calcium, but had no effect on endothelin-1–, bradykinin-, acetylcholine-, or angiotensin II–induced changes in [Ca2+]i. Flufenamic acid reduced only bradykinin-induced increases in [Ca2+]i. Although species and receptor differences may exist, it would be interesting to test the effect of other TAS2R agonists on gelsolin KO mice under various contractile agonist stimulations.
We identified a secondary cellular effect of the absence of gelsolin that could contribute to a contractile (or impaired relaxation) phenotype: increased IP3 synthesis and [Ca2+]i in response to the Gq-coupled agonist, serotonin. This is not due to the expression levels of serotonin receptors in gelsolin KO cells, because immunoblotting revealed similar levels of 5HT2 receptor expression in ASM cells from wild-type and gelsolin KO mice (data not shown).
Gelsolin’s effect on cell migration has been studied in isolated neutrophils and fibroblasts from gelsolin KO mice (19), and its role in ASM cells migration was also suggested (25). Migration of ASM cells contributes to airway remodeling triggered by chronic inflammation (25), which is frequently observed in patients with asthma. It has been reported that the total amount of smooth muscle is increased in asthma (31–33), and increased muscle mass has the potential to increase airway contractions in patients with asthma. Structural plasticity of ASM cells is regulated by an array of cytokines and intracellular signaling pathways (34) evoked by inflammation. Gelsolin’s effect on airway remodeling in response to allergic lung inflammation would be an interesting future study.
Excessive narrowing of the airways in asthma can be due to increases in airway muscle mass, or can be due to the change in the contractile properties of asthmatic ASM cells. Although still lacking experimental support, it has been proposed that dysregulated actin filaments may become longer in asthma. Such elongation of actin is thought to be responsible for the lack of beneficial effects of deep inspirations, which promotes bronchodilation in normal individuals, but not in patients with asthma (35). Using a simple network model of the ASM CSK, Silveira and Fredberg (36) demonstrated that small changes in actin length are able to cause large alterations in both muscle mechanics and dynamics. Therefore, depolymerization of the actin CSK by gelsolin would be an attractive approach in treating the asthmatic airway.
It is interesting that the lack of only one actin-binding protein leads to impaired relaxation. There are two major classes of actin-binding proteins with depolymerization and severing of actin filament properties: gelsolin and actin-depolymerizing factor/cofilin (9). Other actin-severing proteins have been reported (9); however, gelsolin is the most potent actin filament–severing protein identified to date. Therefore, gelsolin would be a better candidate to induce actin severing in the targeted cells among other known actin-severing proteins.
It is well known that gelsolin exists as an intracellular cytoplasmic protein found in most cell types, but there is also a secreted form that is found in the systemic circulation generated primarily by muscle cells (skeletal, cardiac, and smooth muscle cells) (37). Both forms of gelsolin are encoded by the same gene through the use of separate transcription initiation sites, and secreted plasma gelsolin differs from cytoplasmic gelsolin by the addition of a 25–amino acid–long aminoterminal peptide (38, 39). The potential protective function of circulating plasma gelsolin has been studied. The intravenous administration of recombinant gelsolin prevented an increase in pulmonary microvascular permeability in burn-injured rats (40), and gelsolin purified from human plasma reduced the viscosity of sputum from patients with cystic fibrosis (41). In our gelsolin KO model, the synthesis of both cytoplasmic and plasma forms of gelsolin were disrupted (19). We can attribute at least part of the effects seen in the present study to the intracellular form of gelsolin, because impaired cellular relaxation and second messenger synthesis was demonstrated in cultured cells. However, we cannot determine if our effects measured in ex vivo tracheal rings or in vivo lung resistance may also be attributed to a lack of circulating plasma gelsolin. Our results suggest that targeting cytoplasmic gelsolin could be a therapeutic approach in modulating ASM tone. Controlling gelsolin’s expression and activation can be a therapeutic strategy for ASM relaxation by (1) reducing PLCβ-mediated increases in cellular calcium, and (2) increased capping and severing, and thus disassembly of the actin CSK. Because gelsolin is a ubiquitous protein and the actin target is present in virtually all cells, cell-specific targeted expression or uptake would be necessary. Targeted gelsolin overexpression experiments in ASM would be an interesting direction for future research.
In conclusion, tracheal rings from gelsolin KO mice demonstrated impaired relaxation, and their cultured smooth muscle cells demonstrated impaired actin depolymerization in response to both β-agonists and TAS2R ligands, indicating the importance of this actin-capping protein in ASM relaxation mediated by different upstream prorelaxant signaling pathways. Moreover, [Ca2+]i responses and IP3 synthesis were enhanced in response to a Gq-coupled agonist in gelsolin KO ASM cells, suggesting that the absence of gelsolin allows for increased PIP2 substrate availability for phospholipase Cβ conversion to IP3, leading to increased [Ca2+]i and impaired smooth muscle relaxation. Thus, gelsolin plays a critical role in the actin dynamics and calcium responses in ASM relaxation.
Acknowledgments
Acknowledgments
The authors thank Dr. David J. Kwiatkowski (Harvard Medical School, Boston, MA and Division of Pulmonary Medicine, Brigham and Women’s Hospital, Boston, MA) for sharing the gelsolin KO mice.
Footnotes
This work was supported by National Institutes of Health grants GM065281 (C.W.E.), HL122340 (C.W.E.), GM008464 (C.W.E.), DK098120 (S.K.), and HL107361 (S.S.A.), and the Stony Wold-Herbert Fund (M.M.).
Author Contributions: Conception and design—M.M., E.T., S.K., S.S.A., and C.W.E.; acquisition, analysis, and interpretation—M.M., Y.Z., J.D., T.J., H.M.Y., S.S.A., and C.W.E.; drafting or revising the manuscript for important intellectual content—M.M., J.D., E.T., S.S.A., and C.W.E.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0292OC on January 24, 2017
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Janmey PA, Chaponnier C. Medical aspects of the actin cytoskeleton. Curr Opin Cell Biol. 1995;7:111–117. doi: 10.1016/0955-0674(95)80052-2. [DOI] [PubMed] [Google Scholar]
- 2.An SS, Laudadio RE, Lai J, Rogers RA, Fredberg JJ. Stiffness changes in cultured airway smooth muscle cells. Am J Physiol Cell Physiol. 2002;283:C792–C801. doi: 10.1152/ajpcell.00425.2001. [DOI] [PubMed] [Google Scholar]
- 3.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol. 2008;295:C576–C587. doi: 10.1152/ajpcell.00253.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang W, Gunst SJ. Interactions of airway smooth muscle cells with their tissue matrix: implications for contraction. Proc Am Thorac Soc. 2008;5:32–39. doi: 10.1513/pats.200704-048VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Theriot JA. Regulation of the actin cytoskeleton in living cells. Semin Cell Biol. 1994;5:193–199. doi: 10.1006/scel.1994.1024. [DOI] [PubMed] [Google Scholar]
- 6.Davies DE, Wicks J, Powell RM, Puddicombe SM, Holgate ST. Airway remodeling in asthma: new insights. J Allergy Clin Immunol. 2003;111:215–225, quiz 226. doi: 10.1067/mai.2003.128. [DOI] [PubMed] [Google Scholar]
- 7.Pepe C, Foley S, Shannon J, Lemiere C, Olivenstein R, Ernst P, Ludwig MS, Martin JG, Hamid Q. Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol. 2005;116:544–549. doi: 10.1016/j.jaci.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Shore SA. Airway smooth muscle in asthma—not just more of the same. N Engl J Med. 2004;351:531–532. doi: 10.1056/NEJMp048139. [DOI] [PubMed] [Google Scholar]
- 9.Ono S. Mechanism of depolymerization and severing of actin filaments and its significance in cytoskeletal dynamics. Int Rev Cytol. 2007;258:1–82. doi: 10.1016/S0074-7696(07)58001-0. [DOI] [PubMed] [Google Scholar]
- 10.Sun HQ, Yamamoto M, Mejillano M, Yin HL. Gelsolin, a multifunctional actin regulatory protein. J Biol Chem. 1999;274:33179–33182. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- 11.Yin HL, Stossel TP. Control of cytoplasmic actin gel–sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979;281:583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]
- 12.Kinosian HJ, Newman J, Lincoln B, Selden LA, Gershman LC, Estes JE. Ca2+ regulation of gelsolin activity: binding and severing of F-actin. Biophys J. 1998;75:3101–3109. doi: 10.1016/S0006-3495(98)77751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janmey PA, Stossel TP. Modulation of gelsolin function by phosphatidylinositol 4,5-bisphosphate. Nature. 1987;325:362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- 14.Townsend EA, Siviski ME, Zhang Y, Xu C, Hoonjan B, Emala CW. Effects of ginger and its constituents on airway smooth muscle relaxation and calcium regulation. Am J Respir Cell Mol Biol. 2013;48:157–163. doi: 10.1165/rcmb.2012-0231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallos G, Remy KE, Danielsson J, Funayama H, Fu XW, Chang HY, Yim P, Xu D, Emala CW., Sr Functional expression of the TMEM16 family of calcium-activated chloride channels in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2013;305:L625–L634. doi: 10.1152/ajplung.00068.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizuta K, Xu D, Pan Y, Comas G, Sonett JR, Zhang Y, Panettieri RA, Jr, Yang J, Emala CW., Sr GABAA receptors are expressed and facilitate relaxation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1206–L1216. doi: 10.1152/ajplung.00287.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang CH, Lifshitz LM, Uy KF, Ikebe M, Fogarty KE, ZhuGe R. The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol. 2013;11:e1001501. doi: 10.1371/journal.pbio.1001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witke W, Sharpe AH, Hartwig JH, Azuma T, Stossel TP, Kwiatkowski DJ. Hemostatic, inflammatory, and fibroblast responses are blunted in mice lacking gelsolin. Cell. 1995;81:41–51. doi: 10.1016/0092-8674(95)90369-0. [DOI] [PubMed] [Google Scholar]
- 20.Hirshman CA, Zhu D, Pertel T, Panettieri RA, Emala CW. Isoproterenol induces actin depolymerization in human airway smooth muscle cells via activation of an Src kinase and GS. Am J Physiol Lung Cell Mol Physiol. 2005;288:L924–L931. doi: 10.1152/ajplung.00463.2004. [DOI] [PubMed] [Google Scholar]
- 21.An SS, Fabry B, Mellema M, Bursac P, Gerthoffer WT, Kayyali US, Gaestel M, Shore SA, Fredberg JJ. Role of heat shock protein 27 in cytoskeletal remodeling of the airway smooth muscle cell. J Appl Physiol (1985) 2004;96:1701–1713. doi: 10.1152/japplphysiol.01129.2003. [DOI] [PubMed] [Google Scholar]
- 22.Bursac P, Fabry B, Trepat X, Lenormand G, Butler JP, Wang N, Fredberg JJ, An SS. Cytoskeleton dynamics: fluctuations within the network. Biochem Biophys Res Commun. 2007;355:324–330. doi: 10.1016/j.bbrc.2007.01.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.An SS, Fabry B, Trepat X, Wang N, Fredberg JJ. Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness? Am J Respir Cell Mol Biol. 2006;35:55–64. doi: 10.1165/rcmb.2005-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotta K, Emala CW, Hirshman CA. TNF-α upregulates Giα and Gqα protein expression and function in human airway smooth muscle cells. Am J Physiol. 1999;276:L405–L411. doi: 10.1152/ajplung.1999.276.3.L405. [DOI] [PubMed] [Google Scholar]
- 25.Gerthoffer WT. Migration of airway smooth muscle cells. Proc Am Thorac Soc. 2008;5:97–105. doi: 10.1513/pats.200704-051VS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braido F. Failure in asthma control: reasons and consequences. Scientifica (Cairo) 2013;2013:549252. doi: 10.1155/2013/549252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nuttle LC, Farley JM. Frequency modulation of acetylcholine-induced oscillations in Ca++ and Ca(++)-activated Cl− current by cAMP in tracheal smooth muscle. J Pharmacol Exp Ther. 1996;277:753–760. [PubMed] [Google Scholar]
- 28.Prakash YS, van der Heijden HF, Kannan MS, Sieck GC. Effects of salbutamol on intracellular calcium oscillations in porcine airway smooth muscle. J Appl Physiol (1985) 1997;82:1836–1843. doi: 10.1152/jappl.1997.82.6.1836. [DOI] [PubMed] [Google Scholar]
- 29.Camoretti-Mercado B, Pauer SH, Yong HM, Smith DC, Deshpande DA, An SS, Liggett SB. Pleiotropic effects of bitter taste receptors on [Ca2+]i mobilization, hyperpolarization, and relaxation of human airway smooth muscle cells. PLoS One. 2015;10:e0131582. doi: 10.1371/journal.pone.0131582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.An SS, Bai TR, Bates JH, Black JL, Brown RH, Brusasco V, Chitano P, Deng L, Dowell M, Eidelman DH, et al. Airway smooth muscle dynamics: a common pathway of airway obstruction in asthma. Eur Respir J. 2007;29:834–860. doi: 10.1183/09031936.00112606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis. 1993;147:405–410. doi: 10.1164/ajrccm/147.2.405. [DOI] [PubMed] [Google Scholar]
- 32.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma: a 3-D morphometric study. Am Rev Respir Dis. 1993;148:720–726. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- 33.Kuwano K, Bosken CH, Paré PD, Bai TR, Wiggs BR, Hogg JC. Small airways dimensions in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993;148:1220–1225. doi: 10.1164/ajrccm/148.5.1220. [DOI] [PubMed] [Google Scholar]
- 34.Halayko AJ, Amrani Y. Mechanisms of inflammation-mediated airway smooth muscle plasticity and airways remodeling in asthma. Respir Physiol Neurobiol. 2003;137:209–222. doi: 10.1016/s1569-9048(03)00148-4. [DOI] [PubMed] [Google Scholar]
- 35.Solway J, Bellam S, Dowell M, Camoretti-Mercado B, Dulin N, Fernandes D, Halayko A, Kocieniewski P, Kogut P, Lakser O, et al. Actin dynamics: a potential integrator of smooth muscle (Dys-)function and contractile apparatus gene expression in asthma: Parker B. Francis lecture. Chest. 2003;123(3 s) uppl:392S–398S. doi: 10.1378/chest.123.3_suppl.392s. [DOI] [PubMed] [Google Scholar]
- 36.Silveira PS, Fredberg JJ. Smooth muscle length adaptation and actin filament length: a network model of the cytoskeletal dysregulation. Can J Physiol Pharmacol. 2005;83:923–931. doi: 10.1139/y05-092. [DOI] [PubMed] [Google Scholar]
- 37.Kwiatkowski DJ, Mehl R, Izumo S, Nadal-Ginard B, Yin HL. Muscle is the major source of plasma gelsolin. J Biol Chem. 1988;263:8239–8243. [PubMed] [Google Scholar]
- 38.Kwiatkowski DJ, Stossel TP, Orkin SH, Mole JE, Colten HR, Yin HL. Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicated actin-binding domain. Nature. 1986;323:455–458. doi: 10.1038/323455a0. [DOI] [PubMed] [Google Scholar]
- 39.Yin HL, Kwiatkowski DJ, Mole JE, Cole FS. Structure and biosynthesis of cytoplasmic and secreted variants of gelsolin. J Biol Chem. 1984;259:5271–5276. [PubMed] [Google Scholar]
- 40.Rothenbach PA, Dahl B, Schwartz JJ, O’Keefe GE, Yamamoto M, Lee WM, Horton JW, Yin HL, Turnage RH. Recombinant plasma gelsolin infusion attenuates burn-induced pulmonary microvascular dysfunction. J Appl Physiol (1985) 2004;96:25–31. doi: 10.1152/japplphysiol.01074.2002. [DOI] [PubMed] [Google Scholar]
- 41.Vasconcellos CA, Allen PG, Wohl ME, Drazen JM, Janmey PA, Stossel TP. Reduction in viscosity of cystic fibrosis sputum in vitro by gelsolin. Science. 1994;263:969–971. doi: 10.1126/science.8310295. [DOI] [PubMed] [Google Scholar]