Abstract
Myofibroblasts, the primary effector cells that mediate matrix remodeling during pulmonary fibrosis, rapidly assemble an extracellular fibronectin matrix. Tensin (TNS) 1 is a key component of specialized cellular adhesions (fibrillar adhesions) that bind to extracellular fibronectin fibrils. We hypothesized that TNS1 may play a role in modulating myofibroblast-mediated matrix formation. We found that TNS1 expression is increased in fibroblastic foci from lungs with idiopathic pulmonary fibrosis. Transforming growth factor (TGF)-β profoundly up-regulates TNS1 expression with kinetics that parallel the expression of the myofibroblast marker, smooth muscle α-actin. TGF-β–induced TNS1 expression is dependent on signaling through the TGF-β receptor 1 and is Rho coiled-coiled kinase/actin/megakaryoblastic leukemia-1/serum response factor dependent. Small interfering RNA–mediated knockdown of TNS1 disrupted TGF-β–induced myofibroblast differentiation, without affecting TGF-β/Smad signaling. In contrast, loss of TNS1 resulted in disruption of focal adhesion kinase phosphorylation, focal adhesion formation, and actin stress fiber development. Finally, TNS1 was essential for the formation of fibrillar adhesions and the assembly of nascent fibronectin and collagen matrix in myofibroblasts. In summary, our data show that TNS1 is a novel megakaryoblastic leukemia-1–dependent gene that is induced during pulmonary fibrosis. TNS1 plays an essential role in TGF-β–induced myofibroblast differentiation and myofibroblast-mediated formation of extracellular fibronectin and collagen matrix. Targeted disruption of TNS1 and associated signaling may provide an avenue to inhibit tissue fibrosis.
Keywords: megakaryoblastic leukemia-1, tensin 1, fibrillar adhesion, myofibroblast, extracellular matrix
Clinical Relevance
Pulmonary fibrosis is characterized by the formation of excessive extracellular matrix (ECM) by myofibroblasts. This study finds that the fibrillar adhesion molecule tensin (TNS) 1 is essential for myofibroblast differentiation and ECM deposition. Targeting TNS1 and its associated signaling may provide an avenue to inhibit tissue fibrosis.
Myofibroblasts play a key role in propagating fibrogenesis and tissue remodeling in both human and experimental pulmonary fibrosis. They are characterized by de novo expression of smooth muscle and matrix genes in response to profibrotic cytokines, such as transforming growth factor (TGF)-β. We have previously found that the transcription factor, megakaryoblastic leukemia (MKL)-1, and its nuclear target, serum response factor (SRF), are essential for mediating myofibroblast differentiation and the development of bleomycin-induced pulmonary fibrosis (1–3). Myofibroblasts have an enhanced capacity to deposit fibronectin and collagen matrix, independent of their expression of these proteins, compared with their undifferentiated counterparts (4). MKL1-dependent gene expression is essential in facilitating rapid fibronectin matrix formation by myofibroblasts (3, 4). However, it is not known which MKL1-dependent proteins are responsible for this effect.
Tensin (TNS) 1 is a multidomain protein that can interact with the actin cytoskeleton, bind β1-integrin, and serve as a scaffold for adhesion-related signaling (5). TNS1 is recruited to focal adhesion sites (6), where it interacts with the Asn-Pro-X-Tyr motif of the β1-integrin tail via its phosphotyrosine binding domain (7). TNS1 also interacts with several focal adhesion–related kinases, including focal adhesion kinase (FAK), p130 Crk-associated substrate, and phosphoinositide 3-kinase, via its proto-oncogene tyrosine-protein kinase Src homology 2 domain (8). TNS1 is extensively phosphorylated at tyrosine and serine/threonine sites in response to integrin activation by the extracellular matrix (ECM) (9, 10). TNS1 is a component of specialized cell adhesions (fibrillar adhesions) that are critical for the formation of a fibrillar fibronectin matrix (11) and has been implicated in cell migration (12). Recent studies have found a link between a single-nucleotide polymorphism in TNS1 and lung function decline (13). Furthermore, the promoter region of TNS1 was reported to contain at least one potential SRF-binding motif, the so-called CC(A/T)6GG element (14). For these reasons, we hypothesized that TNS1 may play a role in formation of nascent ECM by myofibroblasts, and may be under the control of MKL1/SRF. In this study, we have found that TNS1 is a novel MKL1/SRF–dependent gene that is up-regulated during myofibroblast differentiation and in fibrotic lung. Furthermore, TNS1 plays a key role in both facilitating myofibroblast differentiation and the ability of myofibroblasts to form nascent fibronectin and collagen matrix.
Materials and Methods
Isolation and Primary Culture of Human Pulmonary Fibroblasts
Deidentified tissue samples of normal (nonfibrotic) and fibrotic (idiopathic pulmonary fibrosis [IPF]) lungs were obtained from thoracic surgical resection specimens through Carbone Cancer Center Translational Science BioCore at the University of Wisconsin–Madison (Madison, WI), under Institutional Review Board approval (no. 2011-0840). Human lung fibroblasts (HLFs) were isolated from resection specimens, as described in the online supplement and previously (4).
Western Blot
Whole cell lysis and subsequent Western blot was performed as described in the online supplement and previously (3). Densitometry was performed using ImageJ (Bethesda, MD) (15).
Deoxycholate Extraction
Deoxycholate extraction was performed as described in the online supplement and previously (4).
Small Interfering RNA Knockdown Assays
Before transfection, HLFs were plated at 5 × 104 cells/ml for 24 hours, reaching 70–80% confluency by the time of transfection. Small interfering RNA (siRNA) (Qiagen, Valencia, CA) was transfected using RNAiMAX transfection reagent (13778; Life Technologies, Carlsbad, CA) diluted in Opti-MEM (31985062; Gibco/Life Technologies, Carlsbad, CA) with 1 μl RNAiMAX per 10 pmol of siRNA. Predetermined concentrations of siRNA were used to achieve sufficient knockdown. Cells were incubated for 24 hours, serum starved, stimulated as indicated, and analyzed using real-time PCR, Western blot, or immunocytochemistry. All siRNA sequences were from Qiagen (Hilden, Germany). Sequences are listed in the online supplement.
Immunofluorescence Staining
Immunofluorescence staining was performed as described in the online supplement and previously (4).
Quantification of Focal and Fibrillar Adhesions
Images of immunofluorescence staining were taken at ×60 magnification with five images per condition. Images were analyzed using ImageJ. To determine average focal or fibrillar adhesion lengths, 15 adhesion lengths were measured per image, and the average taken. To determine average number of focal or fibrillar adhesions, numbers of adhesions were counted per image and divided by the cell count.
Fibronectin Assembly Assay
FN assembly assay was performed as per the online supplement and previously (4).
Histology
Histological analysis against TNS1 antibody was performed as described in the online supplement and previously (3). Primary antibody (1:200) was applied for 1 hour before incubation with horseradish peroxidase–conjugated secondary antibody and nuclear staining.
Quantitative Real-Time RT-PCR
Real-time PCR was performed as before (16). Briefly, pretreated cells were lysed in 1 ml RNA STAT-60 (AMS Biotechnology, Milton, Abingdon, UK) to extract total RNA. iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) was used for random-primed reverse transcription of 1 μg of total RNA. iTaq SYBR Green Supermix (Bio-Rad) was used to perform real-time PCR analysis in an ABI 7,500 multicolor real-time PCR detection system (Applied Biosystems, Foster City, CA). Primers are listed in the online supplement. Data are expressed as fold difference using the comparative cycle threshold method.
Statistical Analysis
For all experiments, data are representative of at least three independent experiments. Quantitative data, when comparing two groups, were analyzed by the Student’s paired t test. When more than two treatments or time points were compared, outcome measures were compared using mixed-effect ANOVA models with fixed effects for treatment or time and random effects for experiment to account for within-experiment correlation. Tukey’s procedure was used to control the overall significance level for multiple comparisons.
Results
TNS1 Expression Is Increased in Myofibroblasts and in Fibrotic Lung
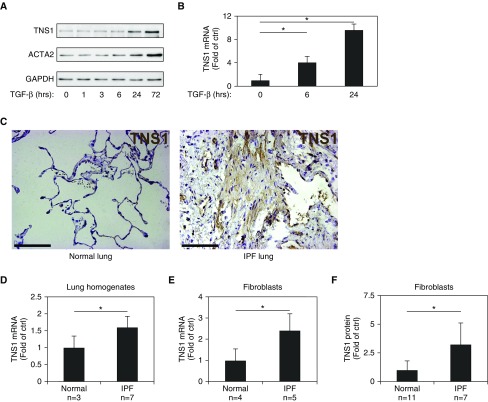
TNS1 expression has been observed in the lung (UniGene EST profile Hs.471381; The National Center for Biotechnology Information, Bethesda, MD, and Reference 17); however its precise localization has not been established. To begin to evaluate the role of TNS1 in myofibroblast biology, we used normal HLFs and assessed the expression of TNS1. As shown in Figure 1A, basal expression of TNS1 under serum-starved conditions was very low, whereas treatment of primary HLFs cultures with TGF-β strongly induced TNS1 protein expression, with similar kinetics to that of the myofibroblast marker gene, smooth muscle α-actin (ACTA2). The induction of TNS1 by TGF-β appeared to be, at least in part, transcriptionally regulated, given the induction of TNS1 mRNA at 6 and 24 hours (Figure 1B). Given the induction of TNS1 by TGF-β, we assessed TNS1 expression in normal and fibrotic lung tissue with immunohistochemistry (Figure 1C). In normal lung (left panel), faint staining was present in the alveolar septa, whereas, in fibrotic lung (right panel), TNS1 expression was more strongly increased in bands of spindle-shaped cells and in areas that morphologically appeared as fibroblastic foci. There was also a significant increase in TNS1 mRNA expression in homogenates of IPF lung compared with normal lung (Figure 1D). Isolated fibroblasts derived from IPF lungs showed increased levels of TNS1 mRNA and protein compared with fibroblasts derived from nonfibrotic lungs (Figures 1E and 1F). Together, these data point to expression of TNS1 within the mesenchymal compartment of the lung and support a transcriptionally regulated increase of TNS1 in myofibroblasts under conditions of TGF-β stimulation or during in vivo fibrosis.
Figure 1.
Tensin (TNS) 1 is up-regulated in myofibroblasts and in idiopathic pulmonary fibrosis (IPF) lung. (A) Western blot of human lung fibroblast (HLF) lysates after 1 ng/ml transforming growth factor (TGF)-β stimulation at indicated time points against TNS1 and smooth muscle α-actin (ACTA2). (B) TNS1 mRNA expression in normal HLFs after 6- or 24-hour treatment with 1 ng/ml TGF-β and compared with time 0 controls (unstimulated). Mixed-effect ANOVA (*P < 0.05) was used for statistical analysis. (C) Immunohistochemical staining of normal and IPF lung samples against TNS1. Scale bars, 100 μm. (D) Total TNS1 mRNA in normal and IPF lung homogenates. Student’s t test (*P < 0.05) was used for statistical analysis. (E and F) Total TNS1 mRNA (E) or protein (F) in lysates from normal and IPF-derived lung fibroblasts. Student’s t test (*P < 0.05) was used for statistical analyses. Data are presented as means (±SD). Ctrl, control.
TGF-β–Induced TNS1 Expression Requires Signaling through TGF-β Receptor I, but not Smads
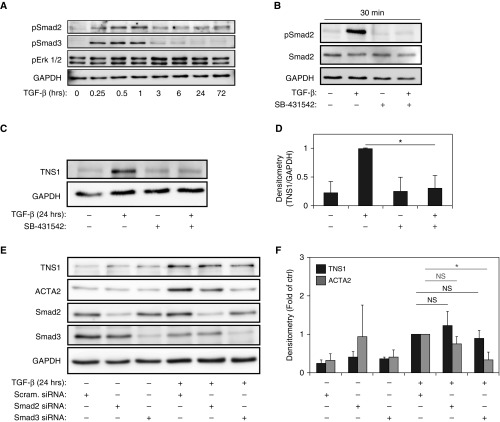
Due to the observed induction of TNS1 by TGF-β, we first investigated the potential involvement of TGF-β receptor/smad-signaling in the regulation of TNS1 gene expression. Figure 1A demonstrates the kinetic response of TNS1 expression after TGF-β stimulation, which is similar to that of ACTA2. Figure 2A, shows the signaling response to TGF-β, with a peak in Smad2 phosphorylation that is observed after 30 minutes of stimulation, with a decrease, but not complete resolution, of Smad2 phosphorylation by 24 and 72 hours. Smad3 phosphorylation has a similar peak at 30 minutes, but completely resolves by 6 hours after stimulation. Of note, no significant induction of extracellular signal-regulated protein kinases 1 and 2 (Erk1/2) was observed in response to TGF-β in our system, although some baseline phosphorylation was evident. Given the observed induction of Smad2/3 phosphorylation to TGF-β stimulation, we hypothesized that TGF-β–induced TNS1 expression was likely occurring via the TGF-β II receptor interactions with the TGF-β type I receptor kinase (activin receptor–like kinase 5). To test this hypothesis, we used the small-molecule inhibitor of activin receptor–like kinase 5, SB431542 (18). As shown in Figure 2B, treatment with SB431542 fully inhibits TGF-β receptor–mediated Smad2 phosphorylation at the 30-minute poststimulation peak. As shown in Figures 2C and 2D, pretreatment with SB431542 fully inhibits TGF-β–induced TNS1 expression, suggesting that TGF-β type I receptor–mediated signaling is required for TNS1 expression. Canonical TGF-β–induced signaling via the TGF-β type I receptor uses the receptor-associated Smads, Smad2 and Smad3 (19). To investigate the role of Smad2 and Smad3 in mediating TGF-β–induced TNS1 expression, we used siRNA-mediated knockdown of each molecule. As shown in Figures 2E and 2F, we unexpectedly found that knockdown of either Smad2 or Smad3 did not alter the expression of TNS1 in response to TGF-β stimulation. In contrast, the TGF-β–induced expression of ACTA2 is significantly attenuated by knockdown of Smad3, but not Smad2, consistent with the findings of Hu and colleagues (20) in rat lung fibroblasts supporting the role of Smad3 in the transcriptional regulation of ACTA2. Interestingly, Smad2 knockdown also increased ACTA2 expression in the absence of TGF-β stimulation (P = 0.04). From these data, we concluded that TNS1 expression requires TGF-β type I receptor activation in response to TGF-β, but that the downstream signaling is independent of the receptor-associated Smad 2/3.
Figure 2.
TGF-β–induced TNS1 expression requires signaling through TGF-β receptor I but not Smads. (A) Western blot of HLF lysates after stimulation with TGF-β (1 ng/ml) at indicated time points against phosphorylated (p) Smad2, pSmad3, and phosphorylated extracellular signal-regulated kinases 1 and 2 (pErk1/2). (B) HLFs were treated with 1 ng/ml TGF-β for 30 minutes in the presence of 10 μM SB531542 or vehicle control, followed by Western blotting for the indicated (phospho) proteins. (C and D) HLFs were treated with 1 ng/ml TGF-β for 24 hours in the presence of 10 μM SB531542 or vehicle control, followed by Western blotting for TNS1 (C) and subsequent densitometry (D). (E and F) HLFs were transfected with Smad2 or Smad3 small interfering (si)RNA or scrambled control followed by treatment with 1 ng/ml TGF-β for 24 hours and Western (E) blotting and densitometry (F) for the indicated proteins. Mixed-effect ANOVA (*P < 0.05; NS, not significant) was used for all statistical analyses. Data are presented as means (± SD). scram., scrambled.
TGF-β–Induced TNS1 Expression Is Mediated by Rho Coiled-Coiled Kinase/Actin/MKL1
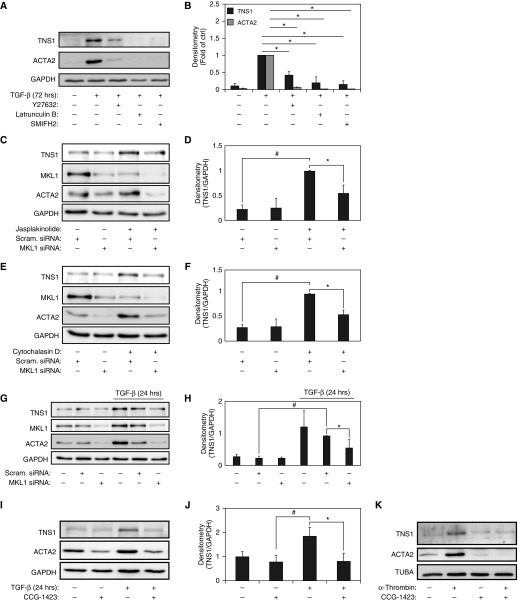
In addition to Smad signaling, TGF-β receptor I can induce the activation of the Rho GTPases and their effector kinase Rho coiled-coiled kinase (ROCK) 1, leading to actin cytoskeletal polymerization, independent of Smad signaling (21). Our group has previously shown that the delayed kinetics of ACTA2 in response to TGF-β results from ROCK and actin-regulated MKL1/SRF activation (2, 16). Thus, we hypothesized that the TGF-β–induced TNS1 expression in myofibroblasts may also be dependent on ROCK/actin/MKL1/SRF signaling, similar to ACTA2. To test this possibility, we examined the role of ROCK in mediating TGF-β–induced TNS1 expression using the small-molecule inhibitor of ROCK1/2, Y27632 (22). Similarly, we assessed the role of actin-dependent signaling in TGF-β–induced TNS1 expression by directly disrupting actin polymerization with latrunculin B or with the formin homology 2 domain inhibitor, 1-(3-bromophenyl)-5-(2-furanylmethylene)dihydro-2-thioxo-4,6(1H,5H)-pyrimidinedione (23). As shown in Figures 3A and 3B, pretreatment with Y27632, latrunculin B, or 1-(3-bromophenyl)-5-(2-furanylmethylene)dihydro-2-thioxo-4,6(1H,5H)-pyrimidinedione inhibited TGF-β–induced TNS1 expression in HLFs, demonstrating that ROCK activation and actin polymerization are required for TNS1 inducibility by TGF-β. To further explore the role of actin polymerization in mediating TNS1 expression, we used jasplakinolide, a known inducer of actin polymerization (24). As shown in Figures 3C and 3D, treatment with jasplakinolide reliably induces the expression of TNS1, similar to its known ability to induce ACTA2 expression (2). Polymerization of actin into filaments by jasplakinolide depletes the pool of monomeric actin, thereby decreasing the sequestration of MKL1 in the cytosol by monomeric actin, allowing it to translocate to the nucleus and activate transcription (25, 26). Thus, treatment with jasplakinolide can also be used to activate MKL1 independently of TGF-β. As shown in Figures 3C and 3D, siRNA-mediated knockdown of MKL1 inhibited the induction of jasplakinolide-induced TNS1 expression, demonstrating that MKL1 is required for TNS1 expression. Similarly, cytochalasin D, a fungal metabolite that can disrupt the actin cytoskeleton (27), has been shown to disrupt monomeric actin–MKL1 association, thereby enabling MKL1 translocation and activation of SRF (25, 26). Treatment with cytochalasin D resulted in a similar induction in TNS1 expression (Figures 3E and 3F). As in Figures 3C and 3D, we confirmed the role of MKL1 in this effect by using siRNA-mediated knockdown of MKL1. As shown in Figures 3E and 3F, siRNA-mediated knockdown of MKL1 resulted in the loss of cytochalasin D–induced TNS1 expression, further supporting the role of MKL1 in TNS1 expression.
Figure 3.
TGF-β–induced TNS1 expression is mediated by Rho coiled-coiled kinase (ROCK)/actin/megakaryoblastic leukemia (MKL)-1. (A and B) HLFs were treated with 1 ng/ml TGF-β for 72 hours in the presence of the ROCK inhibitor Y27632 (10 μM), the actin filament disruptor latrunculin B (500 nM), the formin inhibitor 1-(3-bromophenyl)-5-(2-furanylmethylene)dihydro-2-thioxo-4,6(1H,5H)-pyrimidinedione (SMIFH2) (20 μM), or vehicle control, followed by Western blotting for TNS1 and ACTA2 (A) and densitometry of the indicated bands and compared with time 0 controls (unstimulated) (B). (C–F) HLFs were transiently transfected with MKL1 siRNA or scrambled control before a 14-hour treatment with either the MKL1 actin disruptor cytochalasin D (2 μM [C and D]), or the actin filament stabilizer jasplakinolide (300 nM [E and F]), followed by Western blotting and densitometry. (G and H) Western blot analysis and densitometry of TNS1 and MKL1 expression in HLF lysates after transient transfection with siRNA against MKL1 or scrambled control, and subsequent treatment with TGF-β (1 ng/ml) for 24 hours. (I and J) HLFs were treated with 1 ng/ml TGF-β for 24 hours in the presence of the MKL1 inhibitor N-[2-[4(4-chlorophenyl)amino]-1-methyl-2-oxoethoxy]-3,5-bis(trifluoromethyl)-benzamide (CCG-1423) (10 μM) or vehicle control, followed by Western blotting and densitometry of TNS1. (K) HLFs were treated with 0.5 U/μl human α-thrombin for 24 hours in the presence of the MKL1 inhibitor CCG-1423 (10 μM) or vehicle control, followed by Western blotting against TNS1 and ACTA2. Mixed-effect ANOVA (* and #P < 0.05) was used for all statistical analyses. * and # symbols designate significant difference between the indicated conditions. Data are presented as means (±SD). TUBA, α-tubulin.
To determine if MKL1 was also required for TNS1 expression in response to TGF-β stimulation, we again used siRNA against MKL1. As shown in Figures 3G and 3H, siRNA-mediated knockdown of MKL1 results in loss of TNS1 expression under TGF-β stimulation compared with scrambled siRNA control. Little effect on basal TNS1 expression was seen with MKL1 knockdown. As an alternative approach, we used N-[2-[4(4-chlorophenyl)amino]-1-methyl-2-oxoethoxy]-3,5-bis(trifluoromethyl)-benzamide (CCG-1423), a small-molecule inhibitor of MKL1/SRF, which disrupts the ability of MKL1 to translocate to the nucleus and activate SRF (1, 28). Figures 3I and 3J demonstrate that incubation with CCG-1423 results in a significant decrease in TGF-β–induced TNS1 expression in HLFs. Finally, because MKL1/SRF can also be directly activated by G protein–coupled receptor ligands, including human α-thrombin (26, 29, 30), we examined the effect of human α-thrombin on TNS1 expression. α-Thrombin signals via G protein–coupled protease activated receptor-1 to induce SRF and myofibroblast differentiation (31). We observed induction of TNS1 expression in response to 24-hour stimulation with α-thrombin, which was inhibited by CCG-1423, suggesting that MKL1/SRF is similarly required for α-thrombin–induced TNS1 expression (Figure 3G). Because α-thrombin can also activate TGF-β via integrin-mediated tension-dependent elaboration of latent TGF-β from the ECM (32, 33), we examined Smad phosphorylation by α-thrombin. Very little induction of Smad phosphorylation was observed in response to α-thrombin stimulation during the selected time points up to 24 hours, even with extremely long exposure times (300–400 s, Figure E1), indicating that TGF-β was less likely to be a significant contributor to the effects of α-thrombin in this case, consistent with previously published results in a similar experimental system (31). Altogether, these results show that signaling via ROCK, actin polymerization, and MKL1/SRF activation are essential for TNS1 expression in HLFs.
TNS1 Is Essential for Myofibroblast Differentiation
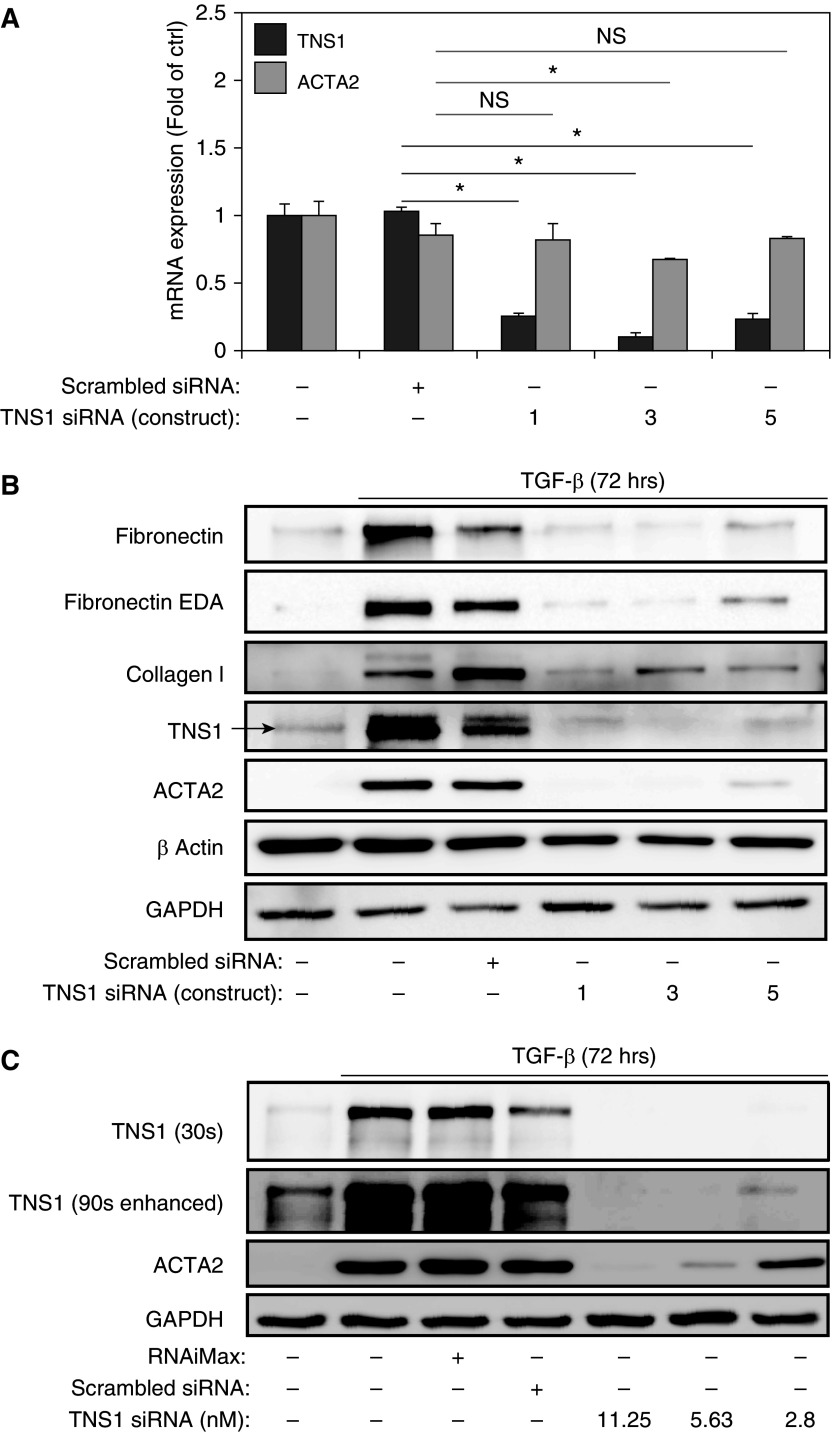
Considering the increased TNS1 expression observed in differentiated myofibroblasts, we then sought to determine whether loss of TNS1 would inhibit myofibroblast differentiation or function. As shown in Figure 4A, we were able to specifically and efficiently knock down TNS1 using an siRNA approach with three distinct siRNA constructs, with no effect on basal ACTA2 expression using constructs 1 and 5. We then used this approach to knock down TNS1 in HLFs during stimulation with TGF-β for 72 hours. We found that, under TGF-β treatment for extended periods of time, we occasionally observed a doublet signal for TNS1 (Figure 4B). The appearance of a second, higher apparent molecular weight band could potentially represent electrophoretic mobility shift due to increased phosphorylation, given the presence of abundant serine/threonine and tyrosine phosphorylation sites on TNS1, including a report of increased phosphorylation at Y1404 in response to TGF-β (9, 34). Unexpectedly, we observed that knockdown of TNS1 using any of these siRNA constructs resulted in loss of TGF-β–induced myofibroblast differentiation, as determined by ACTA2 expression (Figure 4B). Similar effects were seen with collagen, fibronectin, and extra type III domain A containing fibronectin expression. Titration with decreasing siRNA concentrations resulted in the return of some TNS1 expression (see overexposed and contrast-enhanced blot in Figure 4C), which was associated with the partial return of ACTA2 expression in response to TGF-β (Figure 4C). From this, we concluded that a basal amount of TNS1 expression is required to mediate myofibroblast differentiation in response to TGF-β.
Figure 4.
TNS1 is essential for myofibroblast differentiation. (A) Total TNS1 and ACTA2 mRNA expression in HLFs upon TNS1 knockdown using three different siRNA constructs (or treatment with scrambled control). Mixed-effect ANOVA (*P < 0.05; NS, not significant) was used for statistical analysis. (B) HLFs were transfected with the indicated TNS1 siRNAs or scrambled control, followed by treatment with 1 ng/ml TGF-β for 72 hours and Western blotting for the indicated proteins. Arrow indicates the expected TNS1 band. (C) Dose–response of TGF-β–induced (1 ng/ml for 72 h) ACTA2 expression to varying concentrations of TNS1 siRNA. The top TNS1 blot was exposed for a typical length of time (30 s) without saturation of the bands. The lower TNS1 blot was overexposed with increased contrast to visualize low-level TNS1 expression under low concentrations of TNS1 siRNA. Data are presented as means (±SD). EDA, extra domain A.
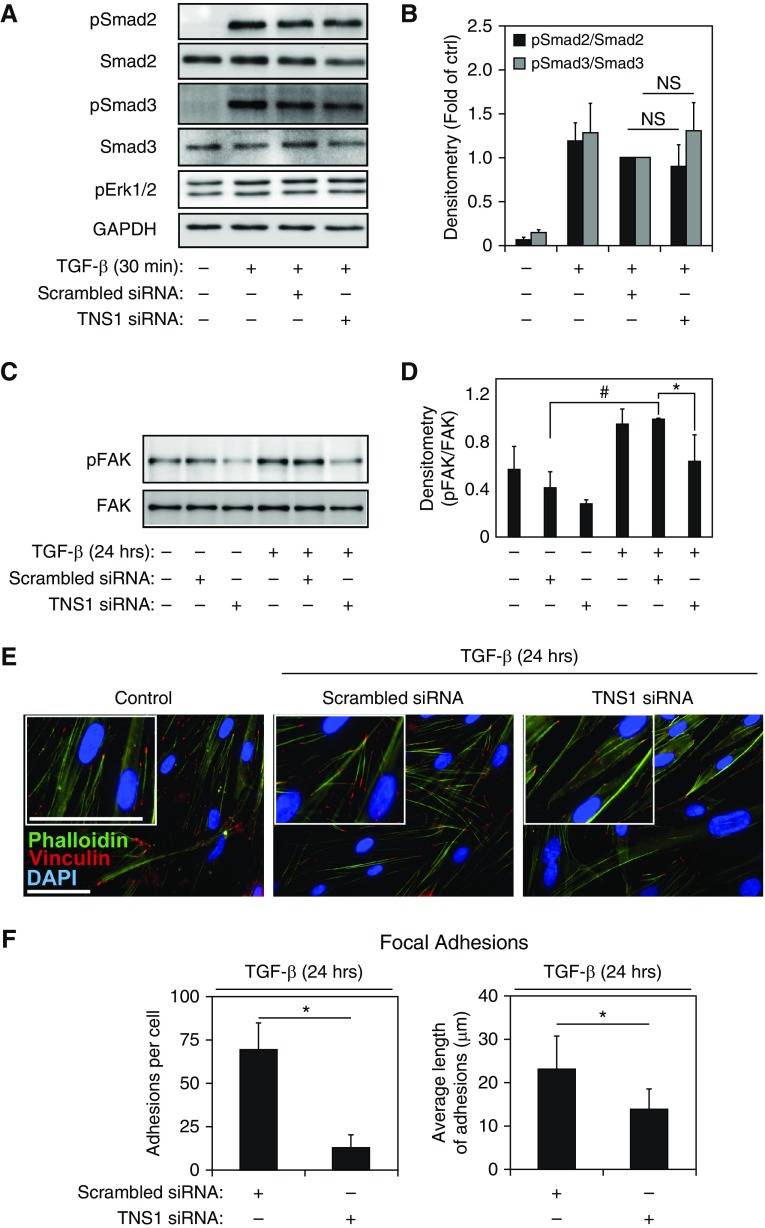
TNS1 Is Dispensable for TGF-β Signaling, but Required for Focal Adhesion–Dependent Signals and Actin Polymerization
As myofibroblast differentiation was inhibited by loss of TNS1 expression, we examined whether TGF-β receptor I-associated signaling was disrupted by loss of TNS1. As shown in Figures 5A and 5B, loss of TNS1 did not affect TGF-β–induced Smad2 or Smad3 phosphorylation at 30 minutes, which is the point of peak Smad phosphorylation in response to TGF-β (Figure 2A). Similarly, we assessed the effect of TNS1 knockdown on Smad 2/3 phosphorylation after 24 hours and found no effect (Figure E2). In addition, although there was no induction of Erk1/2 phosphorylation by TGF-β (Figure 2A), we assessed the effect of TNS1 knockdown on basal Erk1/2 phosphorylation. As shown in Figure 2A, there was no effect on Erk1/2 phosphorylation status. These results strongly suggest that the effect of TNS1 on TGF-β–induced myofibroblast differentiation is independent of Smad and Erk signaling.
Figure 5.
TNS1 is dispensable for TGF-β signaling but required for focal adhesion–dependent signals. (A and B) HLFs were transfected with TNS1 siRNA or scrambled control followed by treatment with 1 ng/ml TGF-β for 30 minutes and Western blotting and densitometry for the indicated (phospho) proteins. Mixed-effect ANOVA (*P < 0.05; NS, not significant) was used for statistical analysis. (C and D) HLFs were transfected with TNS1 siRNA or scrambled control, followed by treatment with 1 ng/ml TGF-β for 24 hours, Western blotting, and densitometry for the phosphorylated tyrosine 397 residue of focal adhesion kinase (FAK). Mixed-effect ANOVA (* and #P < 0.05) was used for statistical analysis. (E) Merged immunocytochemistry images of HLFs stained against phalloidin (green) and vinculin (red) upon TNS1 knockdown using TNS1 siRNA (or treatment with scrambled control) and treatment with 1 ng/ml TGF-β for 24 hours. Scale bar, 50 μm. Insets show digital magnifications of vinculin staining for each condition. Scale bar, 50 μM. (F) Quantitation of number and length of focal (vinculin-containing) adhesions in TGF-β–induced (1 ng/ml for 24 h) myofibroblasts under siRNA-mediated TNS1 knockdown or scrambled control. Student’s t test (*P < 0.05) was used for statistical analysis. * and # symbols designate significant difference between the indicated conditions. Data are presented as means (±SD). DAPI, 4′,6-diamidino-2-phenylindole.
Thannickal and colleagues (35) demonstrated that FAK phosphorylation at tyrosine 397 (Tyr397) in response to TGF-β peaks by 24 hours and that phosphorylation at this residue is required for myofibroblast differentiation. We observed that knockdown of TNS1 resulted in loss of TGF-β–induced FAK phosphorylation at the Tyr397 residue (Figures 5C and 5D) in HLFs. This may account for the inhibitory effect of TNS1 depletion on myofibroblast differentiation. As TNS1 can also localize to focal adhesions (6, 36), we examined the effect of loss of TNS1 on focal adhesion formation in HLFs treated with TGF-β. TGF-β–treated cells that had siRNA-mediated knockdown of TNS1 had fewer, smaller vinculin-containing focal adhesions (Figures 5E and 5F and insets). Staining with phalloidin, which stains all actin isoforms (including ubiquitously expressed β and γ actin), shows an apparent increase in the amount of actin stress fibers in TGF-β–treated HLFs compared with untreated cells, consistent with our previous observations (2). Knockdown of TNS1 expression in TGF-β–treated HLFs resulted in less apparent staining of actin stress fibers compared with the scrambled siRNA control, although some residual diffuse cytosolic staining was still observed (Figure 5E and insets). These results suggest that TNS1 may be important for maturation of focal adhesions and actin cytoskeletal remodeling (37), along with the recruitment and phosphorylation of FAK during myofibroblast differentiation.
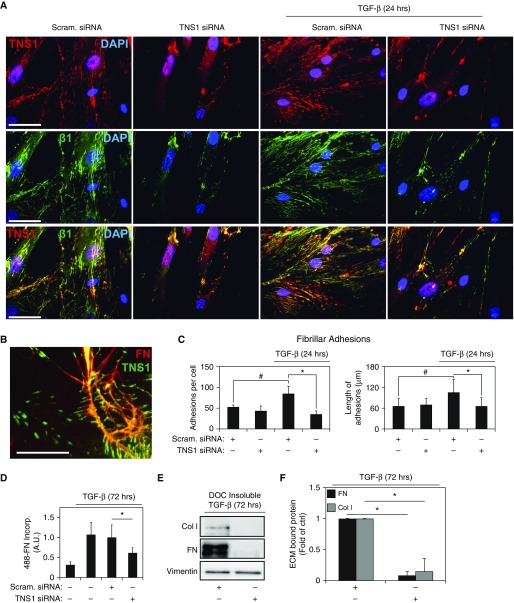
TNS1 Expression Is Required for Fibrillar Adhesion Formation and ECM Deposition in Myofibroblasts
Fibrillar adhesions mediate the formation of nascent fibronectin matrix and are comprised of TNS1 in association with α5β1 integrins (38). We assessed the development of these adhesions under conditions of TNS1 depletion. Figures 6A and 6B show immunofluorescent staining of normal HLFs, demonstrating that TNS1 is localized to both extended plaques with a large aspect ratio (>7:1) and smaller aspect ratio plaques corresponding to fibrillar adhesions and mature classical focal adhesions, respectively (39). In the elongated plaques, TNS1 colocalized with activated β1-integrin, as determined by immunostaining with the 12G10 β1-integrin antibody (Figure 6A, row 3). High-magnification image of TNS1 and fibronectin staining in myofibroblasts demonstrates that TNS1-containing elongated adhesions closely associate with fibrillar fibronectin (Figure 6B, right half of image), also consistent with the morphology of fibrillar adhesions (38, 39), demonstrating the interaction of these structures with the extracellular fibrillar fibronectin matrix. The number of elongated TNS1-containing plaques (fibrillar adhesions) increased upon TGF-β stimulation (Figures 6A and 6C). In TGF-β–treated HLFs, siRNA-mediated knockdown of TNS1 resulted in a decreased number and length of fibrillar adhesions (Figures 6A and 6C), supporting an essential role for TNS1 in the development of these adhesions.
Figure 6.
TNS1 is required for fibrillar adhesion formation and extracellular matrix (ECM) deposition by myofibroblasts. (A) Coimmunostaining for TNS1 and β1-integrin in HLFs transfected with TNS1 siRNA or scrambled control, followed by treatment with 1 ng/ml TGF-β for 24 hours. Scale bars, 50 μm. Immunostaining for the row is indicated in the images of the left column. (B) Coimmunostaining for TNS1 (green) and fibronectin (red, extracellular fibrils) in HLFs. Image digitally zoomed for clarity. Scale bar, 25 μm. (C) Quantitation of number and length of fibrillar (activated β1-integrin–containing) adhesions in TGF-β–induced (1 ng/ml, 24 h) myofibroblasts under siRNA-mediated TNS1 knockdown or scrambled control. Mixed-effect ANOVA (* and #P < 0.05) was used for statistical analysis. (D) HLFs, transfected with TNS1 siRNA or scrambled control, were treated with 1 ng/ml TGF-β for 72 hours, then replated and incubated with Alexa488-labeled fibronectin (488-FN) for 90 minutes, followed by fixation and immunofluorescent microscopy. Mixed-effect ANOVA (* and #P < 0.05) was used for statistical analysis. (E and F) HLFs were transfected with TNS1 siRNA or scrambled control, treated with 1 ng/ml TGF-β for 72 hours, followed by deoxycholate (DOC) buffer extraction to remove non–ECM-bound material. Residual ECM was then solubilized and run via PAGE followed by Western blotting and densitometry of the indicated proteins. Data show reduced DOC-insoluble fibronectin (FN) or collagen I (Col I) fractions. Mixed-effect ANOVA (*P < 0.05) was used for statistical analysis. * and # symbols designate significant difference between the indicated conditions. Data are presented as means (±SD). A.U., arbitrary units.
Given the requirement for TNS1 to form fibrillar adhesions, we then assessed the impact of TNS1 depletion on the formation of nascent ECM. As shown in Figure 6D, siRNA-mediated loss of TNS1 in TGF-β–treated HLFs also resulted in a significant decrease in the assembly of a fibrillar fibronectin matrix from Alexa488-labeled, soluble fibronectin. Using a complimentary approach, we assessed the ability of HLFs to form a deoxycholate-insoluble ECM under conditions of TNS1 knockdown. As shown in Figures 6E and 6F, loss of TNS1 expression resulted in marked attenuation of TGF-β–treated HLF-mediated deposition of fibronectin and collagen into the ECM. These results demonstrate that TNS1 expression plays an essential role in the induction of fibrillar adhesion formation and resulting fibronectin (and collagen) matrix formation by myofibroblasts.
Discussion
A critical function of myofibroblasts is the formation of nascent ECM during the development of pulmonary fibrosis. In our previous studies, we have found that myofibroblasts are characterized by an enhanced ability to form a fibrillar fibronectin matrix, which serves as a scaffold for the incorporation of additional matrix elements (4). In our current study, we have now found that a key component of the fibrillar adhesion, TNS1, is strongly up-regulated by the profibrotic cytokine, TGF-β, and has increased expression in the fibroblastic focus of the fibrotic lung. TNS1 is a multidomain protein that can interact with both the actin cytoskeleton and integrin-containing adhesion plaques, and serve as a scaffold for adhesion-related signaling molecules (40, 41). Because of the putative role of TNS1 in the formation of fibrillar adhesions and the formation of fibronectin matrix, we sought to characterize the biology of this protein in the context of myofibroblast function.
We have found that TNS1 expression is strongly induced by TGF-β in HLFs and is expressed more strongly in spindle-shaped cells in areas of fibroblastic foci in fibrotic lung. Similar to the myofibroblast marker, ACTA2, our work suggests that TNS1 is transcriptionally regulated by signaling via ROCK/actin/MKL1/SRF in response to TGF-β. In contrast to ACTA2, however, there does not appear to be a dependency on Smad2 or Smad3. Signaling via ROCK/actin/MKL1/SRF mediates myofibroblast differentiation in response to G protein–coupled receptor agonists, TGF-β, and biophysical cues, such as matrix stiffness (2, 42, 43). MKL1 is required for the development of experimental fibrosis, likely via its role in determining fibroblast cell fate, antiapoptotic signaling, and matrix remodeling (3, 44, 45). Thus, TNS1 up-regulation may be a key component of MKL1-regulated genes in response to profibrotic cues.
In addition, we unexpectedly found that myofibroblast differentiation in response to TGF-β was inhibited by loss of TNS1. Myofibroblast-associated genes that were examined included ACTA2, along with collagen I, fibronectin, and extra type III domain A–fibronectin. Each of these markers was strongly attenuated by loss of TNS1. TNS1 is known to interact with phosphorylated FAK via its proto-oncogene tyrosine-protein kinase Src homology 2 domain (8), and we found that loss of TNS1 results in attenuation of phosphorylation of FAK at the Tyr397 residue. Because phosphorylation of FAK at Tyr397 is required for myofibroblast differentiation (35), the observed loss of FAK phosphorylation could account for inhibition of myofibroblast differentiation upon TNS1 depletion. These data suggest that TNS1 may serve as a scaffold for recruitment of FAK and other adhesion-associated proteins to the enlarging adhesion plaques during myofibroblast differentiation (46). This role fits with the morphologic alterations that we observed in TNS1-deficient cells. TGF-β–treated HLFs in which TNS1 was depleted were slightly less spread than normal myofibroblasts, had a reduction in the number and size of focal adhesions, and had a less robust actin cytoskeleton. Previous work has established that TNS1 is recruited to nascent adhesions upon integrin ligation along with FAK (6). TNS1 interacts with β1-integrin at its Asn-Pro-X-Tyr domain and interacts with actin filaments via its actin binding domains (7, 40). In this fashion, TNS1 serves as a linker protein coupling the actin cytoskeleton to the ECM-bound integrins. Our studies suggest that increased expression of TNS1 during myofibroblast differentiation results in its recruitment to focal adhesion sites, thereby facilitating both the enlargement of focal adhesions and the formation of actin stress fibers that couple to these enlarged adhesions. MKL1/SRF is required for the proper formation of adhesions in embryonic cells and mouse fibroblasts (47, 48). Thus, our work suggests that TNS1 is a key MKL1/SRF–regulated protein that facilitates focal adhesion development and maturation during myofibroblast differentiation.
In addition to its role in focal adhesion formation, TNS1 also plays a role in the formation of the specialized fibrillar adhesions that promote extension of fibronectin polymers, thereby facilitating assembly of a fibronectin matrix (11). In response to stimulation with TGF-β, we have found that the number and length of fibrillar adhesions increases in HLFs. Loss of TNS1 significantly disrupted increases in fibrillar adhesion formation in response to TGF-β. This translates to loss of both fibronectin and collagen matrix formation by these myofibroblasts. This suggests that the increases in TNS1 expression during myofibroblast differentiation may be critical for the formation of new fibrillar adhesions and the accelerated fibronectin matrix assembly that we have previously observed in myofibroblasts (44). Taken together, our findings suggest that disruption of TNS1 and its associated signaling may be an attractive target to inhibit scar formation in vivo.
Acknowledgments
Acknowledgments
The authors thank the University of Wisconsin (UW) Carbone Cancer Center (Madison, WI) for use of its Shared Services to complete this research, as well as Drew Roenneburg and the UW-Madison Department of Surgery, Histology Core Facility for performing the histological staining.
Footnotes
This work was supported in part by National Institutes of Health (NIH)/NCI p30 CA14520-UW Comprehensive Cancer Center Support, and was supported by the NIH award K08 HL093367 (N.S.), the American Thoracic Society/Pulmonary Fibrosis Foundation/Coalition for Pulmonary Fibrosis Research Award (N.S.), University of Wisconsin Graduate School Research Funding (N.S.), and an American Heart Association Postdoctoral Fellowship (K.B.).
Author Contributions: N.S. conceived of and designed research; K.B., E.E.T., J.K.A., C.R.N., and N.S. performed experiments; K.B., E.E.T., M.D.E., C.R.N., and N.S. analyzed data and interpreted results of experiments; K.B., E.E.T., C.R.N., and N.S. prepared figures and drafted the manuscript; K.B., E.E.T., and N.S. edited and revised the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0104OC on December 22, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Sandbo N, Kregel S, Taurin S, Bhorade S, Dulin NO. Critical role of serum response factor in pulmonary myofibroblast differentiation induced by TGF-β. Am J Respir Cell Mol Biol. 2009;41:332–338. doi: 10.1165/rcmb.2008-0288OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandbo N, Lau A, Kach J, Ngam C, Yau D, Dulin NO. Delayed stress fiber formation mediates pulmonary myofibroblast differentiation in response to TGF-β. Am J Physiol Lung Cell Mol Physiol. 2011;301:L656–L666. doi: 10.1152/ajplung.00166.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernau K, Ngam C, Torr EE, Acton B, Kach J, Dulin NO, Sandbo N. Megakaryoblastic leukemia-1 is required for the development of bleomycin-induced pulmonary fibrosis. Respir Res. 2015;16:45. doi: 10.1186/s12931-015-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torr EE, Ngam CR, Bernau K, Tomasini-Johansson B, Acton B, Sandbo N. Myofibroblasts exhibit enhanced fibronectin assembly that is intrinsic to their contractile phenotype. J Biol Chem. 2015;290:6951–6961. doi: 10.1074/jbc.M114.606186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Ishii A, Wong WK, Chen LB, Lo SH. Molecular characterization of human tensin. Biochem J. 2000;351:403–411. [PMC free article] [PubMed] [Google Scholar]
- 6.Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- 7.McCleverty CJ, Lin DC, Liddington RC. Structure of the PTB domain of tensin1 and a model for its recruitment to fibrillar adhesions. Protein Sci. 2007;16:1223–1229. doi: 10.1110/ps.072798707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis S, Lu ML, Lo SH, Lin S, Butler JA, Druker BJ, Roberts TM, An Q, Chen LB. Presence of an SH2 domain in the actin-binding protein tensin. Science. 1991;252:712–715. doi: 10.1126/science.1708917. [DOI] [PubMed] [Google Scholar]
- 9.Hall EH, Balsbaugh JL, Rose KL, Shabanowitz J, Hunt DF, Brautigan DL. Comprehensive analysis of phosphorylation sites in tensin1 reveals regulation by p38MAPK. Mol Cell Proteomics. 2010;9:2853–2863. doi: 10.1074/mcp.M110.003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bockholt SM, Burridge K. Cell spreading on extracellular matrix proteins induces tyrosine phosphorylation of tensin. J Biol Chem. 1993;268:14565–14567. [PubMed] [Google Scholar]
- 11.Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. Integrin dynamics and matrix assembly: tensin-dependent translocation of α(5)β(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol. 2000;148:1075–1090. doi: 10.1083/jcb.148.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Duncan IC, Bozorgchami H, Lo SH. Tensin1 and a previously undocumented family member, tensin2, positively regulate cell migration. Proc Natl Acad Sci USA. 2002;99:733–738. doi: 10.1073/pnas.022518699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Repapi E, Sayers I, Wain LV, Burton PR, Johnson T, Obeidat M, Zhao JH, Ramasamy A, Zhai G, Vitart V, et al. Wellcome Trust Case Control Consortium; NSHD Respiratory Study Team. Genome-wide association study identifies five loci associated with lung function. Nat Genet. 2010;42:36–44. doi: 10.1038/ng.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Q, Chen G, Streb JW, Long X, Yang Y, Stoeckert CJ, Jr, Miano JM. Defining the mammalian CArGome. Genome Res. 2006;16:197–207. doi: 10.1101/gr.4108706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandbo N, Ngam C, Torr E, Kregel S, Kach J, Dulin N. Control of myofibroblast differentiation by microtubule dynamics through a regulated localization of mDia2. J Biol Chem. 2013;288:15466–15473. doi: 10.1074/jbc.M113.464461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo SH, Yu QC, Degenstein L, Chen LB, Fuchs E. Progressive kidney degeneration in mice lacking tensin. J Cell Biol. 1997;136:1349–1361. doi: 10.1083/jcb.136.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inman GJ, Nicolás FJ, Callahan JF, Harling JD, Gaster LM, Reith AD, Laping NJ, Hill CS. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 19.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 20.Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-β–induced α–smooth muscle actin expression. Am J Respir Cell Mol Biol. 2003;29:397–404. doi: 10.1165/rcmb.2003-0063OC. [DOI] [PubMed] [Google Scholar]
- 21.Vardouli L, Moustakas A, Stournaras C. LIM-kinase 2 and cofilin phosphorylation mediate actin cytoskeleton reorganization induced by transforming growth factor-beta. J Biol Chem. 2005;280:11448–11457. doi: 10.1074/jbc.M402651200. [DOI] [PubMed] [Google Scholar]
- 22.Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- 23.Rizvi SA, Neidt EM, Cui J, Feiger Z, Skau CT, Gardel ML, Kozmin SA, Kovar DR. Identification and characterization of a small molecule inhibitor of formin-mediated actin assembly. Chem Biol. 2009;16:1158–1168. doi: 10.1016/j.chembiol.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holzinger A. Jasplakinolide: an actin-specific reagent that promotes actin polymerization. Methods Mol Biol. 2009;586:71–87. doi: 10.1007/978-1-60761-376-3_4. [DOI] [PubMed] [Google Scholar]
- 25.Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell. 2003;113:329–342. doi: 10.1016/s0092-8674(03)00278-2. [DOI] [PubMed] [Google Scholar]
- 26.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 27.Casella JF, Flanagan MD, Lin S. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature. 1981;293:302–305. doi: 10.1038/293302a0. [DOI] [PubMed] [Google Scholar]
- 28.Evelyn CR, Wade SM, Wang Q, Wu M, Iñiguez-Lluhí JA, Merajver SD, Neubig RR. CCG-1423: a small-molecule inhibitor of RhoA transcriptional signaling. Mol Cancer Ther. 2007;6:2249–2260. doi: 10.1158/1535-7163.MCT-06-0782. [DOI] [PubMed] [Google Scholar]
- 29.Lockman K, Hinson JS, Medlin MD, Morris D, Taylor JM, Mack CP. Sphingosine 1-phosphate stimulates smooth muscle cell differentiation and proliferation by activating separate serum response factor co-factors. J Biol Chem. 2004;279:42422–42430. doi: 10.1074/jbc.M405432200. [DOI] [PubMed] [Google Scholar]
- 30.Mao J, Yuan H, Xie W, Simon MI, Wu D. Specific involvement of G proteins in regulation of serum response factor–mediated gene transcription by different receptors. J Biol Chem. 1998;273:27118–27123. doi: 10.1074/jbc.273.42.27118. [DOI] [PubMed] [Google Scholar]
- 31.Bogatkevich GS, Tourkina E, Silver RM, Ludwicka-Bradley A. Thrombin differentiates normal lung fibroblasts to a myofibroblast phenotype via the proteolytically activated receptor-1 and a protein kinase C–dependent pathway. J Biol Chem. 2001;276:45184–45192. doi: 10.1074/jbc.M106441200. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin–dependent TGF-beta activation and promotes acute lung injury. J Clin Invest. 2006;116:1606–1614. doi: 10.1172/JCI27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okayama A, Miyagi Y, Oshita F, Ito H, Nakayama H, Nishi M, Kurata Y, Kimura Y, Ryo A, Hirano H. Identification of tyrosine-phosphorylated proteins upregulated during epithelial–mesenchymal transition induced with TGF-β. J Proteome Res. 2015;14:4127–4136. doi: 10.1021/acs.jproteome.5b00082. [DOI] [PubMed] [Google Scholar]
- 35.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 36.Lo SH, An Q, Bao S, Wong WK, Liu Y, Janmey PA, Hartwig JH, Chen LB. Molecular cloning of chick cardiac muscle tensin: full-length cDNA sequence, expression, and characterization. J Biol Chem. 1994;269:22310–22319. [PubMed] [Google Scholar]
- 37.Sandbo N, Dulin N. Actin cytoskeleton in myofibroblast differentiation: ultrastructure defining form and driving function. Transl Res. 2011;158:181–196. doi: 10.1016/j.trsl.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zamir E, Katz M, Posen Y, Erez N, Yamada KM, Katz BZ, Lin S, Lin DC, Bershadsky A, Kam Z, et al. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol. 2000;2:191–196. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- 39.Zamir E, Katz BZ, Aota S, Yamada KM, Geiger B, Kam Z. Molecular diversity of cell-matrix adhesions. J Cell Sci. 1999;112:1655–1669. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- 40.Lo SH, Janmey PA, Hartwig JH, Chen LB. Interactions of tensin with actin and identification of its three distinct actin-binding domains. J Cell Biol. 1994;125:1067–1075. doi: 10.1083/jcb.125.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo SH, Weisberg E, Chen LB. Tensin: a potential link between the cytoskeleton and signal transduction. BioEssays. 1994;16:817–823. doi: 10.1002/bies.950161108. [DOI] [PubMed] [Google Scholar]
- 42.Huang X, Yang N, Fiore VF, Barker TH, Sun Y, Morris SW, Ding Q, Thannickal VJ, Zhou Y. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol. 2012;47:340–348. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haak AJ, Tsou PS, Amin MA, Ruth JH, Campbell P, Fox DA, Khanna D, Larsen SD, Neubig RR. Targeting the myofibroblast genetic switch: inhibitors of myocardin-related transcription factor/serum response factor–regulated gene transcription prevent fibrosis in a murine model of skin injury. J Pharmacol Exp Ther. 2014;349:480–486. doi: 10.1124/jpet.114.213520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sisson TH, Ajayi IO, Subbotina N, Dodi AE, Rodansky ES, Chibucos LN, Kim KK, Keshamouni VG, White ES, Zhou Y, et al. Inhibition of myocardin-related transcription factor/serum response factor signaling decreases lung fibrosis and promotes mesenchymal cell apoptosis. Am J Pathol. 2015;185:969–986. doi: 10.1016/j.ajpath.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123:1096–1108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C. α-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell. 2003;14:2508–2519. doi: 10.1091/mbc.E02-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schratt G, Philippar U, Berger J, Schwarz H, Heidenreich O, Nordheim A. Serum response factor is crucial for actin cytoskeletal organization and focal adhesion assembly in embryonic stem cells. J Cell Biol. 2002;156:737–750. doi: 10.1083/jcb.200106008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morita T, Mayanagi T, Sobue K. Reorganization of the actin cytoskeleton via transcriptional regulation of cytoskeletal/focal adhesion genes by myocardin-related transcription factors (MRTFs/MAL/MKLs) Exp Cell Res. 2007;313:3432–3445. doi: 10.1016/j.yexcr.2007.07.008. [DOI] [PubMed] [Google Scholar]