Abstract
The release of neutrophil extracellular traps (NETs) is a major immune mechanism intended to capture pathogens. These histone- and protease-coated DNA structures are released by neutrophils in response to a variety of stimuli, including respiratory pathogens, and have been identified in the airways of patients with respiratory infection, cystic fibrosis, acute lung injury, primary graft dysfunction, and chronic obstructive pulmonary disease. NET production has been demonstrated in the lungs of mice infected with Staphylococcus aureus, Klebsiella pneumoniae, and Aspergillus fumigatus. Since the discovery of NETs over a decade ago, evidence that “NET evasion” might act as an immune protection strategy among respiratory pathogens, including group A Streptococcus, Bordetella pertussis, and Haemophilus influenzae, has been growing, with the majority of these studies being published in the past 2 years. Evasion strategies fall into three main categories: inhibition of NET release by down-regulating host inflammatory responses; degradation of NETs using pathogen-derived DNases; and resistance to the microbicidal components of NETs, which involves a variety of mechanisms, including encapsulation. Hence, the evasion of NETs appears to be a widespread strategy to allow pathogen proliferation and dissemination, and is currently a topic of intense research interest. This article outlines the evidence supporting the three main strategies of NET evasion—inhibition, degradation, and resistance—with particular reference to common respiratory pathogens.
Keywords: neutrophil extracellular traps, immune evasion, deoxyribonuclease, Streptococcus
Clinical Relevance
The importance of neutrophil extracellular traps (NETs) in pathogen killing has received considerable attention, and it is now increasingly clear that many pathogens have evolved mechanisms to evade NET-mediated entrapment and killing. This article highlights this important and rapidly evolving field, and aims to alert respiratory scientists and clinicians to therapeutic opportunities to modulate pathogen–NET interactions.
What Are Neutrophil Extracellular Traps?
Neutrophil granulocytes, comprising over half of all circulating white blood cells, are terminally differentiated phagocytes that form a key first line of defense against invading microbes. Neutrophils engage invading microbes through a multitude of surface receptors, including antibody Fc receptors, complement receptors, and Toll-like receptors (TLRs), which recognize and respond to pathogen-associated molecular patterns (1). Once activated, neutrophils are responsible for phagocytic clearance, and can elaborate an arsenal of antimicrobial peptides, proteases, and reactive oxygen species (ROS) to achieve pathogen killing.
Our understanding of neutrophil “antipathogenic” mechanisms was broadened a decade ago with the finding that neutrophils can release extracellular networks of chromatin coated with granule proteins (2); this response could be triggered by a variety of infectious agents, including bacteria, viruses and parasites, host-derived cytokines, inflammatory mediators, and chemical factors, such as phorbol myristate acetate (PMA; Figure 1). The discovery of these so-called “neutrophil extracellular traps” (NETs) has catalyzed a growing body of research, and the ability of NETs to capture pathogens has been demonstrated in many in vivo contexts, restricting their movement and surrounding them in a microenvironment of concentrated antimicrobial agents (3). The classically described NET pathway commits neutrophils to a specialized form of cell death, “NETosis,” although some pathogens appear to induce a “vital NETosis,” where certain neutrophil chemotactic and phagocytic functions remain temporarily intact, even after the cell becomes anuclear (3).
Figure 1.
Neutrophil extracellular trap (NET) release. (1) Upon stimulation by a variety of triggers, including many pathogens, neutrophils release NETs into the extracellular space. (2) These structures are composed of DNA coated with histones and chromatin, and trap microbes in a concentrated environment of antimicrobial proteins, such as neutrophil elastase and myeloperoxidase. Insets are images showing (left) human NETs released by phorbol myristate acetate–stimulated neutrophils by fluorescence microscopy using Sytox Green (arrows), and (right) green-stained Salmonella typhimurium bacteria (arrows) trapped in murine NETs. (3) As well as exerting antimicrobial effects, NETs may play a proinflammatory role contributing to the pathogenesis of conditions, such as acute lung injury and venous thromboembolism.
In the classical “suicidal pathway” of NETosis, agonists, such as PMA or IL-8, activate the extracellular signal–regulated kinase (ERK) cascade, which stimulates the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase to produce ROS, which, in turn, triggers granule membrane disintegration and release of neutrophil elastase (NE) and myeloperoxidase.
More remains to be discovered about the signaling events that trigger NET formation, as this response is neither uniform nor observed in every situation where NADPH is activated. Nevertheless, when NE translocates to the nucleus, it acts to cleave histones, and, along with myeloperoxidase, decondenses chromatin and degrades the nuclear membrane. This process extrudes DNA, histones, and proteases into the cytosol, where they combine to form NETs, with eventual plasma membrane rupture and NET expulsion from the cell (3). This extracellular release of DNA occurs typically 2–3 hours after stimulation.
A second, NADPH oxidase–independent, calcium-mediated NETosis pathway has also been described, involving the enzyme, protein arginine deiminase 4 (PAD4) (4). Upon the increase of intracellular calcium in response to agents such as calcium ionophores, PAD4 binds calcium, crosses into the nucleus, and deiminates positively charged arginine residues to generate uncharged citrullin on histones H3 and H4. This loss of positive charge leads to the dissociation of the histones from negatively charged DNA, resulting in decondensation of the nucleosome. Citrullination of histones has been shown to be involved in calcium-dependent, but not NADPH oxidase–dependent, NETosis (4). It was demonstrated that PAD4 deficiency or use of Cl-amidine to chemically inhibit PAD4 activity causes a reduction of histone decondensation and NET formation (5). Other studies suggest that PAD4-deficient mice and wild-type mice clear infections equally well (6); therefore, the role of PAD4 in infection-related NETosis is uncertain.
In contrast, vital NETosis appears to be a rapid event seen in response to a more limited group of bacteria (3). Hence, with infection with gram-negative bacteria, such as Escherichia coli, TLR4-activated platelets can stimulate NETs through a direct CD11a interaction with neutrophils, and gram-positive bacteria, such as Staphylococcus aureus, activate neutrophil TLR2 and complement receptor 3, leading to induction of vital NETosis. Much remains to be elucidated regarding the mechanisms underlying this latter process, but fluorescence and electron micrographs show intact plasma membranes despite the release of NETs.
The electrostatic charge of nucleic acid (3) and the presence of pathogen-binding molecules, such as surfactant protein D (7), combine to support bacterial–NET interactions. Intravital microscopy has demonstrated the binding of E. coli to NETs released in the liver microcirculation during sepsis, with inhibition of NET formation resulting in increased bacterial dissemination to distant organs (8). It appears that neutrophils can spread NETs over wide distances in vivo, and Yipp and Kubes (3) suggested that DNA-binding sites on interstitial fibronectin may explain this remarkable ability to disperse NETs over large areas.
A growing body of evidence supports the ability of NETs to kill pathogens. For example, neutrophils treated with cytochalasin D, which inhibits phagocytosis and thus most traditional neutrophil-killing mechanisms, are still bactericidal (2), and treatment of these neutrophils with DNase or anti-histone antibodies diminishes this killing. NETs have also been shown to kill Aspergillus (9), group A Streptococcus (GAS) (10), and yeast and hyphal forms of Candida albicans (11). However, some other studies have failed to show NETs killing, including reports where a subset of S. aureus trapped by NETs continued to proliferate after DNase-induced release (12). What is clear, however, is that the trapping of bacteria by NETs acts to reduce proliferation and prevent bacterial dissemination from certain organs, including the lung (13).
Beyond trapping and killing pathogens, other suggested antipathogenic effects of NETs include their ability to neutralize virulence factors, such as IpaB from Shigella flexneri (2), functional opsonization of Aspergillus fumigatus, and inhibition of microbial growth via calprotectin-mediated chelation of zinc during Aspergillus infection (9).
Pathogen Evasion of NETs
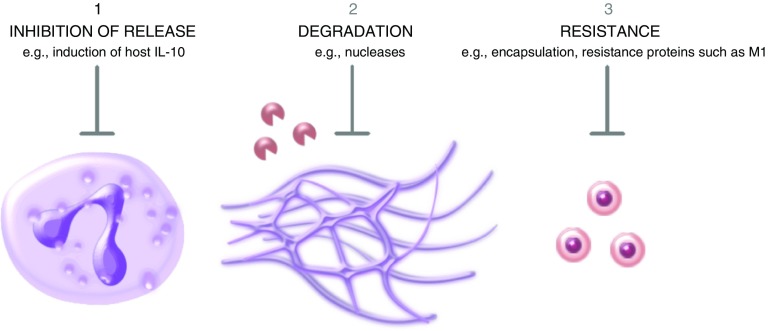
Although the host immune system has evolved numerous strategies to neutralize pathogens, invading microbes have also developed various mechanisms to avoid being attacked by the immune system; examples include inhibiting host inflammatory responses, degrading antimicrobial molecules with catalytic enzymes, and avoiding or resisting host proteases. Research over the past decade has now shown that three comparable mechanisms are also employed by pathogenic bacteria, viruses, and parasites: inhibition of NETs release, degradation of NETs, and resistance to NETs killing (Figure 2). Perhaps the best example of this is GAS, which has evolved mechanisms to evade NETs using all three of these strategies (10, 14–17).
Figure 2.
The three main NET evasion strategies. Certain respiratory pathogens have evolved the ability to evade the antimicrobial effects of NETs, using three main mechanisms: inhibition of the release of NETs; degradation of NETs by the production of pathogen nucleases; and resistance to the effects of the antimicrobial proteins that are enmeshed within NETs.
Inhibition of NETs
The induction of many neutrophil effector functions, including NETs, relies on the generation of ROS. Many pathogens inhibit oxidative pathways to evade immune function, and a handful of studies have shown that this capability may also down-regulate NET induction. ROS inhibition may occur through interference with ERK phosphorylation upstream of NADPH oxidase (18), or by pathogen induction of the NET-suppressive cytokine, IL-10, to block TLR-induced ROS generation (15). Many pathogens are able to induce cellular IL-10, whereas some viruses encode their own IL-10 homologs (19). Although induction of this immunosuppressive cytokine likely benefits infecting pathogens in several ways, the importance of its ability to down-regulate NET induction has yet to be determined. It is likely, however, that attenuation of ROS generation will prove to be a broad NET-evasion strategy employed by a variety of pathogens.
Degradation of NETs
Studies investigating the production of NET-degrading factors by pathogens have focused largely, but not exclusively, on the nucleases produced by common gram-positive respiratory pathogens, including GAS, Streptococcus pneumoniae, and S. aureus (Table 1). Soon after its discovery, the evasion of NETs was suggested as a potential function of GAS nucleases (14), and, in the following year, two groups demonstrated that the degradation of NETs by Streptococcal nucleases resulted in increased bacterial dissemination and pathogenicity (10, 13). Of interest, it was later reported that S. aureus not only degrades NETs, but that the degraded products even support disease progression (20). Most of these studies used NETs induced either by PMA or the pathogen, and to prevent confounding by phagocytosis, the actin-polymerization inhibitor, cytochalasin D, was often added to neutrophils before infection.
Table 1.
Properties of Neutrophil Extracellular Trap–Degrading Pathogen Nucleases
| Pathogen Species (Ref. No.) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus (20, 28) | Streptococcus sanguinis (32) | Streptococcus suis (30, 31) | Streptococcus pyogenes (GAS) (10, 14) | Streptococcus pneumoniae (13) | Streptococcus agalactiae (GBS) (15, 8) | Vibrio cholerae (38) | Neisseria gonorrheae (39) | Leishmania infantum (40) | Lutzomyia longipalpis (41) | |
| Nuclease | Nuc and AdsA | SWAN | SsnA and EndAsuis | Sda1/SdaD2 | EndA | NucA | Dns and Xds | Nuc | 3′ NT/NU | Lundep |
| Location | Secreted | Cell wall anchored | Cell wall–anchored and secreted (SsnA), membrane bound (EndAsuis) | Secreted | Membrane bound | Secreted | Extracellular | Secreted | Membrane anchored | Secreted |
| Pathogen induces NETs | Yes | Not known | Yes | NET induction inhibited by additional mechanism | Yes | NET induction inhibited by additional mechanism | Yes | Yes | Yes | N/A |
| Pathogen trapped/killed | Trapped and killed | Not killed | Trapped not killed | Not killed | Trapped not killed | Not killed | Trapped and killed | Killed | Trapped and killed | N/A |
| Other nuclease function | Biofilm dispersal; NET degradation products trigger macrophage apoptosis | Postulated to be able to bind integrins for a role in attachment | Potential role in nutrition (oligonucleotide production) | Prevents macrophage TLR9 activation; potential role in dissemination by liquefying pus | Necessary for DNA uptake in genetic transformation | Not known | Biofilm formation | Biofilm remodeling | Parasite nutrition (oligonucleotide production) | Lowering blood viscosity; facilitates sandfly feeding |
Definition of abbreviations: AdsA, adenosine synthase A; Dns, deoxyribonuclease; EndA, endonuclease A; EndAsuis, endonuclease A suis; GAS, group A Streptococcus; GBS, group B Streptococcus; Lundep, Lutzomyia NET destroying protein; N/A, not applicable; NET, neutrophil extracellular trap; 3′NT/NU, 3′-nucleotidase/nuclease; Nuc, nuclease; Sda, streptodornase; SsnA, Streptococcus suis-secreted nuclease A; SWAN, Streptococcal wall anchored nuclease; TLR, Toll-like receptor.
Recently, we found evidence of a host strategy to attenuate pathogen degradation of NETs: the antimicrobial peptide, LL-37 (cathelicidin), is constitutively expressed in neutrophils and present at high concentrations in NETs; however, it loses its microbicidal activity when bound to DNA. We found that purified neutrophil DNA and NETs released by PMA-treated neutrophils, when supplemented with LL-37, were resistant to degradation by S. aureus, S. pneumoniae, and GAS nucleases (21). These results were recapitulated using other cationic AMPs, with the cationicity of the agents correlating with the degree of resistance to degradation.
Resistance to NETs
In addition to the prevention of NET formation and the degradation of NETs, certain respiratory pathogens appear to resist NET trapping and killing by other mechanisms, including biofilm formation and electrochemical modification of the bacterial surface. Modification or masking of the negatively charged bacterial surface by bacterial enzymes or a capsule exploits the cationic nature of most NET components, preventing the electrostatic attraction thought to be responsible for bacterial adherence (17). Furthermore, certain bacterial virulence factors have been shown to confer resistance to the antimicrobial peptides present in NETs (22).
Respiratory Pathogens that Employ NET Evasion
GAS: NET Inhibition, Degradation, and Resistance
GAS employs multiple NET evasion mechanisms, including the ability to prevent NET formation, to digest preformed NETs, and to resist killing by microbicidal NET components. First, GAS inhibits NET formation by multiple mechanisms. The GAS pore-forming cytolysin streptolysin O blocks neutrophil ROS generation and, consequently, the generation of NETs (23). GAS also produces a peptide, S. pyogenes cell envelope protease, that cleaves IL-8, a potent neutrophil chemoattractant and activator, resulting in lower levels of neutrophil transmigration as well as reduced NET induction (16). In addition, the GAS hyaluronic acid capsule binds to the inhibitory neutrophil receptor, Sialic acid binding Ig-like lectin 9 (Siglec 9), down-regulating both ROS generation and NET formation (24).
Many gram-positive bacteria express DNases that are associated with virulence (13, 14, 22); however, the pathogenic benefit of these enzymes was unknown for many decades. Sumby and colleagues (14) have shown in murine models that GAS organisms deficient in specific DNases are more susceptible to extracellular killing and clearance compared with wild type. Virulence in the wild type was largely attributed to the activity of Streptodornase 1, the principal extracellular DNase in the M1 serotype GAS strain, which is associated with necrotizing fasciitis (14). The authors of this study were the first to speculate that the DNase may be important in the avoidance of NETs, which has since been confirmed by several reports documenting NET degradation by DNases.
Concrete evidence supporting the ability of pathogens to escape from NETs was first documented in our study on GAS (10), and another paper published concurrently on S. pneumoniae (13). We found that wild-type GAS survived coincubation with NETs significantly better than an Sda1-deficient mutant, and that nonpathogenic Lactococcus lactis heterologously expressing Sda1 on a plasmid vector showed significantly greater survival (10). Confocal microscopy confirmed that this survival was associated with the ability of the pathogen to degrade NETs. Expression of the DNase also made GAS significantly more virulent in a mouse model of necrotizing fasciitis with increased skin lesions and bacterial load at the site of injection. Administration of GAS together with G-actin, a naturally occurring inhibitor of type I family DNases, decreased wild-type bacterial survival to a level comparable to the Sda1-deficient mutant, and substantially decreased the lesion size. Every strain of GAS appears to express at least one DNase (25). Our subsequent study found that the heightened resistance to NET killing in vivo in GAS expressing Sda1 may serve as a selective force to generate hypervirulent bacterial variants (26).
Resistance to NET-associated antimicrobial peptides has also been shown to play an important role in the pathogenesis of invasive GAS infection. In 2009, a novel role for the virulence protein M1 was discovered, involving both stimulation of, and bacterial survival within, NET structures (27). Targeted mutagenesis of the emm1 gene coding for the M1 protein revealed an absence of GAS-induced NET generation in the mutant and an increased susceptibility to NET killing. Resistance could be restored by either an M1-expressing plasmid or recombinant M1 protein, and expression of the M1 gene in the M49 serotype or in L. lactis increased resistance to NET-mediated killing. The ΔM1 mutant was found to be susceptible to killing by the antimicrobial peptide, LL-37, and further investigation using purified recombinant M1 fragments demonstrated this effect to be mediated by sequestration by the M1 N-terminal hypervariable domain. The M1 protein serotype is linked to severe GAS disease, and clinical analysis of a large panel of GAS clinical isolates found all M1 isolates to be cathelicidin resistant, and demonstrated an association between cathelicidin resistance and invasive GAS disease (27).
S. aureus: NET Degradation
In 2012, the Rooijakkers group was the first to show that an S. aureus nuclease confers resistance to extracellular killing by neutrophils, and leads to enhanced disease in vivo (28). After this, an elegant study by Thammavongsa and colleagues (20) demonstrated that S. aureus takes evasion a step further by degrading NETs into a product toxic to macrophages. Lesions containing replicating bacteria surrounded by fibrin form the abscesses commonly found during S. aureus infection. Mice were infected with variant S. aureus with an assortment of silencing inserts in multiple known virulent genes. Whereas neutrophils infiltrated the abscess and formed NETs, macrophages were shown to be typically excluded. However, mice infected with a strain deficient in nuclease (nuc), a nuclease important in S. aureus biofilm dispersal (28), or adenosine synthase A (AdsA) showed increased macrophage penetration into the abscess. The authors found that trypan blue staining of macrophages, which indicates loss of viability, increased significantly in response to a product generated from exposing PMA-induced NETs to S. aureus. This response was attenuated when using the nuc or the adsA mutant, which also generated reduced 2′-deoxyadenosine (dAdo), a deoxyribonucleoside base. Treatment of NETs with purified versions of both nuc and AdsA was necessary to produce dAdo, and purified dAdo also induced caspase-3 activation and apoptosis in macrophages. Taken together, these data show that the S. aureus products, nuc and AdsA, are capable of degrading NETs and producing a toxin, dAdo, which kills macrophages, preventing their infiltration into abscesses and thereby reducing their antimicrobial functions.
S. pneumoniae: NET Degradation and Resistance
Beiter and colleagues (13) were the first to demonstrate in vitro that S. pneumoniae is trapped rather than killed by NETs. The authors found that the pneumococcal strain, TIGR4 (serotype 4), was able to degrade and escape PMA-induced NET capture, whereas a mutant strain deficient in the membrane-bound nuclease endA was captured by intact NETs. Intranasal infection with wild-type and the endA mutant organisms induced neutrophil recruitment, NET release, and the induction of pneumonia in murine lungs, whereas mice infected with the endA mutant showed improved survival. After simultaneous infection with wild-type and mutant bacteria, equal numbers of the two strains were recovered from the upper respiratory tract; however, considerably more wild-type organisms were recovered in the lungs and bloodstream, demonstrating the importance of endA for bacterial invasion. Using the presence of NE in cell supernatants as a reporter of NET degradation, the authors also showed that six of seven pneumococcal strains were able to degrade NETs. These early observations of NET evasion by a pathogen, and its association with virulence, stressed the importance of NET formation as an antipathogenic mechanism, and highlight the therapeutic potential of agents that preserve NET integrity.
More recently, the pneumococcal bacterial capsule has also been shown to confer resistance to NET trapping and killing (17). Encapsulated S. pneumoniae strains show significantly reduced trapping by NETs compared with nonencapsulated strains, and the addition of a capsule was thought to mask the negatively charged bacterial surface, preventing binding to the mostly cationic antimicrobial peptides enmeshed in NETs. In addition, S. pneumoniae has developed the ability to modify its surface charge through the action of a set of enzymes, which incorporate D-alanine into surface lipoteichoic acids (LTAs); these are tethered to the bacterial membrane, and result in a positive charge, which further repels cationic peptides (17). Wartha and colleagues (17) showed that the encapsulation and D-alanylation of LTA is associated with resistance to NET-mediated killing in vitro, and deletion of the dlt operon encoding the enzymes responsible for D-alanylation of LTA increases bacterial sensitivity to NET-mediated killing in nonencapsulated strains. The group went on to show, in a murine model of pneumococcal pneumonia, that mutant strains lacking functional dltA produce a ower bacterial burden in vivo than wild-type organisms.
Group B Streptococcus: NET Degradation and Inhibition
Another streptococcal nuclease, nucA, was found to be secreted by group B Streptococcus (GBS) and demonstrated to be capable of degrading NETs (29). As in the previous examples, the nuclease-deficient mutant was associated with higher bacterial clearance and lower mortality. The up-regulation of the NET-suppressing cytokine, IL-10, and subsequent attenuation of the respiratory burst was also demonstrated to be employed as a NET inhibition strategy by GBS (15). Recently, we found that the sialylated capsular polysaccharide of GBS acts as a mimic “self”-ligand to neutrophil Siglec-9, down-regulating effector responses (15). Blocking of Siglec-9 caused dampening of GBS-induced IL-10 up-regulation, resulting in a stronger oxidative burst, increased NET release and increased extracellular killing.
Other Streptococci: NET Degradation
Streptococcus suis, which induces and is trapped by porcine NETs, expresses Streptococcus suis-secreted nuclease A, a cell wall–anchored DNase that is partially released into the supernatant (30). In 2014, de Buhr and colleagues (30) demonstrated degradation of PMA-induced NETs by S. suis, but not by a mutant that lacked SsnA function, and that wild-type S. suis showed significantly higher viability after incubation with PMA and cytochalasin-treated neutrophils. A second S. suis nuclease homologous to pneumococcal endonuclease A (EndA), designated endonuclease A suis (EndAsuis), was described the following year (31), accounting for residual NET degradation seen in ΔSsnA mutants during the exponential growth phase. However, an EndAsuis mutant did not show increased susceptibility to NET-mediated killing, suggesting that EndAsuis was not as important as SsnA for protection against NETs, and an alternative role was suggested for the second nuclease, potentially to degrade pathogen DNA, thus avoiding TLR9 stimulation.
Morita and colleagues (32) discovered in Streptococcus sanguinis (an organism that colonizes the surfaces of teeth), a cation-dependent nuclease with a cell wall sorting signal, which they termed Streptococcal wall anchored nuclease (SWAN).A SWAN deletion mutant showed a 20% reduction in survival compared with wild type after incubation with PMA and cytochalasin D–treated neutrophils, suggesting a modest contribution to evasion from NETs. Furthermore, expression of SWAN in L. lactis significantly increases bacterial survival.
Pseudomonas aeruginosa: NET Inhibition and Resistance
P. aeruginosa, a significant pathogen in cystic fibrosis (CF), has evolved multiple mechanisms to attenuate NET production and resist NET-mediated killing. The pathogen has been shown to express surface sialic acids that are capable of binding and inducing signaling through neutrophil, Siglec-9, which suppresses the oxidative burst, and thus NET formation (33). The authors in this study showed that treatment with sialidase or use of P. aeruginosa strains lacking in sialic acids led to increased NET production compared with sialic acid–positive strains. Furthermore, paired P. aeruginosa strains isolated from patients with CF at early and late stages of disease showed that resistance to NET-mediated killing evolved over time, and correlated with the development of the mucoid phenotype characterized by excess alginate production (34). However, a mutant P. aeruginosa strain that overexpressed alginate did not show increased survival on incubation with PMA-treated neutrophils, suggesting that the acquired resistance of P. aeruginosa to NET-mediated killing in the CF airway was due to an as-yet unknown mechanism (34).
Haemophilus influenzae: NET Resistance
The respiratory pathogen, H. influenzae, evades NET killing through both the production of antioxidants and the formation of biofilms—surface-adherent bacterial communities that resist clearance by immune cells and antimicrobials through encasement in a polymer matrix (22). Nontypeable H. influenzae (NTHi), a leading cause of chronic otitis media, has been demonstrated to form a biofilm within the middle ear during infection, a process promoted by the production of lipo-oligosaccharides, which is thought to promote bacterial persistence. Biofilm presence was shown to correlate with bacterial load in an experimental model of otitis media (22), with microscopy revealing the presence of NTHi communities with biofilm phenotypes within a DNA lattice containing neutrophils, elastase, and histones, consistent with the appearance of NETs. The bacteria were found to be resistant to phagocytosis and extracellular neutrophil killing in vitro, which was lipo-oligosaccharide dependent.
NTHi also produces the antioxidants, peroxiredoxin–glutaredoxin and catalase, two molecules that confer resistance to hydrogen peroxide in vitro. Mutant strains with a deletion of the two genes encoding these factors, hktE and pdgX, displayed reduced survival in the presence of hydrogen peroxide, increased susceptibility to NET-mediated killing in vitro, and attenuated persistence in the middle ear (35). Susceptibility to NET-mediated killing was partially rescued by the addition of exogenous catalase, and is thought to be due to the antioxidants providing resistance against the microbicidal activity of ROS within NETs. The possibility that the antioxidants may, in fact, prevent NET induction by neutralization of ROS, which are required for NET release, is an intriguing one, although there is, as yet, no evidence supporting this in H. influenzae or other catalase-expressing bacteria.
Bordetella pertussis and Cryptococcus neoformans: NET Inhibition
B. pertussis (18) and the fungus, C. neoformans (36), both inhibit NET formation by the suppression of ROS generation. Wild-type strains do not induce NETs, but mutants of these two pathogens lacking ROS- and NET-suppressing agents can certainly induce NET release. NETs are inhibited by C. neoformans via its capsular polysaccharide glucuronoxylomannan, and in B. pertussis through generation of adenylyl cyclase toxin (ACT). ACT generates high levels of cyclic AMP, which has a wide range of effects, including inhibition of ERK phosphorylation, thus suppressing ROS generation and NET formation. Intriguingly, convalescent-phase sera from patients with pertussis are able to reverse ACT-mediated inhibition of PMA-induced ROS and NETs (18).
Burkholderia pseudomallei: NET Inhibition
Evasion of NET killing has also been demonstrated by the gram-negative bacterium, B. pseudomallei, the causative agent of mellioidosis, an infection endemic to Southeast Asia, with multiple manifestations, including pneumonia. Neutrophils play a crucial role in controlling infection in murine models of the disease, and investigation of the response of human neutrophils to B. pseudomallei showed that the pathogen triggers the induction of NETs, which exert microbicidal activity (37).
Riyapa and colleagues (37) also showed that the bacteria modify the magnitude of NET formation through the action of two known virulence factors, the bacterial type III protein secretion system encoded by the bsa locus, including the components bsaZ and bsaQ, and capsular polysaccharide 1 encoded by the wcb operon. Mutant strains deficient in either of these components induce higher levels of NET production, which result in increased bacterial killing by NETs compared with wild-type bacteria (37). Increased NET production was mirrored by higher levels of phagocytosis of the mutants with an elevated oxidative burst, implying that the type III protein secretion system and capsular polysaccharide 1 attenuate NET release through inhibition of the NADPH oxidase pathway. These mechanisms are postulated to be responsible for the attenuated replication and plaque formation seen with bsaZ and basQ mutants in vitro and the reduced virulence of wcb mutants in murine models of disease.
Other Pathogens: NET Degradation
The degradation of NETs has also been suggested as a function of nucleases in gram-negative bacteria. Seper and colleagues (38) found that a Vibrio cholerae strain deficient for two extracellular nucleases, deoxyribonuclease and xds, was less invasive than wild-type V. cholerae in immunocompetent mice, although no difference between strains was seen in neutropenic mice, and quantitative RT-PCR revealed that the presence of either NETs or DNA was able to up-regulate the expression of deoxyribonuclease and xds (38). Neisseria gonorrheae has also been shown to encode the heat-stable nuclease, nuc, thought to be important for remodeling N. gonorrheae biofilms (39). NETs were shown to be rapidly induced by N. gonorrheae, but thereafter NET integrity decreased over time in nuc-containing strains compared with a Δnuc mutant, as measured by Sytox Green immunofluorescence. The presence of Nuc enhanced survival of the pathogen on exposure to PMA-stimulated neutrophils, implicating nuc as a virulence factor important for host immune evasion.
In 2014, two studies documented NETs degradation in biological kingdoms other than bacteria. The parasite Leishmania infantum was shown to express high levels of 3′-nucleotidase/nuclease when cultured in low-phosphate media, a phenotype that showed higher survival in NETs (40). A highly active nuclease is also present in the saliva of the parasite’s sandfly carrier, Lutzomyia longipalpis (41), and a recombinant form of the nuclease, Lutzomyia NET destroying protein, was shown to degrade NETs and increase parasite survival when coincubated with neutrophils. Coinjection of the recombinant nuclease with Leishmania in mice increased lesion size and parasite load, suggesting that the nuclease may aid promastigote evasion of NETs as well as lower blood viscosity to facilitate sandfly feeding.
Conclusions
Broadly, there exist three main strategies by which respiratory pathogens counteract NETs: inhibition of NETs release, degradation of NETs by pathogen- or vector-encoded nucleases, and resistance to NET-mediated killing, for example, by production of inhibitors of antimicrobial peptides. These effects likely overlap significantly with other functions (40).
The prevalence of NET evasion mechanisms within respiratory pathogens supports the view that NETs are an important antipathogenic mechanism. The relevance of NET evasion by pathogens is further supported by evidence that the body also appears to “fight back”—for example, high concentrations of the antimicrobial peptide, LL-37, within NETs increases resistance to the action of DNases (21), and the presence of anti-ACT in the sera of patients infected with B. pertussis indicates the participation of humoral responses in combating the specific pathways by which pathogens modify NETs.
This body of knowledge must, however, be interpreted acknowledging the limitations of many commonly used NETs assays. For example, although the use of PMA is convenient to assess the effect of a compound on already-released NETs, the physiological relevance of this chemical factor has been repeatedly questioned (42). There are stark differences between PMA-induced NETs and those induced by more physiological agonists; for example, PMA induces all neutrophils to release DNA, whereas only 25% of neutrophils do so with S. aureus. Furthermore, cytochalasin D is often used to inhibit phagocytosis to separate traditional neutrophil killing mechanisms from NET-associated effects; however, phagocytosis is integral to so many neutrophil functions that its inhibition may confound. Hence, Branzk and colleagues (43) show that phagocytosis via dectin-1 can inhibit the release of NETs as part of a neutrophil size–sensing mechanism that determines their antipathogenic response. Therefore, NET-mediated killing that is demonstrated in the presence of cytochalasin D could be misleading, if NET induction would have been inhibited by phagocytosis in its absence. These inherent caveats pertain to many NET studies, and can only be mitigated with the use of multiple complimentary techniques and the inclusion of physiological NET stimulants.
Future work is likely to involve translation of the advances in our understanding of pathogen evasion of NETs into pharmacological therapies. Development of a rapid technique to establish whether a pathogen is NET resistant, or a therapy counteracting common mechanisms of NET evasion by pathogens, could complement antimicrobial approaches. All three pathogen-evasion strategies may be targetable. Therapies aimed at neutralizing bacterial pathways that down-regulate NET induction may hold promise, including targeting the GAS IL-8 protease SpyCep or neutrophil Siglec-9, which is engaged by multiple pathogens to inhibit NETs induction (15). Pathogen-encoded nucleases are another attractive target. G-actin, a natural pharmacologic inhibitor of type I DNases, has been shown to prevent Sda1-mediated degradation of NETs (10) and certain patients with systemic lupus erythematosus produce specific DNase1 inhibitors that act against endogenous serum DNase1 (44). Other therapeutic opportunities involve protecting NETs from degradation—high concentrations of LL-37 increase DNA resistance to pathogen DNases (21), and NETs are protected from degradation in systemic lupus erythematosus due to the deposition of autoantibodies, which are thought to shield them from serum DNase1 (44). Furthermore, targetable bacterial regulators of nuclease expression have been described, including period circadian protein homolog 1, which regulates GAS Sda1 expression (45), and the mgrA and Staphylococcal accessory regulator Z transcriptional regulators, which up-regulate S. aureus nuc expression (46). These are part of a wide family of bacterial peroxide-sensing transcriptional regulators, making this a promising area of research. Bacterial mechanisms of resistance to NETs may also be targeted, including modification of the protective capsule or inhibiting resistance genes, such as the GAS M1 protein.
Lastly, the above studies build a framework that may be used for assessing the many other bacterial species that contain nucleases, such as species of the bacterium Mycoplasma, which all contain surface nucleases. Investigation of their effects on NETs may reveal the evolutionary significance of such genes in evasion of the host innate immune response.
It is also important to recognize that certain immunosuppressive agents, such as the calcineurin inhibitors, have been shown to impair NET formation (47), which could further enhance susceptibility to opportunistic infections, whereas other drugs, such as statins and tamoxifen, appear to increase NET production and promote bacterial clearance both in vitro and in vivo (48). However, despite the positive role that NETs play in mediating infections, NET components have been shown to cause inflammation and tissue damage, and aberrant or excessive NETosis might play a role in inflammatory processes, including acute lung injury and venous thrombosis development (47). Excess NET formation damages the epithelium and leads to lung tissue damage, and has been reported in the lungs of patients with a wide range of pulmonary conditions that are associated with neutrophil infiltrates, including neutrophilic asthma, chronic obstructive pulmonary disease, CF, respiratory syncytial virus bronchiolitis, influenza, and tuberculosis (49). It is thought that NET-induced injury can be particularly severe in the lungs, due to the ability of NETs to expand easily in the alveolar space and to directly induce death in endothelial and epithelial cells by the action of histones. Excessive NET formation can also facilitate blood coagulation, thus disturbing the alveolar microcirculation (49). Furthermore, extracellular DNA has been recognized as a critical component of biofilms with the presence of neutrophil actin and DNA enhancing their formation (50), and NETs as a potential source for the formation of harmful biofilms is deserving of future research. Hence, extreme care will be needed in designing agents that modulate NET biology.
In conclusion, pathogens have evolved a range of mechanisms to evade NETs that can be categorized into three main categories: NET inhibition; NET degradation; and NET resistance. NET formation is now recognized as an important host mechanism to fight respiratory infections, and future research will involve identifying additional mechanisms to resist NETs as well as options to develop therapeutic interventions to counteract evasion.
Footnotes
Author Contributions: D.M.L.S. and J.M.P. co-wrote the manuscript. A.S.C., J.K.J., and A.N. contributed sections, and V.N. and E.R.C. provided editorial input.
Originally Published in Press as DOI: 10.1165/rcmb.2016-0193PS on November 17, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 3.Yipp BG, Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 4.Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci U S A. 2015;112:2817–2822. doi: 10.1073/pnas.1414055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–213. doi: 10.1083/jcb.200806072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinod K, Fuchs TA, Zitomersky NL, Wong SL, Demers M, Gallant M, Wang Y, Wagner DD. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood. 2015;125:1948–1956. doi: 10.1182/blood-2014-07-587709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douda DN, Jackson R, Grasemann H, Palaniyar N. Innate immune collectin surfactant protein D simultaneously binds both neutrophil extracellular traps and carbohydrate ligands and promotes bacterial trapping. J Immunol. 2011;187:1856–1865. doi: 10.4049/jimmunol.1004201. [DOI] [PubMed] [Google Scholar]
- 8.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12:324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, Zychlinsky A, Reichenbach J. Restoration of NET formation by gene therapy in CGD controls Aspergillosis. Blood. 2009;114:2619–2622. doi: 10.1182/blood-2009-05-221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16:396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 11.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 12.Menegazzi R, Decleva E, Dri P. Killing by neutrophil extracellular traps: fact or folklore? Blood. 2012;119:1214–1216. doi: 10.1182/blood-2011-07-364604. [DOI] [PubMed] [Google Scholar]
- 13.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16:401–407. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 14.Sumby P, Barbian KD, Gardner DJ, Whitney AR, Welty DM, Long RD, Bailey JR, Parnell MJ, Hoe NP, Adams GG, et al. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc Natl Acad Sci USA. 2005;102:1679–1684. doi: 10.1073/pnas.0406641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlin AF, Uchiyama S, Chang YC, Lewis AL, Nizet V, Varki A. Molecular mimicry of host sialylated glycans allows a bacterial pathogen to engage neutrophil Siglec-9 and dampen the innate immune response. Blood. 2009;113:3333–3336. doi: 10.1182/blood-2008-11-187302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zinkernagel AS, Timmer AM, Pence MA, Locke JB, Buchanan JT, Turner CE, Mishalian I, Sriskandan S, Hanski E, Nizet V. The IL-8 protease SpyCEP/ScpC of group A Streptococcus promotes resistance to neutrophil killing. Cell Host Microbe. 2008;4:170–178. doi: 10.1016/j.chom.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wartha F, Beiter K, Albiger B, Fernebro J, Zychlinsky A, Normark S, Henriques-Normark B. Capsule and D-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol. 2007;9:1162–1171. doi: 10.1111/j.1462-5822.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- 18.Eby JC, Gray MC, Hewlett EL. Cyclic AMP-mediated suppression of neutrophil extracellular trap formation and apoptosis by the Bordetella pertussis adenylate cyclase toxin. Infect Immun. 2014;82:5256–5269. doi: 10.1128/IAI.02487-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouyang P, Rakus K, van Beurden SJ, Westphal AH, Davison AJ, Gatherer D, Vanderplasschen AF. IL-10 encoded by viruses: a remarkable example of independent acquisition of a cellular gene by viruses and its subsequent evolution in the viral genome. J Gen Virol. 2014;95:245–262. doi: 10.1099/vir.0.058966-0. [DOI] [PubMed] [Google Scholar]
- 20.Thammavongsa V, Missiakas DM, Schneewind O. Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science. 2013;342:863–866. doi: 10.1126/science.1242255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neumann A, Völlger L, Berends ET, Molhoek EM, Stapels DA, Midon M, Friães A, Pingoud A, Rooijakkers SH, Gallo RL, et al. Novel role of the antimicrobial peptide LL-37 in the protection of neutrophil extracellular traps against degradation by bacterial nucleases. J Innate Immun. 2014;6:860–868. doi: 10.1159/000363699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong W, Juneau RA, Pang B, Swords WE. Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus influenzae persistence in the chinchilla model for otitis media. J Innate Immun. 2009;1:215–224. doi: 10.1159/000205937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchiyama S, Döhrmann S, Timmer AM, Dixit N, Ghochani M, Bhandari T, Timmer JC, Sprague K, Bubeck-Wardenburg J, Simon SI, et al. Streptolysin O rapidly impairs neutrophil oxidative burst and antibacterial responses to group A Streptococcus. Front Immunol. 2015;6:581. doi: 10.3389/fimmu.2015.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Secundino I, Lizcano A, Roupé KM, Wang X, Cole JN, Olson J, Ali SR, Dahesh S, Amayreh LK, Henningham A, et al. Host and pathogen hyaluronan signal through human Siglec-9 to suppress neutrophil activation. J Mol Med (Berl) 2016;94:219–233. doi: 10.1007/s00109-015-1341-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira BT, Benchetrit LC, De Castro AC, Batista TG, Barrucand L. Extracellular deoxyribonucleases of streptococci: a comparison of their occurrence and levels of production among beta-hemolytic strains of various serological groups. Zentralbl Bakteriol. 1992;277:493–503. doi: 10.1016/s0934-8840(11)80474-3. [DOI] [PubMed] [Google Scholar]
- 26.Walker MJ, Hollands A, Sanderson-Smith ML, Cole JN, Kirk JK, Henningham A, McArthur JD, Dinkla K, Aziz RK, Kansal RG, et al. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med. 2007;13:981–985. doi: 10.1038/nm1612. [DOI] [PubMed] [Google Scholar]
- 27.Lauth X, von Köckritz-Blickwede M, McNamara CW, Myskowski S, Zinkernagel AS, Beall B, Ghosh P, Gallo RL, Nizet V. M1 protein allows group A streptococcal survival in phagocyte extracellular traps through cathelicidin inhibition. J Innate Immun. 2009;1:202–214. doi: 10.1159/000203645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berends ET, Horswill AR, Haste NM, Monestier M, Nizet V, von Köckritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. 2010;2:576–586. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Derré-Bobillot A, Cortes-Perez NG, Yamamoto Y, Kharrat P, Couvé E, Da Cunha V, Decker P, Boissier MC, Escartin F, Cesselin B, et al. Nuclease A (Gbs0661), an extracellular nuclease of Streptococcus agalactiae, attacks the neutrophil extracellular traps and is needed for full virulence. Mol Microbiol. 2013;89:518–531. doi: 10.1111/mmi.12295. [DOI] [PubMed] [Google Scholar]
- 30.de Buhr N, Neumann A, Jerjomiceva N, von Köckritz-Blickwede M, Baums CG. Streptococcus suis DNase SsnA contributes to degradation of neutrophil extracellular traps (NETs) and evasion of NET-mediated antimicrobial activity. Microbiology. 2014;160:385–395. doi: 10.1099/mic.0.072199-0. [DOI] [PubMed] [Google Scholar]
- 31.de Buhr N, Stehr M, Neumann A, Naim HY, Valentin-Weigand P, von Köckritz-Blickwede M, Baums CG. Identification of a novel DNase of Streptococcus suis (EndAsuis) important for neutrophil extracellular trap degradation during exponential growth. Microbiology. 2015;161:838–850. doi: 10.1099/mic.0.000040. [DOI] [PubMed] [Google Scholar]
- 32.Morita C, Sumioka R, Nakata M, Okahashi N, Wada S, Yamashiro T, Hayashi M, Hamada S, Sumitomo T, Kawabata S. Cell wall–anchored nuclease of Streptococcus sanguinis contributes to escape from neutrophil extracellular trap-mediated bacteriocidal activity. PLoS One. 2014;9:e103125. doi: 10.1371/journal.pone.0103125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khatua B, Bhattacharya K, Mandal C. Sialoglycoproteins adsorbed by Pseudomonas aeruginosa facilitate their survival by impeding neutrophil extracellular trap through siglec-9. J Leukoc Biol. 2012;91:641–655. doi: 10.1189/jlb.0511260. [DOI] [PubMed] [Google Scholar]
- 34.Young RL, Malcolm KC, Kret JE, Caceres SM, Poch KR, Nichols DP, Taylor-Cousar JL, Saavedra MT, Randell SH, Vasil ML, et al. Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PLoS One. 2011;6:e23637. doi: 10.1371/journal.pone.0023637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juneau RA, Pang B, Armbruster CE, Murrah KA, Perez AC, Swords WE. Peroxiredoxin-glutaredoxin and catalase promote resistance of nontypeable Haemophilus influenzae 86-028NP to oxidants and survival within neutrophil extracellular traps. Infect Immun. 2015;83:239–246. doi: 10.1128/IAI.02390-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rocha JD, Nascimento MT, Decote-Ricardo D, Côrte-Real S, Morrot A, Heise N, Nunes MP, Previato JO, Mendonça-Previato L, DosReis GA, et al. Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Sci Rep. 2015;5:8008. doi: 10.1038/srep08008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riyapa D, Buddhisa S, Korbsrisate S, Cuccui J, Wren BW, Stevens MP, Ato M, Lertmemongkolchai G. Neutrophil extracellular traps exhibit antibacterial activity against Burkholderia pseudomallei and are influenced by bacterial and host factors. Infect Immun. 2012;80:3921–3929. doi: 10.1128/IAI.00806-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seper A, Hosseinzadeh A, Gorkiewicz G, Lichtenegger S, Roier S, Leitner DR, Röhm M, Grutsch A, Reidl J, Urban CF, et al. Vibrio cholerae evades neutrophil extracellular traps by the activity of two extracellular nucleases. PLoS Pathog. 2013;9:e1003614. doi: 10.1371/journal.ppat.1003614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Juneau RA, Stevens JS, Apicella MA, Criss AK. A thermonuclease of Neisseria gonorrhoeae enhances bacterial escape from killing by neutrophil extracellular traps. J Infect Dis. 2015;212:316–324. doi: 10.1093/infdis/jiv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guimarães-Costa AB, DeSouza-Vieira TS, Paletta-Silva R, Freitas-Mesquita AL, Meyer-Fernandes JR, Saraiva EM. 3′-nucleotidase/nuclease activity allows Leishmania parasites to escape killing by neutrophil extracellular traps. Infect Immun. 2014;82:1732–1740. doi: 10.1128/IAI.01232-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chagas AC, Oliveira F, Debrabant A, Valenzuela JG, Ribeiro JM, Calvo E. Lundep, a sand fly salivary endonuclease increases Leishmania parasite survival in neutrophils and inhibits XIIa contact activation in human plasma. PLoS Pathog. 2014;10:e1003923. doi: 10.1371/journal.ppat.1003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nauseef WM, Kubes P. Pondering neutrophil extracellular traps with healthy skepticism. Cell Microbiol. 2016;18:1349–1357. doi: 10.1111/cmi.12652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, Brown GD, Papayannopoulos V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol. 2014;15:1017–1025. doi: 10.1038/ni.2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci USA. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang CH, Chiang-Ni C, Kuo HT, Zheng PX, Tsou CC, Wang S, Tsai PJ, Chuang WJ, Lin YS, Liu CC, et al. Peroxide responsive regulator PerR of group A Streptococcus is required for the expression of phage-associated DNase Sda1 under oxidative stress. PLoS One. 2013;8:e81882. doi: 10.1371/journal.pone.0081882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen PR, Nishida S, Poor CB, Cheng A, Bae T, Kuechenmeister L, Dunman PM, Missiakas D, He C. A new oxidative sensing and regulation pathway mediated by the MgrA homologue SarZ in Staphylococcus aureus. Mol Microbiol. 2009;71:198–211. doi: 10.1111/j.1365-2958.2008.06518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta AK, Giaglis S, Hasler P, Hahn S. Efficient neutrophil extracellular trap induction requires mobilization of both intracellular and extracellular calcium pools and is modulated by cyclosporine A. PLoS One. 2014;9:e97088. doi: 10.1371/journal.pone.0097088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corriden R, Hollands A, Olson J, Derieux J, Lopez J, Chang JT, Gonzalez DJ, Nizet V. Tamoxifen augments the innate immune function of neutrophils through modulation of intracellular ceramide. Nat Commun. 2015;6:8369. doi: 10.1038/ncomms9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Porto BN, Stein RT. Neutrophil extracellular traps in pulmonary diseases: too much of a good thing? Front Immunol. 2016;7:311. doi: 10.3389/fimmu.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker TS, Tomlin KL, Worthen GS, Poch KR, Lieber JG, Saavedra MT, Fessler MB, Malcolm KC, Vasil ML, Nick JA. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect Immun. 2005;73:3693–3701. doi: 10.1128/IAI.73.6.3693-3701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]