Abstract
Aging is associated with metabolic diseases such as type 2 diabetes mellitus, cardiovascular disease, cancer, and neurodegeneration. Aging contributes to common processes including metabolic dysfunction, DNA damage, and reactive oxygen species generation. Although glycolysis has been linked to cell growth and proliferation, the mechanisms by which the activation of glycolysis by aging regulates fibrogenesis in the lung remain unclear. The objective of this study was to determine if glucose transporter 1 (GLUT1)–induced glycolysis regulates age-dependent fibrogenesis of the lung. Mouse and human lung tissues were analyzed for GLUT1 and glycolytic markers using immunoblotting. Glycolytic function was measured using a Seahorse apparatus. To study the effect of GLUT1, genetic inhibition of GLUT1 was performed by short hairpin RNA transduction, and phloretin was used for pharmacologic inhibition of GLUT1. GLUT1-dependent glycolysis is activated in aged lung. Genetic and pharmacologic inhibition of GLUT1 suppressed the protein expression of α-smooth muscle actin, a key cytoskeletal component of activated fibroblasts, in mouse primary lung fibroblast cells. Moreover, we demonstrated that the activation of AMP-activated protein kinase, which is regulated by GLUT1-dependent glycolysis, represents a critical metabolic pathway for fibroblast activation. Furthermore, we demonstrated that phloretin, a potent inhibitor of GLUT1, significantly inhibited bleomycin-induced lung fibrosis in vivo. These results suggest that GLUT1-dependent glycolysis regulates fibrogenesis in aged lung and that inhibition of GLUT1 provides a potential target of therapy of age-related lung fibrosis.
Keywords: idiopathic pulmonary fibrosis, glucose metabolism, bleomycin
Clinical Relevance
Aging contributes to common processes including metabolic dysfunction, DNA damage, and reactive oxygen species generation. Although glycolysis has been linked to cell growth and proliferation, the mechanisms by which the activation of glycolysis by aging regulates fibrogenesis in lung remain unclear. In this study, we found that glucose transporter 1 (GLUT1)–dependent glycolytic phenotype in aged mouse lungs is significantly higher than in young mice. We showed that genetic and pharmacologic inhibition of GLUT1 suppressed the activation of glycolysis and the protein expression of α-smooth muscle actin, a key cytoskeletal component of activated fibroblasts, in primary mouse lung fibroblast cells. We demonstrated that the activation of AMP-activated protein kinase by inhibition of GLUT1-dependent glycolysis represents a critical metabolic pathway for regulating fibroblast activation. Phloretin, a potent inhibitor of GLUT1, significantly suppressed bleomycin-induced lung fibrosis in vivo.
Fibrotic interstitial lung diseases, including idiopathic pulmonary fibrosis (IPF), are more prevalent in aging populations (1, 2). IPF is a rapidly progressive, fatal lung disease with a median survival of <3 years after diagnosis (3). Fibrosis is characterized by the accumulation of extracellular matrix (ECM) proteins, activated myofibroblasts, and inflammatory cells (4). We and other groups have reported recently that aged mice, compared with their younger counterparts, have greater lung injury and fibrosis (5, 6). Glycolytic reprogramming has been shown to play an important role in the pathogenesis of lung fibrosis (7). Recent studies have reported that patients with IPF exhibit higher glycolytic activity in fibrotic areas, with positive retention index values being a strong predictor of deterioration in pulmonary function and of higher mortality (8, 9).
Glycolysis is a critical pathway in glucose metabolism that provides intermediates for energy generation (10–12). Cellular metabolic pathways, including glycolysis and fatty acid synthesis, have been shown to play an important role in lung injury (13, 14). Glucose transporter 1 (GLUT1), a member of the facilitative GLUT transporter family (SLC2), is the most highly conserved and widely distributed glucose transporter in mammalian cells (15, 16). Changes in metabolic state and oxidative stress can regulate GLUT1 expression (17). Previous work has shown several signaling molecules, such as cAMP, p53, phosphoinositide 3-kinase, and Akt, can regulate GLUT1 expression and function (18–20). Despite these findings, the regulation of fibrogenesis by the metabolic changes in aging cells has not been well elucidated.
The AMP-activated protein kinase (AMPK) is a central energy sensor that exists as a heterotrimer composed of a catalytic α subunit with regulatory β and γ subunits (21–23). When cellular energy supply is low, AMPK obtains the kinase activity by phosphorylation of the α subunit at Thr172 (24). The sensitivity of AMPK activation declines with aging, and the increasing responsiveness of AMPK activation plays a crucial role in extending lifespan in some species (25–28). Recently, it has been suggested that reagents that enhance AMPK activation reduce organ fibrosis (29).
In the current study, we demonstrate that GLUT1-dependent glycolysis regulates the activation of fibrogenesis in aged lung in vivo and in vitro. GLUT1-dependent glycolysis was activated in lung tissues of aged mice relative to young mice. Genetic and pharmacologic inhibition of GLUT1 suppressed the protein expression of α-smooth muscle actin (α-SMA), which is a cytoskeletal component of contractile actin stress fibers, in mouse primary fibroblasts. The activation of AMPK, which is a key metabolic regulator, by GLUT1 inhibition significantly reduced the protein expression of α-SMA in mouse primary fibroblasts. Pharmacologic inhibition of GLUT1 by phloretin, a potent GLUT1 inhibitor, suppressed bleomycin-induced lung fibrosis in vivo. Taken together, our results suggest that the activation of GLUT1-dependent glycolysis by aging is critical for fibrogenesis in the lung.
Materials and Methods
Reagents
Bleomycin and phloretin were obtained from Cayman Chemical (Ann Arbor, MI). Recombinant mouse transforming growth factor-β (TGF-β) 1 was obtained from R&D Systems (Minneapolis, MN). MG132, 2-deoxyglucose (2DG), 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR), and dorsomorphin were obtained from Sigma-Aldrich (St. Louis, MO).
Animal Studies
Male C57BL/6 mice of 2 months and 18 months of age were purchased from the National Institute on Aging Mouse Colony. Mice were exposed to phosphate-buffered saline or bleomycin (0.01 mg/mouse) via oropharyngeal aspiration. Phloretin (10 mg/kg) was started on the day of bleomycin administration or on Day 7 after bleomycin and was given by intraperitoneal injection every other day for 14 days. All animal experimental protocols were approved by the institutional animal care and use committee at Weill Cornell Medicine.
Isolation and Culture of Murine Lung Fibroblasts
Primary lung fibroblasts were isolated and cultured as described previously (30).
Transduction of GLUT1 Short Hairpin RNA
For stable knockdown of mouse glut1, two independent short hairpin RNAs (shRNAs) (TRCN00000305719 and TRCN00000311413, Sigma-Aldrich) were used. Aged mouse lung fibroblasts (2 × 105 cells/well) were seeded in 6-well plates and were transduced with shRNA lentiviral constructs against mouse GLUT1 or nontarget shRNA (SHC016, Sigma-Aldrich) controls.
Immunoblot Analysis
Immunoblotting was performed as described previously (5). Anti–β-actin (4,967), –α-tubulin (2,144), -AMPKα (5,831), –phospho-AMPKα (2,535), -Smad3 (9,513), –phospho-Smad3 (9,520) antibodies and a glycolytic sampler kit (8,337) were obtained from Cell Signaling (Danvers, MA). Anti-GLUT1 (ab40084), -collagen type 1 (ab21286) antibodies were from Abcam (Cambridge, MA). Antifibronectin (sc-6952) was from Santa Cruz Biotechnology (Dallas, TX). Anti–α-SMA (A2547) was from Sigma-Aldrich.
Quantitative Reverse Transcriptase–Polymerase Chain Reaction
Total RNA was isolated from cultured cells and tissues using the TRIzol reagent (Thermo Fischer Scientific, Waltham, MA), per manufacturer’s instructions. Quantitative reverse transcriptase–polymerase chain reaction was performed as described in our previous study (5).
Assessment of Fibrosis
Lung tissues were formalin fixed and hematoxylin and eosin stained by the Research Pathology Laboratory at Weill Cornell Medical College. Fibrosis was evaluated histologically using Masson trichrome stains and biochemically using the Sircol collagen assay kit (S1000; Biocolor, County Antrim, UK) or hydroxyproline assay (MAK008; Sigma-Aldrich) according to the manufacturer’s instructions.
Immunohistochemistry
Paraffin-embedded murine lung tissue sections went through antigen retrieval using target retrieval buffer (Dako; Carpinteria, CA). Anti-GLUT1 (ab40084, Abcam) antibody and biotinylated goat antimouse antibody (Vector Laboratories Inc., ON, Canada) were used. Slides were developed using Vector NovoRed (Vector Laboratories Inc., Burlington, ON, Canada), and were counterstained with Gill’s hematoxylin (Sigma-Aldrich).
Glycolytic Function Assay
Primary murine cells were plated on XF96 cell culture microplates (101085–004, Seahorse Bioscience, North Billerica, MA). The extracellular acidification rate (ECAR) or oxygen consumption rate (OCR) as parameters of glycolytic flux were measured on a Seahorse XF96 bioanalyzer, using the XF Glycolysis Stress Test kit according to the manufacturer’s instructions (102194–100, Seahorse Bioscience) (see online supplement for a detailed experimental protocol).
Statistical Analysis
Error bars represent the SEM, as indicated in figure legends. All statistical tests were analyzed by Student’s two-tailed t test for comparison of two groups and by analysis of variance (with post hoc comparisons using Dunnett’s test) for comparison of multiple groups using GraphPad Prism v. 6.0 (La Jolla, CA). P < 0.05 was considered statistically significant.
Results
GLUT1-Dependent Glycolysis Is Activated in Aged Lung
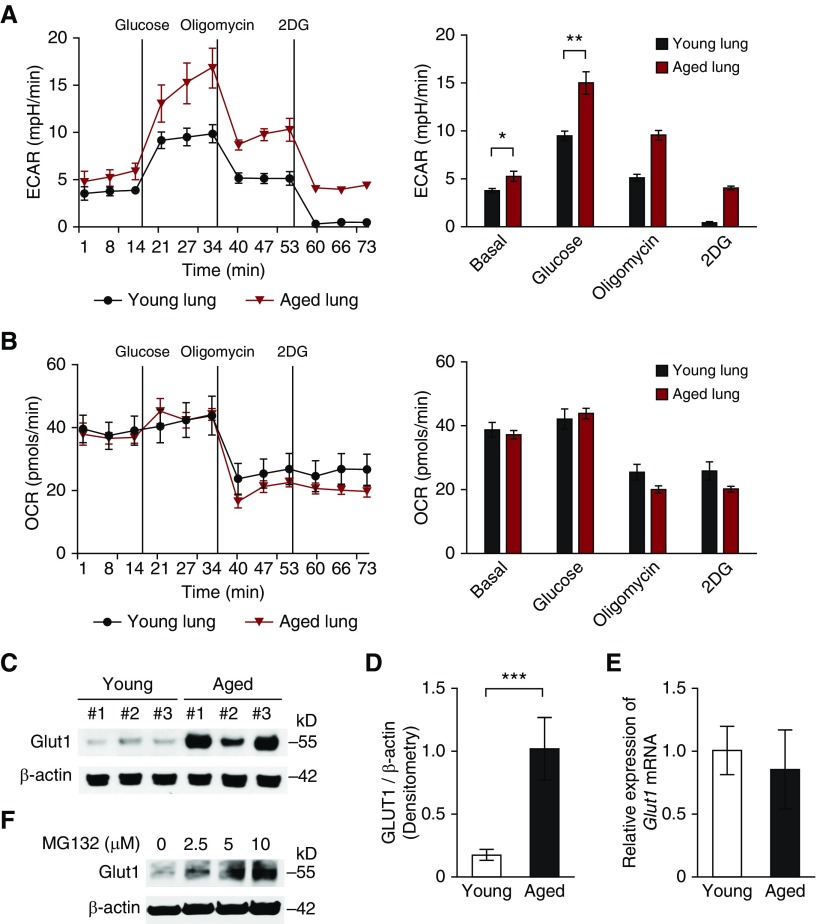
To investigate the metabolic changes in aged lung, we first analyzed the glycolytic function of single-cell lung cell suspensions isolated from young (2-month-old) and aged (18-month-old) murine lung using the GentleMacs tissue dissociation apparatus. We assessed the ECAR as a measure of lactate production (a surrogate for the glycolytic rate) and the mitochondrial OCR. The rate of glycolysis is the ECAR after the addition of glucose. Oligomycin (2 μM) was injected after glucose (10 mM) to inhibit mitochondrial respiration. 2DG (100 mM) injection reduced ECAR down to baseline by inhibiting hexokinase. Importantly, aged cells had high ECAR when compared with young cells (Figure 1A), whereas the OCR was comparable (Figure 1B). Of note, although oligomycin generally shifts energy production to glycolysis to increase ECAR in transformed cells, there was decreased ECAR after oligomycin injection in both primary young and aged cells. These results suggest that the glycolytic phenotype is elevated in aged lung relative to young lung.
Figure 1.
Glucose transporter 1 (GLUT1)–associated glycolysis is activated in aged lung. (A) The extracellular acidification rate (ECAR) was measured in young (2-month-old) and aged (18-month-old) cells isolated from whole lung (5 × 104 cells/well). Data are presented as mean ± SEM. *P < 0.05, **P < 0.01 by ANOVA. (B) The oxygen consumption rate (OCR) was measured in young and aged cells isolated from whole lung (5 × 104 cells/well). (C) Immunoblot assay of GLUT1 for lung lysates from young and aged mice. β-actin served as the standard. (D) Densitometry of immunoblot assay for GLUT1 in young and aged lung cells. Data are presented as mean ± SEM. ***P < 0.001 by ANOVA. (E) Analysis of GLUT1 mRNA expression in young and aged lung cells. Data are presented as mean ± SEM. Results are representative of three or more independent experiments. (F) Immunoblot assay for GLUT1 in aged lung fibroblasts treated with MG132 (0, 2.5, 5, 10 μM) for 1 hour. β-actin served as the standard. 2DG, 2-deoxyglucose.
To demonstrate the target of high glycolytic phenotype in aged lung, we analyzed the expression of enzymes in glycolytic pathway in lung tissues. Glucose transporters are responsible for modulating the uptake and use of glucose. Importantly, the protein expression of GLUT1 in lung tissues from aged mice was significantly increased compared with that from young mice (Figures 1C and 1D), whereas the Glut1 gene expression was unchanged (Figure 1E). We next investigated whether defective function of the ubiquitin proteasome system, a major degradation pathway of intracellular proteins, could contribute to altered posttranslational regulation of GLUT1. In response to MG132, a well-characterized proteasome inhibitor, protein levels of GLUT1 in aged lung fibroblasts were further increased (Figure 1F). These results suggest that GLUT1 induction is regulated by mechanisms involving proteasomal degradation. We next examined whether other glycolytic enzymes were responsible for the augmented glycolysis in the aged cells. Interestingly, the protein expressions of other glycolytic enzymes were comparable between aged and young lung tissues (see Figure E1A in the online supplement). Altogether, these results suggest that the induction of GLUT1 protein expression is responsible for the high glycolytic phenotype in aged lung.
Age-Dependent Lung Fibrogenesis Is Associated with the Expression of GLUT1
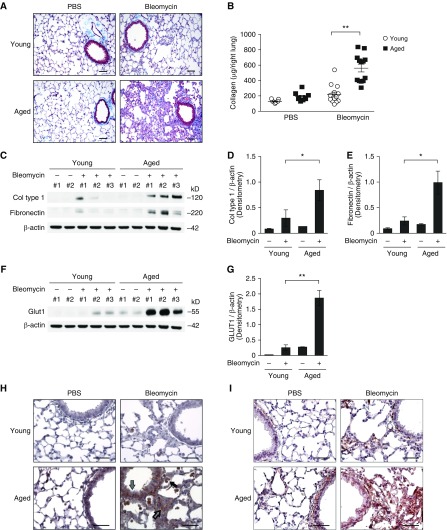
We evaluated the fibroproliferative activity of aged lung cells in response to bleomycin-induced lung injury. Fibrotic changes and collagen deposition in lung tissues after bleomycin instillation were measured using Masson trichrome staining. There was a detectable accumulation of collagen in the subepithelial area and interalveolar septum forming fibrotic foci at 14 days postinstillation (Figure 2A). To validate these results, we measured the acid-soluble collagen levels in lung tissues by Sircol assay. Consistently, the level of acid-soluble collagen was elevated significantly after bleomycin treatment in aged lung when compared with young lung (Figures 2B and E2A). Similarly, protein expression of collagen and fibronectin was also higher in aged lung tissue lysates (Figures 2C–2E). Moreover, aged mice showed progressive loss of body weight after bleomycin instillation, whereas young mice maintained their body weight (Figure E2B).
Figure 2.
Age-dependent lung fibrosis is associated with the expression of GLUT1 in vivo. Young and aged mice were instilled with bleomycin (0.01 mg/mouse via oropharyngeal aspiration) for 14 days. (A) Representative lung sections of young and aged mice stained with Masson trichrome staining. Scale bars, 200 μm. (B) Total lung collagen (Col) was quantified by Sircol assay (young/PBS, n = 11; aged/PBS, n = 9; young/bleomycin, n = 14, aged/bleomycin, n = 14). Data are presented as mean ± SEM. **P < 0.01 by ANOVA. (C) Immunoblot assay for Col type 1 and fibronectin in young and aged lung tissue lysates. β-actin served as the standard. (D) Densitometry of immunoblot assay for Col type 1 in young and aged lung tissue lysates. Densitometry of immunoblot assay for Col type 1 in young and aged lung tissue lysates. Data are presented as mean ± SEM. *P < 0.05 by ANOVA. (E) Densitometry of immunoblot assay for fibronectin in young and aged lung tissue lysates. Densitometry of immunoblot assay for fibronectin in young and aged lung tissue lysates. Data are presented as mean ± SEM. *P < 0.05 by ANOVA. (F) Immunoblot assay for GLUT1 in young and aged lung tissue lysates. β-actin served as the standard. (G) Densitometry of immunoblot assay for GLUT1 in young and aged lung tissue lysates. Densitometry of immunoblot assay for GLUT1 in young and aged lung tissue lysates. Data are presented as mean ± SEM. **P < 0.01 by ANOVA. Immunohistochemical staining of (H) GLUT1 and (I) α-smooth muscle actin in aged lung reveals enhanced signal in fibrotic foci (open arrow), epithelial cells (gray arrow), and inflammatory cells (solid arrow). Scale bars, 200 μm. Results are representative of three or more independent experiments.
To examine whether fibrotic changes in response to bleomycin-induced lung injury are linked to GLUT1 expression, we next examined GLUT1 protein levels in young and aged lung tissue after bleomycin instillation, using immunoblot and immunohistochemical analysis. Protein expression of GLUT1 was increased significantly in the aged lung, not only at baseline but also with bleomycin treatment (Figures 2F, 2G, and E2C). More specifically, GLUT1 expression was increased in various structural cells, including fibroblast foci, epithelial cells, subepithelial cells, and inflammatory cells, in aged lung tissues when compared with young lung tissues (Figure 2H). Importantly, protein expression of GLUT1 was markedly increased in fibrotic areas with high collagen deposits (Figures 2H and 2I). Taken together, these results suggest that age-driven lung fibrogenesis is associated with increased expression of GLUT1.
Glycolysis Is a Critical Metabolic Pathway for the Activation of Aged Fibroblasts
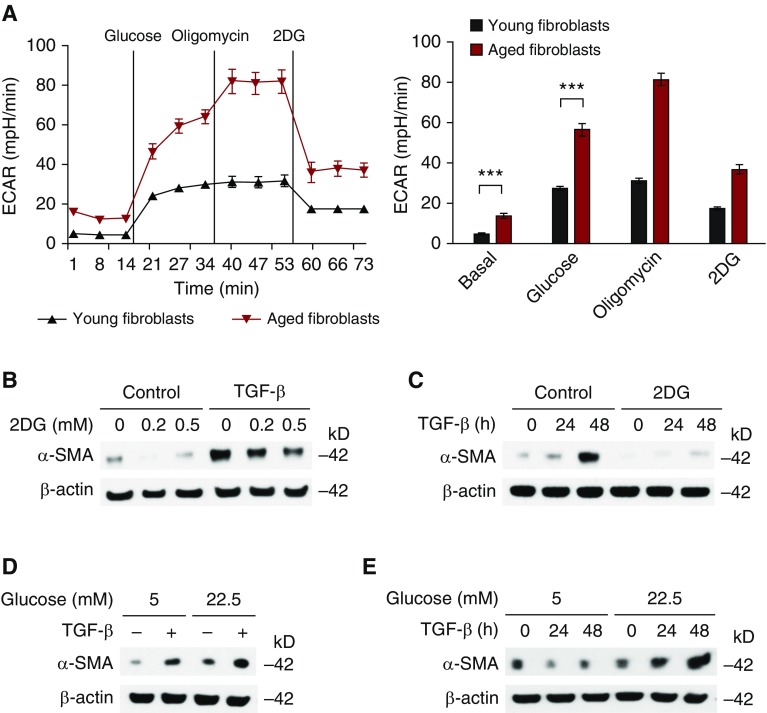
To investigate the effect of biological aging on glycolysis in fibroblasts, we measured the ECAR in primary young and aged lung fibroblasts. Consistent with high GLUT1 protein expression, the ECAR was significantly increased in aged fibroblasts (Figures 3A and S3). To examine the role of glycolysis activation in the stimulation and differentiation of lung fibroblasts, we treated primary aged lung fibroblasts with 2DG, a potent glycolysis inhibitor (31). Then we evaluated the stimulation and differentiation of aged lung fibroblasts in response to TGF-β, a key cytokine responsible for the activation of fibroblasts to myofibroblasts. 2DG significantly suppressed the protein expression of α-SMA in TGF-β–stimulated primary aged fibroblasts in a dose- and time-dependent manner (Figures 3B and 3C). Furthermore, an increase in glucose concentration augmented α-SMA expression in primary aged fibroblasts (Figures 3D and 3E). Taken together, these results suggest that glycolysis is a critical pathway for the activation of lung fibroblasts in response to TGF-β.
Figure 3.
Glycolysis is a critical metabolic pathway for the activation of lung fibroblasts. (A) ECAR was measured in young and aged lung fibroblasts (5 × 104 cells/well). Data are presented as mean ± SEM. ***P < 0.001 by ANOVA. (B) Immunoblot assay for α-smooth muscle actin (α-SMA) in aged lung fibroblasts pretreated with 2DG (0.2, 0.5 mM) for 1 hour before transforming growth factor-β (TGF-β) (20 ng/ml, 48 h) stimulation. (C) Immunoblot assay for α-SMA in aged lung fibroblasts pretreated with 2DG (0.5 mM) for 1 hour before TGF-β (20 ng/ml, 0, 24, 48 h) stimulation. (D) Immunoblot assay for α-SMA in cell lysates from aged lung fibroblasts pretreated with different concentrations of glucose (5.0 and 22.5 mM) before stimulation with TGF-β (20 ng/ml, 48 h). (E) Immunoblot assay for α-SMA in cell lysates from aged lung fibroblasts pretreated with different concentrations of glucose (5.0, and 22.5 mM) before stimulation with TGF-β (20 ng/ml, 0, 24, 48 h). β-actin served as the standard. Results are representative of three or more independent experiments.
GLUT1-Dependent Glycolysis is Critical for the Activation of Aged Fibroblasts
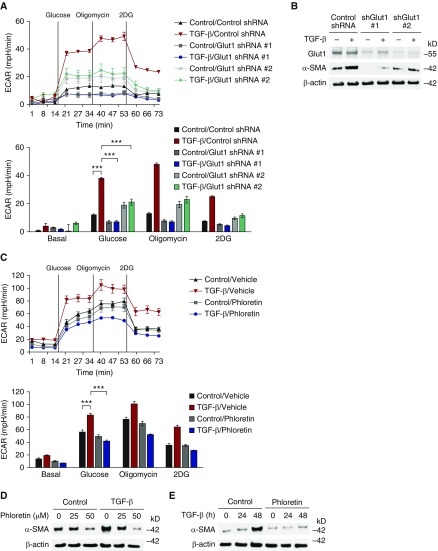
To analyze whether enhanced GLUT1 expression plays a critical role in the activation of aged lung fibroblasts, we next investigated the impact of GLUT1 deficiency on responses to TGF-β stimulation. To this end, primary aged lung fibroblasts were transfected with two independent GLUT1-specific shRNA before stimulation. ECAR induction in response to TGF-β was suppressed significantly in aged fibroblasts transduced with GLUT1 shRNA when compared with control (Figure 4A). Consistently, protein expression of α-SMA in response to TGF-β was also decreased by GLUT1 shRNA relative to control shRNA (Figures 4B, E4A, and E4B).
Figure 4.
GLUT1-dependent glycolysis is critical for the activation of lung fibroblasts and lung fibrogenesis. (A) ECAR was measured in aged lung fibroblasts (4 × 104 cells/well) transduced with lentiviruses expressing nontarget short hairpin RNA (shRNA) (control shRNA) or two independent shRNAs for GLUT1 before TGF-β (20 ng/ml, 48 h) stimulation. Data are presented as mean ± SEM. ***P < 0.001 by ANOVA. (B) α-SMA in cell lysates from aged lung fibroblasts transduced with lentiviruses expressing nontarget shRNA (control shRNA) or two independent shRNA for GLUT1 before TGF-β (20 ng/ml, 4 h) stimulation. β-actin served as the standard. (C) ECAR was measured in cell lysates from aged lung fibroblasts (4 × 104 cells/well) pretreated with phloretin (0, 25, 50 nM) 1 hour before TGF-β (20 ng/ml, 48 h) stimulation. Data are presented as mean ± SEM. ***P < 0.001 by ANOVA. (D) Immunoblot assay for α-SMA in cell lysates from aged lung fibroblasts pretreated with phloretin (0, 25, 50 nM) 1 hour before TGF-β (20 ng/ml, 4 h) stimulation. (E) Immunoblot assay for α-SMA in cell lysates from aged lung fibroblasts pretreated with phloretin (25 nM) 1 hour before TGF-β (20 ng/ml, 0, 24, 48 h) stimulation. β-actin served as the standard. Results are representative of three or more independent experiments. shGlut1, GLUT1 specific short hairpin RNA.
We next investigated the impact of pharmacologic inhibition of GLUT1 in aged fibroblast responses to TGF-β stimulation. We inhibited the activity of GLUT1 in aged fibroblasts via treatment with phloretin, a competitive GLUT1 inhibitor. As with GLUT1 shRNA, phloretin suppressed the induction of ECAR by TGF-β (Figure 4C). Moreover, the protein expression of α-SMA in response to TGF-β was decreased in a time- and dose-dependent manner when compared with vehicle control (Figures 4D, 4E, E4C, and E4D). These results suggest that GLUT1-dependent glycolysis is critical for the activation of aged lung fibroblasts in response to TGF-β.
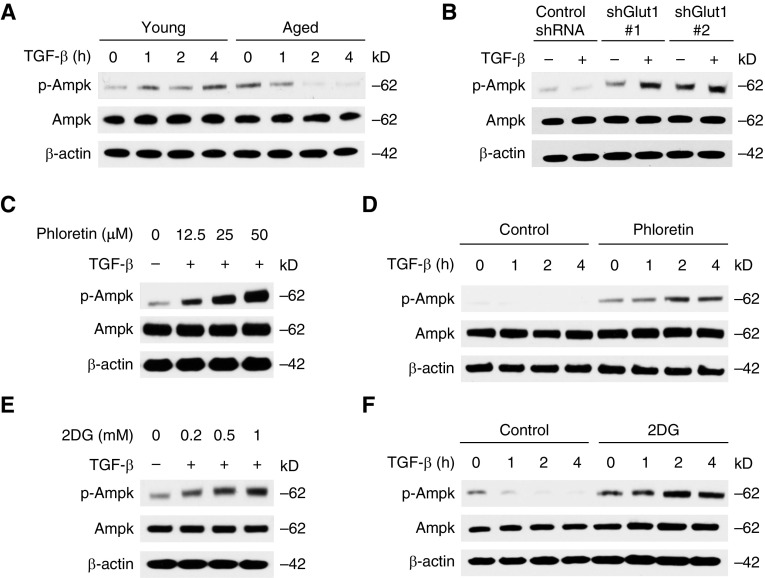
GLUT1-Dependent Glycolysis Inhibits the Activation of AMPK in Aged Fibroblasts
Next, we sought to investigate whether AMPK activation can serve as a downstream mechanism for GLUT1-dependent glycolysis. The phosphorylation of AMPK was suppressed significantly in the aged fibroblasts in response to TGF-β (Figures 5A and E5A). To determine the effect of GLUT1 on the activation of AMPK in aged lung fibroblasts, we used two independent shRNA for GLUT1. AMPK phosphorylation was enhanced by GLUT1 knockdown when compared with vehicle control (Figures 5B and E5B). We next investigated the impact of pharmacologic inhibition of GLUT1 in AMPK activation. Phloretin induced AMPK phosphorylation in a dose- and time-dependent manner (Figures 5C, 5D, E5C, and E5D). We also examined the role of glycolysis in AMPK activation by 2DG treatment to inhibit glycolysis. Similar to the effect of phloretin, 2DG increased AMPK phosphorylation (Figures 5E, 5F, E5E, and E5F). However, it had no effect on TGF-β–induced Smad3 phosphorylation (Figures E6A and E6B). These results suggest that GLUT1-dependent glycolysis regulates the activation of AMPK.
Figure 5.
Inhibition of GLUT1-dependent glycolysis induces AMP-activated protein kinase (AMPK) activation. (A) Immunoblot analysis for phosphorylated AMPK (p-AMPK) and AMPK in cell lysates from young and aged lung fibroblasts treated with TGF-β (5 ng/ml) for 1–4 hours. (B) Immunoblot analysis for p-AMPK and AMPK in cell lysates from aged lung fibroblasts transduced with lentiviruses expressing nontarget shRNA (control shRNA) or two independent shRNA for GLUT1 before TGF-β (5 ng/ml, 2 h) stimulation. (C) Immunoblot analysis for p-AMPK and AMPK in cell lysates from aged lung fibroblasts pretreated with phloretin (0, 12.5, 25, 50 nM) 1 hour before TGF-β (5 ng/ml, 2 h) stimulation. (D) Immunoblot analysis for p-AMPK and AMPK in cell lysates from aged lung fibroblasts pretreated with phloretin (25 nM) 1 hour before TGF-β (5 ng/ml, 0, 1, 2, 4 h) stimulation. (E) Immunoblot analysis for p-AMPK and AMPK in cell lysates from aged lung fibroblasts pretreated with 2DG (0.2, 0.5, 1 mM) for 1 hour before TGF-β (5 ng/ml, 2 h) stimulation. (F) Immunoblot analysis for p-AMPK and AMPK in cell lysates from aged lung fibroblasts pretreated with 2DG (0.5 mM) for 1 hour before TGF-β (5 ng/ml, 0, 1, 2, 4 h) stimulation. Results are representative of three or more independent experiments.
Phosphorylation of AMPK Attenuates the Activation of Aged Lung Fibroblasts
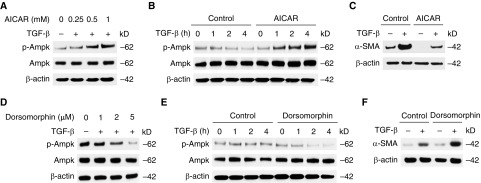
Because GLUT1-dependent glycolysis regulates AMPK activation, we examined whether the activation of AMPK can suppress lung fibroblast activation. We activated the AMPK signaling pathway using AICAR, an activator of AMPK. Consistent with previous observation, the phosphorylation of AMPK was enhanced in AICAR-stimulated lung fibroblasts in a dose-and time-dependent manner (Figures 6A, 6B, E7A, and E7B). Importantly, the protein expression of α-SMA in response to TGF-β was attenuated by AICAR treatment compared with vehicle control (Figures 6C and E7C). Next, we examined the effect of AMPK activation by using dorsomorphin, a potent AMPK inhibitor. Treatment with dorsomorphin significantly decreased AMPK phosphorylation in young fibroblasts (Figures 6D, 6E, E7D, and E7E). Unlike the case of AICAR treatment, the protein expression of α-SMA in response to TGF-β was enhanced by dorsomorphin treatment in young fibroblasts (Figures 6F and E7F). These results suggest that AMPK activation is a critical metabolic pathway involved in fibroblast activation in response to TGF-β.
Figure 6.
The phosphorylation of AMPK attenuates the activation of lung fibroblasts. (A) Immunoblot analysis for p-AMPK and AMPK in cell lysates from aged lung fibroblasts pretreated with 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR) (0, 0.25, 0.5, 1 mM) for 1 hour before TGF-β (5 ng/ml, 2 h) stimulation. (B) Immunoblot analysis for p-AMPK and AMPK in cell lysates from aged lung fibroblasts pretreated with AICAR (0.5 mM) for 1 hour before TGF-β (5 ng/ml, 0, 1, 2, 4 h) stimulation. (C) Immunoblot assay for α-SMA in cell lysates from aged lung fibroblasts pretreated with AICAR (0.5 mM) for 1 hour before TGF-β (20 ng/ml, 48 h) stimulation. (D) Immunoblot analysis for p-AMPK and AMPK in cell lysates from aged lung fibroblasts pretreated with dorsomorphin (0, 1, 2, 5 μM) for 1 hour before TGF-β (5 ng/ml, 2 h) stimulation. (E) Immunoblot analysis for p-AMPK and AMPK in cell lysates from aged lung fibroblasts pretreated with dorsomorphin (2 μM) for 1 hour before TGF-β (5 ng/ml, 0, 1, 2, 4 h) stimulation. (F) Immunoblot assay for α-SMA in cell lysates from aged lung fibroblasts pretreated with dorsomorphin (2 μM) for 1 hour before TGF-β (20 ng/ml, 48 h) stimulation. β-actin served as the standard. Results are representative of three or more independent experiments.
Pharmacologic Inhibition of GLUT1 Suppressed Lung Fibrogenensis in Aged Mice after Bleomycin Challenge
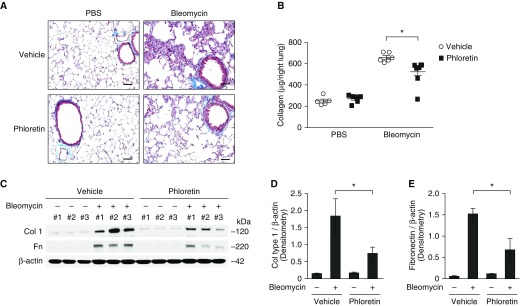
To investigate whether pharmacologic inhibition of GLUT1 can suppress lung fibrogenesis in vivo, we examined the impact of phloretin treatment on bleomycin-induced lung fibrosis. When compared with control, phloretin treatment inhibited the accumulation of collagen in the subepithelial area and interalveolar septum forming fibrotic foci (Figure 7A). The induction of acid-soluble collagen by bleomycin treatment was also decreased significantly by phloretin treatment when compared with vehicle control (Figure 7B). Moreover, phloretin treatment decreased the protein levels of collagen and fibronectin in aged lung tissues in response to bleomycin when compared with vehicle control (Figures 7C–7E). Furthermore, phloretin improved morbidity in bleomycin-instilled aged mice, whereas inflammatory responses were comparable between phloretin-treated and control mice (Figures E8A and E8B). These results suggest that pharmacologic inhibition of GLUT1-dependent glycolysis in aged lung results in decreased fibrogenesis in response to bleomycin instillation.
Figure 7.
Pharmacologic inhibition of GLUT1 attenuates lung fibrogenesis in vivo. Aged mice were treated with intraperitoneal injection of phloretin (10 mg/kg, every other day) after oropharyngeal bleomycin instillation (0.01 mg/mouse). (A) Representative lung sections of aged mice stained with Masson trichrome staining. Scale bars, 200 μm. (B) Total lung collagen was quantified by Sircol assay (PBS/vehicle, n = 5; PBS/phloretin, n = 6; bleomycin/vehicle, n = 6; bleomycin/phloretin, n = 8). Data are presented as mean ± SEM. *P < 0.05 by ANOVA. (C) Immunoblot assay for collagen type 1, fibronectin (Fn) in tissue lysates from aged lung at baseline and also after bleomycin challenge. β-actin served as the standard. (D) Densitometry of immunoblot assay for collagen type 1 in aged lung at baseline and also after bleomycin challenge. Data are presented as mean ± SEM. *P < 0.05 by ANOVA. (E) Densitometry of immunoblot assay for Fn in young and aged lung tissue lysates. Data are presented as mean ± SEM. *P < 0.05 by ANOVA.
Discussion
Fibrotic interstitial lung diseases, including IPF, are more prevalent in aging populations, with a sharp increase in incidence in those older than 50 years. To date, there is evidence that patients with IPF exhibit higher glycolytic activity in fibrotic areas; however, the mechanisms that contribute to this phenotype have not been well explored. In this report, we demonstrate that GLUT1-dependent glycolysis provides a critical mechanism for age-driven lung fibrogenesis. We also demonstrate that GLUT1-dependent glycolysis is a crucial metabolic pathway that is required for the activation and differentiation of lung fibroblasts. Furthermore, we show that pharmacologic inhibition of GLUT1 decreases collagen accumulation and lung fibrogenesis in response to bleomycin instillation.
Fibroblasts are the most common cell type of the connective tissues and are the principal source of the extensive ECM in the lung. Our current study illustrates that increased GLUT1 expression in primary aged fibroblasts contributes to elevated glycolytic rates and lactate production, which may increase glucose-dependent metabolic pathways within these cells. Furthermore, pharmacologic inhibition of GLUT1 resulted in decreased lung fibrogenesis in aged lung in response to bleomycin instillation. Enhanced GLUT1 expression in aged fibroblasts may serve as an age-associated mechanism to increase glucose availability while limiting oxidative metabolism of excess pyruvate and fatty acids when excess energetic requirements are placed on a cell. An age-associated increase in GLUT1-mediated glucose metabolism may contribute to alterations in metabolic programming and increased fibrotic accumulation of ECM in response to prolonged tissue injury and chronic inflammation.
Overexpression of GLUT1 has been observed in many human cancers, and GLUT1 expression levels have been inversely correlated with prognosis (32, 33). The function of GLUT1 in the context of aging and the molecular mechanisms leading to constitutive up-regulation of aerobic glycolysis in the aged cell have not been well defined. Here we demonstrate that GLUT1 expression and glycolysis are increased significantly in both untreated and bleomycin-instilled aged lung. These findings support the notion that aging is a steady adaptation process to cellular senescence and mitochondrial dysfunction. Augmenting glucose influx instead of increasing glycolytic enzymes in aged cells may serve as a mechanism to avert oncogenic transformation, which could occur via the indiscriminate, global up-regulation of glycolytic enzymes. However, it is possible that elevated GLUT1-mediated glucose uptake and metabolism may propel aged fibroblasts into a hyperinflammatory state, with increased activation of proinflammatory mediators. Previous work has illustrated a mechanistic link between GLUT1 overexpression and PAI-1, a known nuclear factor-κB target gene (34). It is plausible that age-associated enhancements in GLUT1 expression in fibroblasts may contribute to increased inflammation and fibrotic lesion development in aged lung in response to bleomycin instillation. Regulation of glucose transporters in aged cells may prove to be an important and unique metabolic phenomenon that can be targeted in developing treatments for age-related diseases.
In this study, we demonstrated that GLUT1 expression in aged lung was significantly increased without substantial changes in the levels of its mRNA. The question then arises as to how the protein expression of GLUT1 is up-regulated in aged lung. The answer could be provided by previous publications that show that GLUT1 expression, at least in part, is regulated by a protein degradation pathway (35, 36). Alterations in protein turnover during biological aging have been correlated with decreased activity of the proteasome system and autophagy (37), which may account for the increase in GLUT1 expression in the aged lung. In line with previous reports, our preliminary data demonstrate that enhanced GLUT1 expression in aged lung is regulated, at least in part, by proteasomal degradation. We can speculate that significantly decreased proteasome expression and activity in aged lung tissues contribute to heightened GLUT1-mediated glycolysis and fibrogenesis, when compared with young controls. Nonetheless, determining the specific mechanism responsible for GLUT1 degradation will require further study.
AMPK is a key metabolic regulator in several intracellular metabolic pathways including the use of glucose, fatty acids oxidation, and fatty acid synthesis (21, 22). The activation of AMPK is a critical event for homeostasis of cellular energy levels by regulating anabolic or catabolic energy pathways (21, 22). Although AMPK has been linked to aging and lifespan (38), little is known about the role of AMPK in aged lung. Interestingly, our results showed the down-regulation of AMPK activation in aged lung compared with young lung. These results suggest that aging mediates the metabolic alteration in the lung via changes in various cellular pathways that control AMPK.
Glycolysis in media was determined by ECAR, which is predominantly from the excretion of lactic acid per unit time after its conversion from pyruvate. ECAR measurements reflect a change in the pH of the assay medium. It is important to note that additional metabolic processes in cells, such as CO2 production by the trichloroacetic acid cycle, may alter pH and thereby confound ECAR measurements. To increase the accuracy of our measurements, we examined the impact of inhibitor treatment on ECAR responses in aged fibroblasts. Specifically, in response to GLUT1 inhibition by phloretin, glucose transport 4 inhibition (data not shown), or knockdown by shRNA, there is a significant reduction in ECAR when compared with vehicle-treated aged fibroblasts (Figure 4). Taken together, our results illustrate that although additional metabolic processes may contribute to ECAR, glycolysis-mediated lactate production underlies the significant differences between young and aged fibroblasts. Future work will need to be performed to examine the impact of aging on additional metabolic processes and the contribution of these responses to residual ECAR detection in fibroblasts.
Age-associated metabolic dysfunction is linked to risk factors for a variety of diseases such as type 2-diabetes, cardiovascular disease, and stroke (39, 40). The respiratory function of the lung has also been shown to decrease with biological aging (41). Our unpublished data show that the expression of genes in mechanistic target of rapamycin and fatty acid synthesis were decreased significantly in aged lung tissues. It is possible that as the supply of oxygen and respiratory function is deceased in aged lung (42), the aerobic metabolic functions, including oxidative phosphorylation in mitochondria or fatty acid metabolism, are also similarly suppressed (42–45).
Conclusions
In conclusion, inhibition of lung fibrogenesis by pharmacologic inhibition of GLUT1-dependent glycolysis provides a potential target of therapy for age-driven fibrotic disease. These findings may have broad implications for therapeutic targeting in human diseases, not only those of the lung, but also those in other fibrosis-susceptible organ systems.
Footnotes
This work was supported by National Institutes of Health (NIH) grants NIH K01AG034999 (H.W.S.-D.), NIH R21AG044755 (H.W.S.-D.), and NIH P01 HL114501 (A.M.K.C.), and by a Department of Medicine Seed Grant for Innovative Research, WCMC (H.W.S.-D.).
Author Contributions: Conception and design: S.J.C., J.-S.M., and H.W.S.-D.; analysis and interpretation: S.J.C., J.-S.M., C.-M.L., and H.W.S.-D.; drafting of the manuscript and review for important intellectual content: S.J.C., J.-S.M., A.M.K.C., and H.W.S.-D.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2016-0225OC on December 20, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 2.Fell CD, Martinez FJ, Liu LX, Murray S, Han MK, Kazerooni EA, Gross BH, Myers J, Travis WD, Colby TV, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:832–837. doi: 10.1164/rccm.200906-0959OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stout-Delgado HW, Cho SJ, Chu SG, Mitzel DN, Villalba J, El-Chemaly S, Ryter SW, Choi AM, Rosas IO. Age-dependent susceptibility to pulmonary fibrosis is associated with NLRP3 inflammasome activation. Am J Respir Cell Mol Biol. 2016;55:252–263. doi: 10.1165/rcmb.2015-0222OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, Meldrum E, Sanders YY, Thannickal VJ. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6:231ra47. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie N, Tan Z, Banerjee S, Cui H, Ge J, Liu RM, Bernard K, Thannickal VJ, Liu G. Glycolytic reprogramming in myofibroblast differentiation and lung fibrosis. Am J Respir Crit Care Med. 2015;192:1462–1474. doi: 10.1164/rccm.201504-0780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umeda Y, Demura Y, Morikawa M, Anzai M, Kadowaki M, Ameshima S, Tsuchida T, Tsujikawa T, Kiyono Y, Okazawa H, et al. Prognostic value of dual-time-point 18F-FDG PET for idiopathic pulmonary fibrosis. J Nucl Med. 2015;56:1869–1875. doi: 10.2967/jnumed.115.163360. [DOI] [PubMed] [Google Scholar]
- 9.Win T, Thomas BA, Lambrou T, Hutton BF, Screaton NJ, Porter JC, Maher TM, Endozo R, Shortman RI, Afaq A, et al. Areas of normal pulmonary parenchyma on HRCT exhibit increased FDG PET signal in IPF patients. Eur J Nucl Med Mol Imaging. 2014;41:337–342. doi: 10.1007/s00259-013-2514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon JS, Hisata S, Park MA, DeNicola GM, Ryter SW, Nakahira K, Choi AM. mTORC1-induced HK1-dependent glycolysis regulates NLRP3 inflammasome activation. Cell Reports. 2015;12:102–115. doi: 10.1016/j.celrep.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Moon JS, Lee S, Park MA, Siempos II, Haslip M, Lee PJ, Yun M, Kim CK, Howrylak J, Ryter SW, et al. UCP2-induced fatty acid synthase promotes NLRP3 inflammasome activation during sepsis. J Clin Invest. 2015;125:665–680. doi: 10.1172/JCI78253. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Thorens B, Mueckler M. Glucose transporters in the 21st century. Am J Physiol Endocrinol Metab. 2010;298:E141–E145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen LQ, Cheung LS, Feng L, Tanner W, Frommer WB. Transport of sugars. Annu Rev Biochem. 2015;84:865–894. doi: 10.1146/annurev-biochem-060614-033904. [DOI] [PubMed] [Google Scholar]
- 17.Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem. 2001;276:9519–9525. doi: 10.1074/jbc.M010144200. [DOI] [PubMed] [Google Scholar]
- 18.Kim MO, Lee YJ, Park JH, Ryu JM, Yun SP, Han HJ. PKA and cAMP stimulate proliferation of mouse embryonic stem cells by elevating GLUT1 expression mediated by the NF-κB and CREB/CBP signaling pathways. Biochim Biophys Acta. 2012;1820:1636–1646. doi: 10.1016/j.bbagen.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Zhang C, Liu J, Liang Y, Wu R, Zhao Y, Hong X, Lin M, Yu H, Liu L, Levine AJ, et al. Tumour-associated mutant p53 drives the Warburg effect. Nat Commun. 2013;4:2935. doi: 10.1038/ncomms3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wieman HL, Wofford JA, Rathmell JC. Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking. Mol Biol Cell. 2007;18:1437–1446. doi: 10.1091/mbc.E06-07-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, et al. Structure of mammalian AMPK and its regulation by ADP. Nature. 2011;472:230–233. doi: 10.1038/nature09932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 25.Reznick RM, Zong H, Li J, Morino K, Moore IK, Yu HJ, Liu ZX, Dong J, Mustard KJ, Hawley SA, et al. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18:3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greer EL, Banko MR, Brunet A. AMP-activated protein kinase and FoxO transcription factors in dietary restriction-induced longevity. Ann N Y Acad Sci. 2009;1170:688–692. doi: 10.1111/j.1749-6632.2009.04019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenesen D, Suh JM, Seo J, Yu K, Lee KS, Kim JS, Min KJ, Graff JM. Adenosine nucleotide biosynthesis and AMPK regulate adult life span and mediate the longevity benefit of caloric restriction in flies. Cell Metab. 2013;17:101–112. doi: 10.1016/j.cmet.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H, Moon SY, Kim JS, Baek CH, Kim M, Min JY, Lee SK. Activation of AMP-activated protein kinase inhibits ER stress and renal fibrosis. Am J Physiol Renal Physiol. 2015;308:F226–F236. doi: 10.1152/ajprenal.00495.2014. [DOI] [PubMed] [Google Scholar]
- 30.Seluanov A, Vaidya A, Gorbunova V. Establishing primary adult fibroblast cultures from rodents. J Vis Exp. 2010;5:2033. doi: 10.3791/2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 32.Kunkel M, Reichert TE, Benz P, Lehr HA, Jeong JH, Wieand S, Bartenstein P, Wagner W, Whiteside TL. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97:1015–1024. doi: 10.1002/cncr.11159. [DOI] [PubMed] [Google Scholar]
- 33.Carvalho KC, Cunha IW, Rocha RM, Ayala FR, Cajaíba MM, Begnami MD, Vilela RS, Paiva GR, Andrade RG, Soares FA. GLUT1 expression in malignant tumors and its use as an immunodiagnostic marker. Clinics (Sao Paulo) 2011;66:965–972. doi: 10.1590/S1807-59322011000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freemerman AJ, Johnson AR, Sacks GN, Milner JJ, Kirk EL, Troester MA, Macintyre AN, Goraksha-Hicks P, Rathmell JC, Makowski L. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289:7884–7896. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez-Serra P, Marcilla M, Villanueva A, Ramos-Fernandez A, Palau A, Leal L, Wahi JE, Setien-Baranda F, Szczesna K, Moutinho C, et al. A DERL3-associated defect in the degradation of SLC2A1 mediates the Warburg effect. Nat Commun. 2014;5:3608. doi: 10.1038/ncomms4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernandes R, Carvalho AL, Kumagai A, Seica R, Hosoya K, Terasaki T, Murta J, Pereira P, Faro C. Downregulation of retinal GLUT1 in diabetes by ubiquitinylation. Mol Vis. 2004;10:618–628. [PubMed] [Google Scholar]
- 37.Kevei É, Hoppe T. Ubiquitin sets the timer: impacts on aging and longevity. Nat Struct Mol Biol. 2014;21:290–292. doi: 10.1038/nsmb.2806. [DOI] [PubMed] [Google Scholar]
- 38.Burkewitz K, Zhang Y, Mair WB. AMPK at the nexus of energetics and aging. Cell Metab. 2014;20:10–25. doi: 10.1016/j.cmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Houtkooper RH, Argmann C, Houten SM, Cantó C, Jeninga EH, Andreux PA, Thomas C, Doenlen R, Schoonjans K, Auwerx J. The metabolic footprint of aging in mice. Sci Rep. 2011;1:134. doi: 10.1038/srep00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teramoto S, Matsuse T, Ouchi Y. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;14:1454–1455. [PubMed] [Google Scholar]
- 42.Bueno M, Lai YC, Romero Y, Brands J, St Croix CM, Kamga C, Corey C, Herazo-Maya JD, Sembrat J, Lee JS, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest. 2015;125:521–538. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida GJ. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res. 2015;34:111. doi: 10.1186/s13046-015-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown K, Liu Y, Chen D. Aging: the mitochondrial connection. J Clin Exp Pathol. 2012;S4 [Google Scholar]