Abstract
α6β4 integrin is localized in a unique punctate distribution at the cell–substratum interface along the leading front of single, front–rear–polarized A549 cells. These puncta are interspersed between focal adhesions and lack association with the actin cytoskeleton. Knockdown of β4 integrin in A549 cells inhibits their directed migration, with knockdown cells exhibiting large focal adhesions and reduced actin dynamics. Despite these changes, the speed of knockdown cells is equivalent to control cells. Interestingly, in such cells, α6 integrin retains its punctate distribution. Moreover, in β4 integrin knockdown cells, we observe a loss of β1 integrin from focal adhesions and an enhanced association with α6 integrin. We confirmed the switch in the β integrin binding partner of α6 integrin in the knockdown cells by immunoprecipitation. We next investigated the role of β4 integrin in collective cell migration. Wounded monolayers of β4 integrin knockdown cells exhibit reduced collective migration compared with controls. When we forced expression of β4 integrin in the leader cells of wounded monolayers, collective migration was restored. Similarly, forced expression of β4 integrin in primary rat alveolar epithelial cells also promotes collective cell migration. In addition, we interrogated the pathway by which β4 integrin regulates A549 cell–directed migration. Constitutively active Ras-related C3 botulinum toxin substrate 1 rescues motility defects resulting from β4 integrin deficiency. Together, our results support the hypothesis that α6β4 integrin is a positive regulator of collective cell migration of A549 cells through influence on signal pathways in leader cells.
Keywords: cell motility, cell adhesion, cytoskeleton, directed motility, matrix adhesions
Clinical Relevance
The impact of this research relates to the role of an important integrin expressed by progenitor cells in the lung and lung tumor cells. We demonstrate for the first time that integrin β4 is a regulator of sheets of epithelial cells.
β4 integrin is the most unusual of all the β integrin subunits, because its cytoplasmic domain is over 1,000 amino acids long (1). It is best known as a component of the hemidesmosome, a stable adhesion device that anchors epithelial cells to the basement membrane in a variety of tissues (2).
α6 integrin is an obligate partner of β4 integrin and α6β4 integrin heterodimers bind to laminin in the extracellular matrix (3). α6β4 integrin is found in a variety of normal cell types that lack bona fide hemidesmosomes both in vitro and in vivo (4, 5). In such cells, the functions of α6β4 integrin are controversial, at least with respect to its role in motility versus stable attachment (6–11). For example, we have reported that, in single epidermal cells, α6β4 integrin supports motile behavior, whereas others have reported that loss of β4 integrin promotes migration (7, 12). Nonetheless, it has been well established that α6β4 integrin regulates tumor cell migration (1, 13). This helps explain the correlation between α6β4 integrin expression levels and poor prognosis in several tumor types. For example, high expression of β4 integrin is associated with aggressiveness of lung tumors (14–16). In this regard, the widely used chemotherapeutic agent, all-trans retinoic acid (ATRA), reduces β4 integrin expression, while at the same time impeding the collective migration of the Lewis lung cancer cell line C87 (17). Together, these findings lead us to hypothesize that β4 integrin not only regulates single, but also collective, epithelial cell motility. Here, we tested this hypothesis using A549 cells.
Materials and Methods
Cell Culture
A549 cells, purchased from American Type Culture Collection (ATCC), and 804G (rat bladder) cells were cultured as described elsewhere (18, 19). Scrambled (SCR) and β4 integrin knockdown (β4KD) A549 cells were generated by transduction of wild-type (WT) cells with a lentiviral vector, encoding scrambled short hairpin RNA (shRNA) or β4 integrin–specific shRNA, respectively (Sigma-Aldrich, St. Louis, MO). Primary rat alveolar type II cells were isolated as detailed previously (18). Adenovirus encoding green fluorescent protein (GFP), GFP-tagged β4 integrin (GFPβ4), shRNA against α6 integrin, GFP-tagged full-length α6 integrin, dominant-negative Ras-related C3 botulinum toxin substrate 1 (Rac1), or constitutively active Rac1 were described previously (7, 20). Adenovirus encoding red fluorescent protein–tagged LifeAct was purchased (Ibidi USA, Inc., Madison, WI).
Quantitative PCR
Quantitative PCR reactions were performed as detailed elsewhere (20).
Drug Treatments
ATRA, mitomycin C, and epidermal growth factor (EGF) were purchased from Sigma-Aldrich.
Immunocytochemistry and Flow Cytometry
Immunocytochemistry and flow cytometry were undertaken as detailed previously (20). Mouse monoclonal antibodies against β1 and β4 integrin (6S6 and 450-11A) were purchased from EMD Millipore (Billerica, MA) and BD Biosciences (San Jose, CA). Monoclonal rabbit anti-paxillin antibody (Y113) was purchased from Abcam (Cambridge, MA), and the rat antibody against α6 integrin (J1b5) was described previously (20). Actin was localized using rhodamine-conjugated phalloidin (Thermo Fisher, Rockford, IL). Secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). Images were captured using a TCS SP5 confocal microscope (Leica Microsystems, Buffalo Grove, IL).
Immunoprecipitation, SDS-PAGE, and Immunoblotting
Western blotting and immunoprecipitation experiments were performed as outlined elsewhere (8).
Rac1 Activity Assay
Rac1 activity was assayed using a Rac1 G-LISA kit (Cytoskeleton Inc., Denver, CO).
Actin Dynamics Assay
Kymographs were generated from images of live cells infected with adenovirus encoding red fluorescent protein–LifeAct (Ibidi USA, Inc.) and used to determine the speed of actin arc movement (21).
Single Cell Motility and Conventional Scratch Assays
These assays were performed using previously described procedures (7). Laminin-332–coated surfaces were generated as detailed elsewhere (7).
Hybrid Sheet Scratch Assays
A published protocol was modified to generate scratched hybrid sheets (22). β4KD2 cells were infected with adenovirus encoding GFPβ4 and, 24 hours later, were plated into a single well. Cells on the right half of the well were scraped away 6 hours later and then β4KD2 cells were plated on top. After 21 hours, the media were supplemented with 4′,6-diamidino-2-phenylindole (Thermo Fisher) and mitomycin C to inhibit proliferation. At 3 hours later, a second scrape was made at a shallow angle relative to the first, leaving two regions of interest. The first region had β4KD2 leader and follower cells, whereas the second region had β4KD2 leader cells expressing GFPβ4 and nonexpressing β4KD2 follower cells. Phase images and fluorescent images were captured of the scrape area every 10 minutes for 12 hours. The positions of cells at the front of the scratch were determined using the TrackMate plugin (http://fiji.sc/trackmate) for Fiji (21). Scratch displacement was defined as the average displacement of these cells in the direction perpendicular to the leading front.
Statistical Analysis
Statistical significance was determined by ANOVA or t test as indicated. P less than 0.05 was considered significant.
Results
α6β4 Integrin Localization in A549 Cells
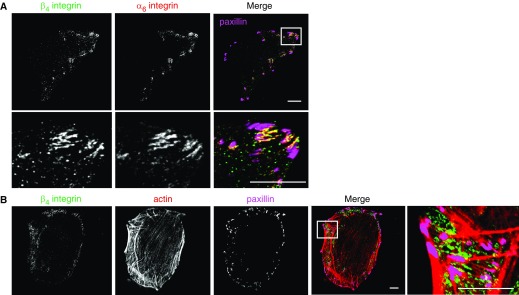
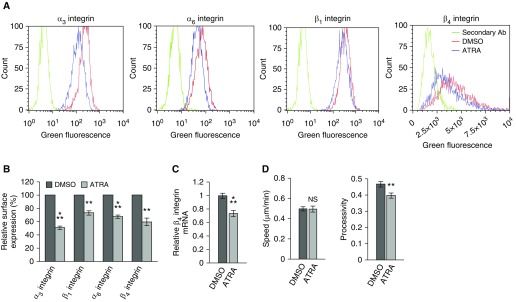
In A549 cells, β4 integrin codistributes with α6 integrin along their basal surface in punctate arrays near the membrane edge (Figure 1A). These arrays are spatially distinct from focal adhesions and are distributed more extensively between actin bundles at the edges of cells, rather than at their termination points (Figures 1A and 1B). Consistent with the work of others using lung cancer cell types, the retinoid ATRA reduces both expression of β4 integrin and the motility of A549 cells (Figure 2) (17). Specifically, ATRA-treated cells exhibit a 45% reduction of β4 integrin surface expression and a 25% reduction of β4 integrin message level (Figures 2A–2C). In addition, the ATRA-treated cells also exhibit reduced processivity, a measure of directed migration (Figure 2D). Processivity was defined as: maximum displacement from the cell’s origin divided by the length of the path traveled (8). However, because ATRA treatment also alters the expression of the integrins α3, α6, and β1 (Figures 2A and 2B), these studies do not allow us to determine whether effects of ATRA on motility are due to β4 integrin loss or loss of another integrin. To resolve this issue, we selectively knocked down β4 integrin in A549 cells and assayed the motility of the knockdown cells.
Figure 1.
α6β4 integrin exhibits a polarized distribution in A549 cells. (A) Confocal images of β4 integrin (green), α6 integrin (red), and the focal adhesion protein paxillin (pink). Scale bars, 10 µm. Lower panel, high magnification of the region in the inset. (B) Confocal images of β4 integrin (green), rhodamine-phalloidin as a marker of F-actin (red), and paxillin (pink). Scale bars, 10 µm. Right panel, high magnification of the region in the inset.
Figure 2.
All-trans retinoic acid (ATRA) reduces integrin expression in A549 cells. Cells were treated with 100 nM ATRA or vehicle every 24 hours for 48 hours and then prepared for (A and B) flow cytometry, (C) quantitative PCR (qPCR; three experiments, three replicates per experiment), and (D) 2-hour motility assays. (A) Flow cytometry for integrins α3, α6, β1, and β4. (B) The relative average surface expression of the indicated integrins was calculated from flow cytometry data (3 experiments, 4 × 103 events per sample). (D) Quantification of the speed and processivity of ATRA- and vehicle-treated cells (3 experiments, >140 cells). Results are presented as means (±SEM). Statistical analyses: t test. NS, not significant; **P < 0.01, ⁂P < 0.001.
Knockdown of β4 Integrin Reduces Directed Migration
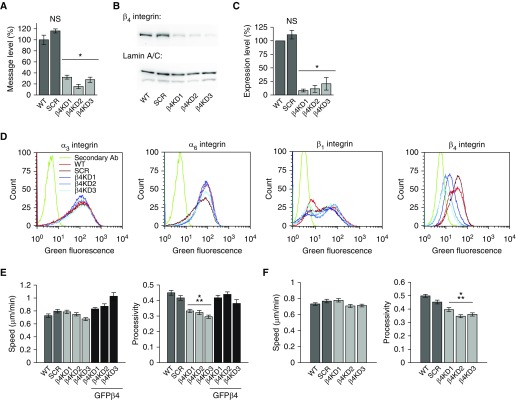
Three stable β4KD A549 clones (β4KD1–3) and a scrambled shRNA-expressing control (SCR) were generated by lentiviral means and selected for experimentation (Figure 3). All β4KDs exhibit knockdown of β4 integrin by greater than 70% at the message level and greater than 75% at the protein level (Figures 3A–3C). In addition, compared with WT and SCR controls, they exhibit reduced β4 integrin surface expression and no differences in the surface expression of α3, α6, and β1 integrins (Figure 3D). Moreover, β4KD cells migrated less processively than controls (Figure 3E). Furthermore, this phenotype is rescued upon infection with an adenovirus encoding GFPβ4, the message of which is refractory to the shRNA used for knockdown (Figure 3E). Because the preferred ligand of β4 integrin is laminin-332, we also compared the motility of control and β4KD cells plated onto dishes coated with laminin-332 (Figure 3F). Although both speed and processivity are increased compared with cells plated onto tissue culture plastic, our findings, that β4KD results in a reduction in processivity, are recapitulated when cells move on a laminin-332 matrix (Figure 3F). Together, these data indicate that β4 integrin supports the motility of A549 cells.
Figure 3.
β4 integrin knockdown (β4KD) inhibits the directed migration of A549 cells. (A) β4 integrin mRNA levels in wild-type (WT) and scrambled shRNA-expressing (SCR) controls and β4KD cells by qPCR (three experiments, three replicates per experiment). (B) Protein extracts were made from control and β4KD cells and processed for immunoblotting. Immunoblotting was performed using antibodies against β4 integrin and lamin A/C (loading control). A representative blot from three experiments is shown. (C) Quantification of results presented in B. (D) Surface expression of α3, α6, β1, and β4 integrin subunits (three experiments, 105 events). (E) Speed and processivity of WT, SCR, β4KD, and β4KD cells expressing green fluorescent protein (GFP)–tagged β4 integrin (GFPβ4; 3 experiments, >70 cells). (F) Speed and processivity of WT, SCR, and β4KD cells on laminin-332–rich matrix (3 experiments, >200 cells). Results are presented as means (±SEM). Statistical analyses: t test (A and C); ANOVA (E and F). *P < 0.05, ⁂P < 0.001. shRNA, short hairpin RNA.
β1 Integrin Associates with α6 Integrin in β4KD Cells
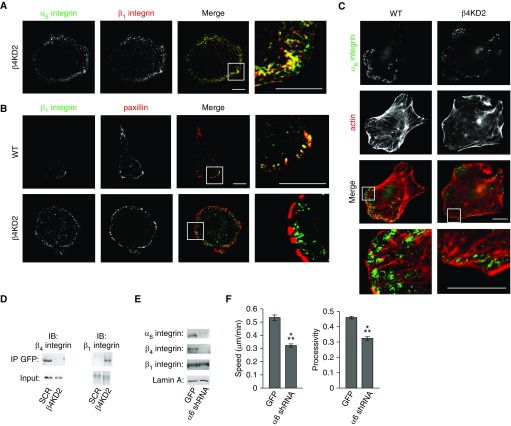
Remarkably, even in β4KDs, α6 integrin localizes in the same distinct punctate arrays along the substratum-attached surface of the cells as it does in controls (Figure 4A). In control cells, β1 integrin localizes to a subset of focal adhesions, primarily at the leading front of single cells (Figure 4B). Surprisingly, β1 integrin appears to be lost from the focal adhesions of β4KDs (Figure 4B). Rather, in β4KDs, α6 and β1 integrin are found together and group around actin bundles, rather than localize at their endpoints (Figure 4C). We quantified β1 integrin association with focal adhesions by comparing the number of β1 integrin–positive focal adhesions to the total number of focal adhesions in 11 control and 11 knockdown cells, in two separate experiments in which cells were immunostained for β1 integrin and paxillin 24 hours after plating onto coverslips. β1 integrin localized to 53% of 750 paxillin–positive focal adhesions in control cells, whereas β1 integrin localized with only 7% of 605 focal adhesions in β4KD2 cells (Figure 4B).
Figure 4.
β1 integrin associates with α6 integrin in β4KD cells. (A) Confocal images of a β4KD2 cell expressing GFP-tagged α6 integrin (green) and immunostained for β1 integrin (red). Right panel, high magnification of the region in the inset. Scale bars, 10 µm. (B) Confocal images of WT and β4KD2 cells immunostained for β1 integrin (green) and paxillin (red) show that β1 integrin colocalizes with paxillin in a subset of focal adhesions in WT cells but not in β4KD cells. Right panels, high magnification of the regions in the insets. Scale bars, 10 µm. (C) Confocal images of WT and β4KD2 cells immunostained for α6 integrin (green) and rhodamine-phalloidin to mark F-actin (red). Lower panels, high magnification of the regions in the insets. Scale bars, 10 µm. (D) SCR and β4KD2 cells were infected with adenovirus encoding GFP-tagged α6 integrin. At 2 days after infection, cells were lysed in radioimmunoprecipitation assay buffer and immunoprecipitated with anti-GFP antibodies conjugated to agarose beads. Samples were then processed via SDS-PAGE and probed with antibodies against β1 and β4 integrin. IB, immunoblot; IP, immunoprecipitation. (E) Protein extracts were prepared from WT cells infected with adenovirus encoding GFP or α6 shRNA. After SDS-PAGE and transfer to a nitrocellulose membrane, extracts were immunoblotted with antibodies against the indicated proteins (three experiments). (F) Quantification of speed and processivity of GFP and α6 shRNA–expressing cells (3 experiments, >130 cells). Results are presented as means (±SEM). Statistical analyses: t test; ⁂P < 0.001.
Focal adhesion organization is also perturbed in knockdown cells. Specifically, focal adhesions tend to be oriented parallel to the cell edge rather than perpendicular to it (Figure 4B). Finally, we confirmed that α6 integrin switches β subunit binding partners as a result of β4KD by immunoprecipitation, implying that α6 integrin acts as a sink for β1 integrin in the absence of β4 integrin (Figure 4D). Moreover, because there is no increase in total β1 integrin cell surface expression, these data indicate that β4KD results in a depletion of β1 integrin from focal adhesions.
Thus, there are two explanations for the defects in motility that we detail in β4KD cells. These are: (1) loss of α6β4 integrin complexes has a direct negative impact on migration; or (2) formation of α6β1 integrin complexes negatively regulates migration. To distinguish between these possibilities, we disrupted α6β4 integrin complexes by knocking down α6 integrin in control cells (Figure 4E). α6 integrin knockdown cells exhibit reduced total and cell surface levels of β4 integrin, but no change in α3 or β1 integrin (Figure 4E and data not shown). Moreover, they exhibit a 25% reduction in speed and processivity (Figure 4F). These data support the notion that α6β4 integrin is a positive regulator of processivity.
β4 Integrin Modulates EGF-Mediated Motility and Rac1 Activity
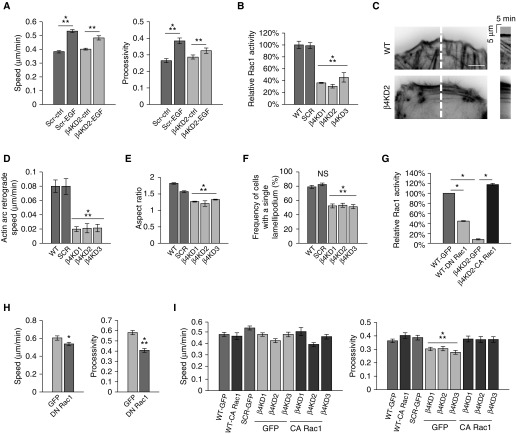
β4 integrin has been reported to be necessary for EGF-induced motility via Rac1 (23). Thus, we tested whether this is the case in A549 cells. Specifically, we assayed the motility of serum-starved A549 WT and β4KD cells in the presence or absence of EGF (Figure 5A). Though EGF increased the speed and processivity of WT cells by 60%, it increased the speed and processivity of β4KD2 cells by less than 30%. Thus, our results indicate that β4 integrin is a positive regulator of EGF-mediated motility, consistent with the work of others (23, 24).
Figure 5.
β4 integrin expression regulates Ras-related C3 botulinum toxin substrate 1 (Rac1) signaling and actin dynamics. (A) After a 1-hour incubation in vehicle or 10 ng/ml epidermal growth factor (EGF), the motility of SCR and β4KD2 cells was assayed. (B) A Rac1 G-LISA activation assay was performed on cells 2 days after plating (two experiments). (C) Actin arc bundle retrograde movement was visualized in cells expressing red fluorescent protein–tagged LifeAct. Kymographs (right panels) were generated along the 5-pixel-wide line (red) shown in the left panels. Scale bar, 10 µm. (D) Quantification of kymographs, two examples of which are shown in C (3 experiments, >10 cells per experiment). (E) Quantification of cellular aspect ratio (3 experiments, >100 cells). (F) Quantification of the number of cells with exactly one lamellipodium (3 experiments, >100 cells per experiment). (G) A Rac1 G-LISA activation assay was performed on cells 2 days after infection with adenovirus encoding the indicated protein (two experiments). (H) Quantification of the speed and processivity of GFP or dominant-negative Rac1 (DN Rac1)–expressing A549 cells (3 experiments, >25 cells per experiment). (I) Quantification of the speed and processivity of WT, SCR, β4KD cells, and both WT and β4KD cells expressing constitutively active Rac1 (CA Rac1; 3 experiments, >25 cells per experiment). Results are presented as means (±SEM). Statistical analyses: t test (H); ANOVA (B, D–F, and I). Because ANOVA indicated differences between groups in A and G, specific groups were compared by t test, as indicated. *P < 0.05, **P < 0.01, ⁂P < 0.001.
A number of studies indicate that EGF regulates Rac1 activity, and thereby determines directed migration by modifying actin dynamics and cell polarity (23, 25–27). We therefore assayed Rac1 activity in control and β4KD cells. Rac1 activity was reduced 55% in β4KDs compared with controls (Figure 5B). Consistent with this finding, β4KDs also exhibited reduced actin dynamics and polarity (Figures 5C–5E). Kymographic analyses of movies generated from control and β4KD cells expressing the fluorescently tagged F-actin marker, LifeAct, revealed that the speed of actin arc bundle retrograde movement in β4KDs was 25% of the speed of controls (Figures 5C and 5D). Knockdown cells also exhibited reduced aspect ratio (Figure 5E). In addition, whereas roughly 80% of control cells possessed one lamellipodium, only about 50% of knockdown cells did so (Figure 5F). Together, these data indicate that β4KDs are less polarized than controls.
The above results suggest that β4 integrin regulates A549 cell migration via an impact on Rac1 activation. To test this hypothesis, we first assayed the motility of A549 cells infected with either an adenovirus encoding a dominant-negative or constitutively active Rac1 construct. We confirmed the efficacy of these constructs using a Rac1 G-LISA kit (Figure 5G). Cells expressing the dominant-negative Rac1 construct exhibited a less than 15% reduction in speed and a 30% reduction in processivity compared with controls (Figure 5H). The processivity of β4KDs infected with an adenovirus encoding constitutively active Rac1 was restored to the same level as controls, whereas speed was unaffected (Figure 5I).
β4 Integrin Regulates Collective Migration
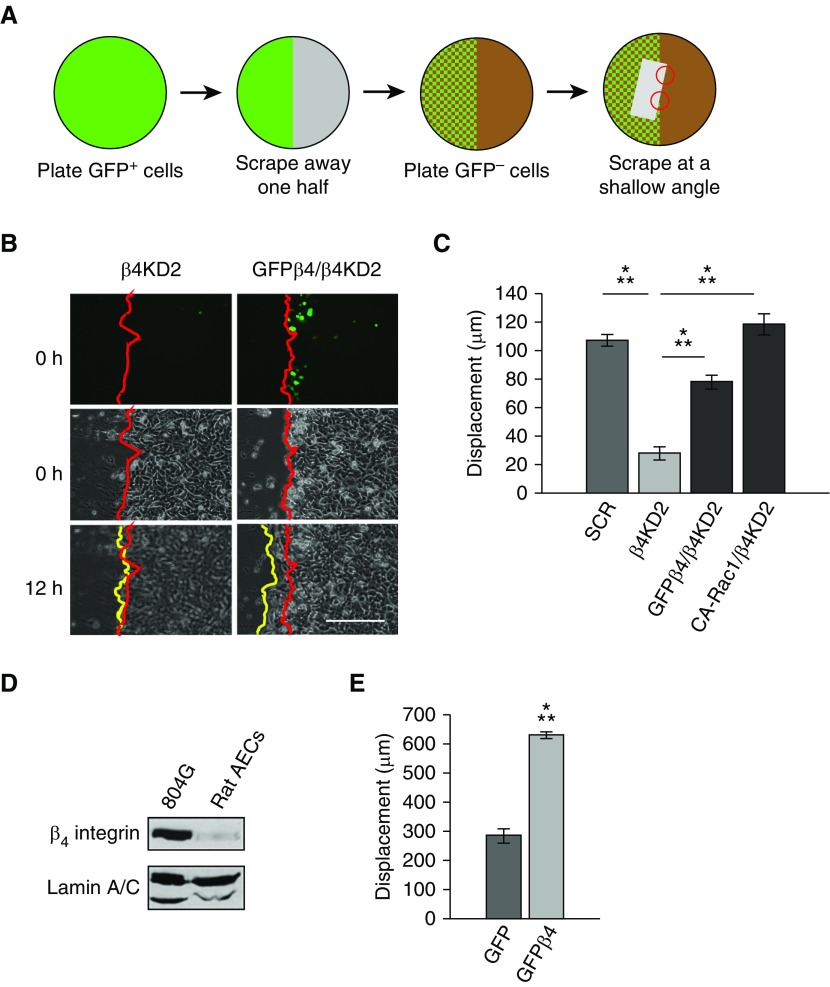
Thus far, our results have focused on single-cell migration. However, in vivo, processes, such as development, wound healing, and metastasis, are all products of collective migration (28–31). In particular, these processes tend to be driven by cells situated at the leading front of a cell sheet (leader cells), a phenotype that has been shown to be mediated by Rac1 (22, 30, 32–36). Therefore, we sought to determine the role of β4 integrin in leader cells during collective cell migration. To do this, we modified the procedure used by Chapnick and Liu (22) to develop scratched “hybrid sheets” of β4KD cells in which the leader cells were either β4KD2 cells or β4KD2 cells forced to express GFPβ4 (Figures 6A and 6B). Next, we performed time-lapse microscopy for 12 hours and compared scratch closure (Figure 6B). Scratched sheets of β4KD2 cells migrated 20% as far as did scratched sheets of control cells (Figure 6C). Moreover, scratched sheets of β4KD2 cells in which the leader cells expressed either GFPβ4 or constitutively active Rac1 migrated nearly as much as controls (Figure 6C).
Figure 6.
Hybrid monolayers. (A) Schematic of procedure to create hybrid cell monolayers. A scrape (indicated by the gray rectangle) was made in a hybrid monolayer composed of β4KD and β4KD cells expressing GFP-tagged β4 integrin (GFPβ4) or CA-Rac1. The angle of the scrape allowed us to analyze healing of sheets of knockdown cells in which all follower cells were β4KD cells and the leading cells were β4KD cells (upper red circle in A) or either knockdown cells expressing GFPβ4 (GFPβ4/β4KD) or CA-Rac1 (CA-Rac1/β4KD) (lower red circle in A). (B) Fluorescence and phase–contrast images of the two different regions in A. Red lines indicate the edges of the original scratch, whereas the yellow lines indicate the wound edge at 12 hours after wounding. In the upper right panel, several GFPβ4 integrin–positive cells are close to the leading edge. Note that the GFPβ4/KD2 cell combination has begun to move over the wound, whereas the KD cells have not. Scale bar, 300 µm. (C) Quantification of hybrid sheet experiments (3 experiments, >10 scratches). ANOVA indicated differences between groups. Specific groups were compared by t test, as indicated. (D) Western blot of β4 integrin in rat bladder 804G cells and primary rat alveolar epithelial cells (AECs) shows low expression levels in freshly isolated cells. (E) Quantification of the leading-edge displacement of scratched epithelial sheets of rat AECs infected with adenovirus encoding GFP or GFPβ4 (17 scratches, t test). Results are presented as means (±SEM). ⁂P < 0.001.
Finally, we also assayed the role of β4 integrin in the collective migration of primary rat alveolar epithelial cells. These cells expressed little endogenous β4 integrin (Figure 6D), consistent with previous reports (18). Intriguingly, there is a dramatic and significant increase in their collective migration after the forced expression of GFPβ4 (Figure 6E).
Discussion
The role of β4 integrin in motility is controversial, with some published studies reporting that it is a positive regulator and others indicating that it is a negative regulator (6). For example, in migrating keratinocytes, α6β4 integrin assembles into punctate arrays at their leading fronts, which appear to facilitate cell “steering” (7, 8). However, these complexes have also been reported to inhibit motility when associated with keratins (9). Interestingly, there are also conflicting reports concerning the motility functions of β4 integrin in the A549 cell line (10, 11). One of the objectives of the present study was to address this controversy. To do so, we investigated the function of β4 integrin in both single and collective cell migration. Our data indicate that β4 integrin is a positive regulator of motility by determining directed migration. This is consistent with several reports of the role of β4 integrin in keratinocytes (25). Moreover, our finding that loss of β4 integrin impacts processivity, whereas loss of α6 integrin impacts both processivity and speed, implies that α6 integrin–containing heterodimers, regardless of the β integrin binding partner, determines speed.
Remarkably, our study also indicates that the expression of β4 integrin in leader cells is sufficient to induce migration of an entire epithelial cell sheet. The importance of leader cells in determining collective migration in tissue remodeling has been well established (32, 34). Most notably, work by Wang and colleagues (37) has shown that the selective activation of Rac1 in leader cells is sufficient to induce collective migration. Our finding, that expressing either β4 integrin or constitutively active Rac1 in the leader cells of sheets of β4KDs rescues collective migration, is consistent with both this notion and reports by ourselves and others that β4 integrin regulates Rac1 activity (27, 32, 36, 38, 39).
A key feature of cell migration is cytoskeletal remodeling (40, 41). Rac1 is known to be a regulator of cytoskeletal remodeling (42). Thus, our finding, that Rac1 activity is reduced in β4KD cells, suggested that cytoskeleton dynamics are also reduced in these cells compared with control cells. This is indeed the case with regard to actin dynamics. Actin dynamics could also be modulated via association of β4 integrin with actin. Previous studies have reported that β4 integrin physically interacts with actin (13). However, in A549 cells we observe no clear colocalization of actin with α6β4 integrin. Thus, rather than support a direct role of β4 integrin in cytoskeletal cell surface anchorage, our findings support the hypothesis that β4 integrin regulates cytoskeletal dynamics and, hence, motility, indirectly via its effect on Rac1 activation.
In a number of cell types, α6β1 integrin has been observed in focal adhesions (43, 44). Thus, we had expected the loss of β4 integrin in A549 cells to result in either a concomitant loss of α6 integrin expression or the association of α6 integrin with β1 integrin in focal adhesions. We were therefore surprised to find that loss of β4 integrin induced β1 integrin to leave focal adhesions and associate with distinct α6 integrin puncta near the cell edge. This finding implicates α6 integrin as the mediator of α6β4/α6β1 integrin localization. Moreover, our data, showing that α6 integrin knockdown cells exhibit reduced speed and processivity, support our hypothesis that α6β4 integrin is a positive regulator of migration, rather than α6β1 integrin being a negative regulator.
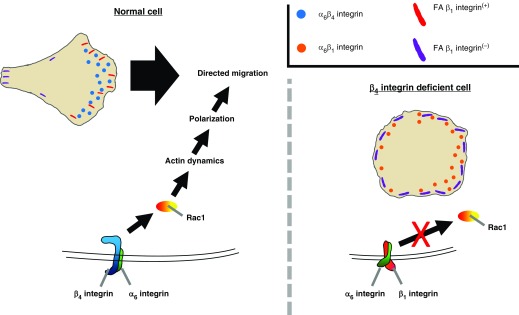
Together, our results lead us to propose the following model (Figure 7). α6β4 integrin induces Rac1 activation. Rac1, in turn, promotes actin dynamics, front–rear polarization, and, as a result, directed migration. In the absence of β4 integrin, however, β1 integrin is recruited from focal adhesions to α6 integrin. In contrast to α6β4 integrin, α6β1 integrin fails to induce Rac1 activation. Thus, β4KD cells fail to migrate in a directed fashion and exhibit significantly reduced collective migration.
Figure 7.
Diagram of β4 integrin–mediated motility. In β4 integrin–expressing cells, α6β4 integrin induces Rac1 activation, which leads to directed migration. In β4 integrin–deficient cells, α6 integrin partners with β1 integrin and does not activate Rac1. Thus, β4 integrin–deficient cells do not exhibit directed migration. FA β1 integrin, focal adhesion β1 integrin.
Because A549 cells are tumorigenic and collective migration has been reported to enhance tumor cell invasion, our results are directly applicable to cancer biology (30, 45, 46). In addition, the recent identification of β4 integrin as a marker for rare alveolar progenitor cells and its up-regulation during the repair of the alveolar epithelium, suggests that β4 integrin also plays a role in alveolar repair (47). Because β4 integrin does not affect the proliferation of alveolar epithelial cells, we suspect that the function of β4 integrin in the alveolar epithelium is to enhance tissue regeneration by promoting collective migration, a view that is supported by our results in which we assayed the migration of primary alveolar epithelial cells forced to express β4 integrin (47).
Acknowledgments
Acknowledgments
The authors thank Dr. Cathryn Hogarth (Washington State University, Pullman, WA) for advice on the use of all-trans retinoic acid, Dr. Jessica Eisenberg (currently at Loyola University, Chicago, IL) for her assistance with the analysis of primary alveolar cells, and Dr. Yuan Wang (Washington State University, Pullman, WA) for advice on statistical analyses.
Footnotes
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health Award RO1 AR AR054184.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Author Contributions: Z.T.C. performed the experiments, developed the modified hybrid sheet procedures, analyzed all data, and drafted the manuscript; J.C.R.J. designed the studies, reviewed data, and helped write the manuscript.
Originally Published in Press as DOI: 10.1165/rcmb.2016-0313OC on December 6, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mercurio AM, Rabinovitz I, Shaw LM. The α6β4 integrin and epithelial cell migration. Curr Opin Cell Biol. 2001;13:541–545. doi: 10.1016/s0955-0674(00)00249-0. [DOI] [PubMed] [Google Scholar]
- 2.Sawamura D, Nakano H, Matsuzaki Y. Overview of epidermolysis bullosa. J Dermatol. 2010;37:214–219. doi: 10.1111/j.1346-8138.2009.00800.x. [DOI] [PubMed] [Google Scholar]
- 3.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hieda Y, Nishizawa Y, Uematsu J, Owaribe K. Identification of a new hemidesmosomal protein, HD1: a major, high molecular mass component of isolated hemidesmosomes. J Cell Biol. 1992;116:1497–1506. doi: 10.1083/jcb.116.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langhofer M, Hopkinson SB, Jones JC. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. J Cell Sci. 1993;105:753–764. doi: 10.1242/jcs.105.3.753. [DOI] [PubMed] [Google Scholar]
- 6.Geuijen CAW, Sonnenberg A. Dynamics of the α6β4 integrin in keratinocytes. Mol Biol Cell. 2002;13:3845–3858. doi: 10.1091/mbc.02-01-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sehgal BU, DeBiase PJ, Matzno S, Chew T-L, Claiborne JN, Hopkinson SB, Russell A, Marinkovich MP, Jones JCR. Integrin β4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J Biol Chem. 2006;281:35487–35498. doi: 10.1074/jbc.M606317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiroyasu S, Colburn ZT, Jones JCR. A hemidesmosomal protein regulates actin dynamics and traction forces in motile keratinocytes. FASEB J. 2016;30:2298–2310. doi: 10.1096/fj.201500160R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seltmann K, Roth W, Kröger C, Loschke F, Lederer M, Hüttelmaier S, Magin TM. Keratins mediate localization of hemidesmosomes and repress cell motility. J Invest Dermatol. 2013;133:181–190. doi: 10.1038/jid.2012.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawano S, Mizutani K, Miyata M, Ikeda W, Takai Y. Interaction of integrin α(6)β(4) with ErbB3 and implication in heregulin-induced ErbB3/ErbB2-mediated DNA synthesis. Genes Cells. 2010;15:995–1001. doi: 10.1111/j.1365-2443.2010.01438.x. [DOI] [PubMed] [Google Scholar]
- 11.Hsu Y-L, Wu C-Y, Hung J-Y, Lin Y-S, Huang M-S, Kuo P-L. Galectin-1 promotes lung cancer tumor metastasis by potentiating integrin α6β4 and Notch1/Jagged2 signaling pathway. Carcinogenesis. 2013;34:1370–1381. doi: 10.1093/carcin/bgt040. [DOI] [PubMed] [Google Scholar]
- 12.Raymond K, Kreft M, Janssen H, Calafat J, Sonnenberg A. Keratinocytes display normal proliferation, survival and differentiation in conditional β4-integrin knockout mice. J Cell Sci. 2005;118:1045–1060. doi: 10.1242/jcs.01689. [DOI] [PubMed] [Google Scholar]
- 13.Rabinovitz I, Mercurio AM. The integrin α6β4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol. 1997;139:1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart RL, O’Connor KL. Clinical significance of the integrin α6β4 in human malignancies. Lab Invest. 2015;95:976–986. doi: 10.1038/labinvest.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart RL, West D, Wang C, Weiss HL, Gal T, Durbin EB, O’Connor W, Chen M, O’Connor KL. Elevated integrin α6β4 expression is associated with venous invasion and decreased overall survival in non–small cell lung cancer. Hum Pathol. 2016;54:174–183. doi: 10.1016/j.humpath.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leng C, Zhang ZG, Chen WX, Luo HP, Song J, Dong W, Zhu XR, Chen XP, Liang HF, Zhang BX. An integrin beta4–EGFR unit promotes hepatocellular carcinoma lung metastases by enhancing anchorage independence through activation of FAK-AKT pathway. Cancer Lett. 2016;376:188–196. doi: 10.1016/j.canlet.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 17.Gaetano C, Melchiori A, Albini A, Benelli R, Falcioni R, Modesti A, Modica A, Scarpa S, Sacchi A. Retinoic acid negatively regulates β 4 integrin expression and suppresses the malignant phenotype in a Lewis lung carcinoma cell line. Clin Exp Metastasis. 1994;12:63–72. doi: 10.1007/BF01784335. [DOI] [PubMed] [Google Scholar]
- 18.Jones JCR, Lane K, Hopkinson SB, Lecuona E, Geiger RC, Dean DA, Correa-Meyer E, Gonzales M, Campbell K, Sznajder JI, et al. Laminin-6 assembles into multimolecular fibrillar complexes with perlecan and participates in mechanical-signal transduction via a dystroglycan-dependent, integrin-independent mechanism. J Cell Sci. 2005;118:2557–2566. doi: 10.1242/jcs.02395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Factor P, Senne C, Dumasius V, Ridge K, Jaffe HA, Uhal B, Gao Z, Sznajder JI. Overexpression of the Na+,K+-ATPase α1 subunit increases Na+,K+-ATPase function in A549 cells. Am J Respir Cell Mol Biol. 1998;18:741–749. doi: 10.1165/ajrcmb.18.6.2918. [DOI] [PubMed] [Google Scholar]
- 20.Kligys KR, Wu Y, Hopkinson SB, Kaur S, Platanias LC, Jones JCR. α6β4 integrin, a master regulator of expression of integrins in human keratinocytes. J Biol Chem. 2012;287:17975–17984. doi: 10.1074/jbc.M111.310458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapnick DA, Liu X. Leader cell positioning drives wound-directed collective migration in TGFβ-stimulated epithelial sheets. Mol Biol Cell. 2014;25:1586–1593. doi: 10.1091/mbc.E14-01-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Russell AJ, Fincher EF, Millman L, Smith R, Vela V, Waterman EA, Dey CN, Guide S, Weaver VM, Marinkovich MP. α6β4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of α3β1 integrin. J Cell Sci. 2003;116:3543–3556. doi: 10.1242/jcs.00663. [DOI] [PubMed] [Google Scholar]
- 24.Mainiero F, Pepe A, Yeon M, Ren Y, Giancotti FG. The intracellular functions of alpha6beta4 integrin are regulated by EGF. J Cell Biol. 1996;134:241–253. doi: 10.1083/jcb.134.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pullar CE, Baier BS, Kariya Y, Russell AJ, Horst BAJ, Marinkovich MP, Isseroff RR. β4 integrin and epidermal growth factor coordinately regulate electric field–mediated directional migration via Rac1. Mol Biol Cell. 2006;17:4925–4935. doi: 10.1091/mbc.E06-05-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamill KJ, Hopkinson SB, DeBiase P, Jones JCR. BPAG1e maintains keratinocyte polarity through β4 integrin–mediated modulation of Rac1 and cofilin activities. Mol Biol Cell. 2009;20:2954–2962. doi: 10.1091/mbc.E09-01-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cruz-Monserrate Z, O’Connor KL. Integrin α6β4 promotes migration, invasion through Tiam1 upregulation, and subsequent Rac activation. Neoplasia. 2008;10:408–417. doi: 10.1593/neo.07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riahi R, Yang Y, Zhang DD, Wong PK. Advances in wound-healing assays for probing collective cell migration. J Lab Autom. 2012;17:59–65. doi: 10.1177/2211068211426550. [DOI] [PubMed] [Google Scholar]
- 29.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 30.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10:445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 31.McLennan R, Dyson L, Prather KW, Morrison JA, Baker RE, Maini PK, Kulesa PM. Multiscale mechanisms of cell migration during development: theory and experiment. Development. 2012;139:2935–2944. doi: 10.1242/dev.081471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaguchi N, Mizutani T, Kawabata K, Haga H. Leader cells regulate collective cell migration via Rac activation in the downstream signaling of integrin β1 and PI3K. Sci Rep. 2015;5:7656. doi: 10.1038/srep07656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trepat X, Wasserman MR, Angelini TE, Millet E, Weitz DA, Butler JP, Fredberg JJ. Physical forces during collective cell migration. Nat Phys. 2009;5:426–430. [Google Scholar]
- 34.Haeger A, Wolf K, Zegers MM, Friedl P. Collective cell migration: guidance principles and hierarchies. Trends Cell Biol. 2015;25:556–566. doi: 10.1016/j.tcb.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Poujade M, Grasland-Mongrain E, Hertzog A, Jouanneau J, Chavrier P, Ladoux B, Buguin A, Silberzan P. Collective migration of an epithelial monolayer in response to a model wound. Proc Natl Acad Sci USA. 2007;104:15988–15993. doi: 10.1073/pnas.0705062104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zegers MM, Friedl P. Rho GTPases in collective cell migration. Small GTPases. 2014;5:e28997. doi: 10.4161/sgtp.28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X, He L, Wu YI, Hahn KM, Montell DJ. Light-mediated activation reveals a key role for Rac in collective guidance of cell movement in vivo. Nat Cell Biol. 2010;12:591–597. doi: 10.1038/ncb2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayor R, Etienne-Manneville S. The front and rear of collective cell migration. Nat Rev Mol Cell Biol. 2016;17:97–109. doi: 10.1038/nrm.2015.14. [DOI] [PubMed] [Google Scholar]
- 39.Zahir N, Lakins JN, Russell A, Ming W, Chatterjee C, Rozenberg GI, Marinkovich MP, Weaver VM. Autocrine laminin-5 ligates α6β4 integrin and activates RAC and NFκB to mediate anchorage-independent survival of mammary tumors. J Cell Biol. 2003;163:1397–1407. doi: 10.1083/jcb.200302023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Y-L, Lu S, Szeto KW, Sun J, Wang Y, Lasheras JC, Chien S. FAK and paxillin dynamics at focal adhesions in the protrusions of migrating cells. Sci Rep. 2014;4:6024. doi: 10.1038/srep06024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mouneimne G, Hansen SD, Selfors LM, Petrak L, Hickey MM, Gallegos LL, Simpson KJ, Lim J, Gertler FB, Hartwig JH, et al. Differential remodeling of actin cytoskeleton architecture by profilin isoforms leads to distinct effects on cell migration and invasion. Cancer Cell. 2012;22:615–630. doi: 10.1016/j.ccr.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nobes CD, Hall A. Rho, Rac, and CDC42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 43.Goel HL, Pursell B, Standley C, Fogarty K, Mercurio AM. Neuropilin-2 regulates α6β1 integrin in the formation of focal adhesions and signaling. J Cell Sci. 2012;125:497–506. doi: 10.1242/jcs.094433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goel HL, Mercurio AM. Enhancing integrin function by VEGF/neuropilin signaling: implications for tumor biology. Cell Adhes Migr. 2012;6:554–560. doi: 10.4161/cam.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bronsert P, Enderle-Ammour K, Bader M, Timme S, Kuehs M, Csanadi A, Kayser G, Kohler I, Bausch D, Hoeppner J, et al. Cancer cell invasion and EMT marker expression: a three-dimensional study of the human cancer–host interface. J Pathol. 2014;234:410–422. doi: 10.1002/path.4416. [DOI] [PubMed] [Google Scholar]
- 46.Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol. 2015;36:13–22. doi: 10.1016/j.ceb.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]