Abstract
Hemophagocytic lymphohistiocytosis (HLH) and newly diagnosed malignant infiltration of liver are rare presentations of acute liver failure associated with poor prognosis. We report a case of a patient with acute liver failure caused by malignant infiltration by diffuse large B-cell lymphoma and secondary HLH.
Introduction
Hematological malignancies such as Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, acute and chronic leukemias, and malignant histiocytosis can cause malignant infiltration of the liver. Malignant hepatic infiltration and hemophagocytic lymphohistiocytosis (HLH) can rarely independently present initially as acute liver failure (ALF) and carry a poor prognosis with mortality rates between 67–100%.1-12 One of the largest single-center studies reported incidence of ALF due to malignant infiltration by hematological malignancies of 0.44%, with a mortality of 94%.10 If no obvious etiology identified, presence of B symptoms, hepatosplenomegaly and lymphadenopathy on physical exam, severe lactic acidosis, and rapidly fatal course should raise the index of suspicion toward malignancy as the possible etiology for ALF.
Case Report
A 57-year-old man presented with a 2-week history of generalized weakness, fatigue, confusion, dizziness, and mild jaundice. His past medical history was significant for hypertension, diabetes mellitus, hyperlipidemia, and hypothyroidism. He was a former smoker and consumed alcohol occasionally. Labs on presentation revealed anemia, thrombocytopenia, acute liver injury, and acute renal failure necessitating intermittent hemodialysis.
He was transferred to a medical intensive care unit (ICU) for lactic acidosis, sepsis, and worsening hepatic encephalopathy. He was febrile to 39°C with hemoglobin 7.5 g/dL, platelets 26,000/mm3, creatinine 4.34 mg/dL, total bilirubin 19 mg/dL, alanine aminotransferase 395 U/L, aspartate aminotransferase 261 U/L, serum lactate 10 mmol/L, serum ferritin >40,000 ng/mL, and serum triglycerides 556 mg/dL. He was also coagulopathic, with international normalized ratio 4.2 and fibrinogen <70 mg/dL. His model for end-stage liver disease (MELD) score was >40, and emergent liver transplant evaluation was started. Workup for his liver failure was unrevealing, and computed tomography of the abdomen and pelvis showed hepatic steatosis without focal hepatic lesions, splenomegaly with multiple splenic infarcts, and right axillary and retropectal lymphadenopathy.
His ICU course was complicated by the need for mechanical ventilation for airway protection, circulatory shock requiring vasopressor support, and worsening renal failure requiring continuous renal replacement therapy. Bone marrow biopsy did not show any evidence of leukemia, lymphoma, or HLH. The patient was removed from the transplant list after transjugular liver biopsy came back positive for diffuse large B-cell lymphoma (Figure 1). He also met criteria for HLH with fever, bicytopenia, splenomegaly, ferritin level, and hypertriglyceridemia. He was started on emergent chemotherapy for lymphoma and HLH with dexamethasone, ifosfamide, carboplatin, and etoposide. Despite chemotherapy and supportive care, he developed worsening metabolic acidosis, hypoglycemia, shock, subdural hemorrhages, and generalized tonic-clonic seizures. The family decided on comfort care measures, and the patient died on ICU day 5.
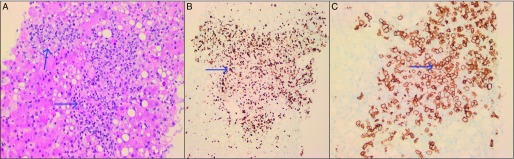
Figure 1.
Liver biopsy pathology. (A) Hematoxylin & eosin stain (20x) showing lymphoma infiltration of the liver. (B) Ki67 (20x) showing the diffuse large B-cell lymphoma with high proliferation index (greater than 90%). (C) Positive expression of CD20 (20x) showing diffuse large B-cell lymphoma.
Discussion
In addition to diffuse large B-cell lymphoma, our patient also presented with secondary HLH, which is a clinical syndrome characterized by a hyperinflammatory condition resulting in increased production of cytokines, impaired cytotoxic T and NK cell production, and stimulation of mononuclear cells.9 It can present as a primary or secondary disorder. Abnormal immune activation and excessive inflammation cause host tissue destruction. Primary or familial HLH, usually seen in infants and children, is caused by gene mutations, whereas secondary HLH is seen in adults, usually caused by triggers like viral infections, malignancy, or autoimmune process. HLH is primarily a pediatric syndrome and but is now increasingly recognized in adults. The Histiocyte Society proposed a standard definition of HLH in 1994, which was later revised for the HLH-2004 trial and serves as the current de facto definition of HLH.13
Hemophagocytosis on bone marrow, spleen, liver, or lymph node biopsy is neither pathognomonic of nor required for the diagnosis of HLH. These criteria have been validated in pediatric patients, but their specificity and sensitivity in adult HLH patients remain to be demonstrated.9
Diagnosis of HLH is challenging due to its rare occurrence, variable presentation, and nonspecific findings. H score is a diagnostic score consisting of 10 variables designed to estimate an individual’s risk of having HLH.14 The probability ranges from <1% with H score of ≤90 to >99% for H score of ≥250. Our patient fulfilled 5 of 8 criteria and had an H score of 250, making the probability of HLH 99%. Per the HLH-2004 trial protocol, patients are treated with 8 weeks of induction therapy with etoposide and dexamethasone, followed by bone marrow transplantation in cases of primary HLH.15
Liver involvement in HLH is common and results in elevation of liver enzymes.9 There have been case reports of HLH presenting as acute liver failure, which is associated with poor prognosis.1,2,5,9,11 The mechanism of liver injury is related to infiltration by hemophagocytic histiocytes or due to overproduction of cytokines. In one case series, 7 of 8 patients presenting with HLH causing acute liver failure died.9
Malignancy-associated secondary HLH is mostly described with T/NK-cell lymphomas and Hodgkin’s lymphomas, but is relatively uncommon in patients with B-cell non-Hodgkin’s lymphomas.16-19 Only one case of newly diagnosed malignant lymphoma with HLH presenting as acute hepatic failure has been described in the literature prior to this report.5 That patient had liver failure due to T-cell lymphoma infiltrating the liver with secondary HLH resulting in a rapidly fatal course, with death on the sixth day of admission. Overall, newly diagnosed malignant lymphoma and HLH very rarely present as acute liver failure and are associated with a poor prognosis. A high index of suspicion and prompt initiation of chemotherapy can be lifesaving but still carries a high mortality.
Disclosures
Author contributions: R. Patel and S. Kapoor wrote the manuscript. H. Patel and W. Mulvoy reviewed the literature. S. Kapoor is the article guarantor.
Financial disclosure: None to report.
Informed consent could not be obtained due to hardship contacting the patient’s family. All identifying information has been removed to protect patient privacy.
References
- 1.Abdullatif H, Mohsen N, El-Sayed R, El-Mougy F, El-Karaksy H. Haemophagocytic lymphohistiocytosis presenting as neonatal liver failure: A case series. Arab J Gastroenterol. 2016;17(2):105–9. [DOI] [PubMed] [Google Scholar]
- 2.Bravo-Jaimes KM. Hemophagocytic lymphohistiocytosis presenting as acute liver failure in a patient with hodgkin lymphoma: Case report and review of the literature. Rev Gastroenterol Peru. 2015;35(3):256–7. [PubMed] [Google Scholar]
- 3.Grille S, Boada M, Bove V, et al. . Diffuse large B cell lymphoma presenting with acute liver failure: A case report. Case Reports in Clinical Pathology. 2015;2(3):53–8. [Google Scholar]
- 4.Haider FS, Smith R, Khan S. Primary hepatic lymphoma presenting as fulminant hepatic failure with hyperferritinemia: A case report. J Med Case Rep. 2008;2:279.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hino T, Sata M, Arima N, et al. . A case of malignant lymphoma with hemophagocytic syndrome presenting as hepatic failure. Kurume Med J. 1997;44:53–60. [DOI] [PubMed] [Google Scholar]
- 6.Kapuria D, Strasser K, Qasem A. Diffuse large B-cell lymphoma causing acute liver failure: A rare case of survival. BMJ Case Rep. 2015;2015:bcr2015209328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kheyri Z, Ali Asgari A, Zare Mehrjerdi A, Zamani F, Ajdarkosh H. Fulminant hepatic failure due to primary hepatic lymphoma: A case report. Middle East J Dig Dis. 2013;5:168–170. [PMC free article] [PubMed] [Google Scholar]
- 8.Lettieri CJ, Berg BW. Clinical features of non-Hodgkins lymphoma presenting with acute liver failure: A report of five cases and review of published experience. Am J Gastroenterol. 2003;98(7):1641–6. [DOI] [PubMed] [Google Scholar]
- 9.Lin S, Li Y, Long J, Liu Q, Yang F, He Y. Acute liver failure caused by hemophagocytic lymphohistiocytosis in adults: A case report and review of the literature. Medicine (Baltimore). 2016;95(47):e5431.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowbotham D, Wendon J, Williams R. Acute liver failure secondary to hepatic infiltration: A single centre experience of 18 cases. Gut. 1998;42:576–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneier A, Stueck AE, Petersen B, Thung SN, Perumalswami P. An unusual cause of acute liver failure: Three cases of hemophagocytic lymphohistiocytosis presenting at a transplant center. Semin Liver Dis. 2016;36(1):99–105. [DOI] [PubMed] [Google Scholar]
- 12.Yeshurun M, Isnard F, Garderet L, et al. . Acute liver failure as initial manifestation of low-grade non-hodgkin's lymphoma transformation into large-cell lymphoma. Leuk Lymphoma. 2001;42(3):555–9. [DOI] [PubMed] [Google Scholar]
- 13.Henter JI, Horne A, Aricó M, et al. . HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–131. [DOI] [PubMed] [Google Scholar]
- 14.Fardet L, Galicier L, Lambotte O, et al. . Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613–20. [DOI] [PubMed] [Google Scholar]
- 15.Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011;118(15):4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adler NR, Sia CS, Polchleb C, Jane S, Aung AK. Intravascular large B cell lymphoma with haemophagocytic syndrome: A double lethal masquerade. Intern Med J. 2015;45(12):1310–2. [DOI] [PubMed] [Google Scholar]
- 17.Alaoua A, Gilbert G, Ghannouchi N, Houchlef M, Letaief A, Bahri F. Primary bilateral adrenal lymphoma revealed by hemophagocytic syndrome. Ann Endocrinol (Paris). 2011;72(3):247–50. [DOI] [PubMed] [Google Scholar]
- 18.Czuchlewski DR, Oupadia SL, Zhang QY. Diffuse large B-cell lymphoma with florid hemophagocytosis. Int J Hematol. 2009;89(1):1–2. [DOI] [PubMed] [Google Scholar]
- 19.Davidson-Moncada JK, McDuffee E, Roschewski M. CD5+ diffuse large B-cell lymphoma with hemophagocytosis. J Clin Oncol. 2013;31(6):e76–9. [DOI] [PMC free article] [PubMed] [Google Scholar]