Abstract
In the brains of individuals with Alzheimer's disease (AD) and chronic traumatic encephalopathy, tau pathology is accompanied usually by intracellular aggregation of transactive response DNA-binding protein 43 (TDP-43). However, the role of TDP-43 in tau pathogenesis is not understood. Here, we investigated the role of TDP-43 in tau expression in vitro and in vivo. We found that TDP-43 suppressed tau expression by promoting its mRNA instability through the UG repeats of its 3΄-untranslated region (3΄-UTR). The C-terminal region of TDP-43 was required for this function. Neurodegenerative diseases-causing TDP-43 mutations affected tau mRNA instability differentially, in that some promoted and others did not significantly affect tau mRNA instability. The expression levels of tau and TDP-43 were inverse in the frontal cortex and the cerebellum. Accompanied with cytoplasmic accumulation of TDP-43, tau expression was elevated in TDP-43M337V transgenic mouse brains. The level of TDP-43, which is decreased in AD brains, was found to correlate negatively with the tau level in human brain. Our findings indicate that TDP-43 suppresses tau expression by promoting the instability of its mRNA. Down-regulation of TDP-43 may be involved in the tau pathology in AD and related neurodegenerative disorders.
INTRODUCTION
Amyloid plaque and neurofibrillary tangles (NFTs) are two hallmarks of Alzheimer's disease (AD). Plaques are extracellular deposits of β-amyloid peptides, which are proteolytic products of amyloid precursor protein (APP). NFTs are intraneuronal fibrillary aggregates of hyperphosphorylated microtubule-associated protein tau (1,2). Clinicopathological correlation studies have shown that NFTs, rather than amyloid plaques, correlate with the clinical symptoms of AD (3–5). Recent studies suggest that the pathological changes of tau appear to be essential to neurodegeneration and to the propagation of neurofibrillary pathology in AD (6). Inhibition of tau expression in some mouse models protects them against cognitive impairment (7–9).
In addition to NFTs, intracellular aggregation and deposition of transactive response DNA-binding protein 43 (TDP-43) are found in the brains of individuals with AD and chronic traumatic encephalopathy (CTE) (10–15). Approximately 57% of AD cases and 85% of CTE cases have TDP-43 deposits in the brain (14,16). TDP-43 pathology is associated with major features of AD—memory loss and medial temporal atrophy (13). Substantial evidence suggests that TDP-43 may be involved in tau pathogenesis in AD (17,18). However, the exact role of TDP-43 in tau pathology remains elusive.
TDP-43 is a ubiquitously expressed nuclear protein and belongs to the family of heterogeneous nuclear ribonucleoproteins (hnRNPs). It has a high-binding affinity for the TG repeats [(TG)n] in DNA or UG repeats [(UG)n] in RNA and is involved in gene transcription, pre-mRNA splicing, mRNA stability, and mRNA transport (19). TDP-43 consists of an N-terminal domain, nuclear localization signal, two RNA recognition motifs (RRM1 and RRM2, with a nuclear export signal in RRM2), and a C-terminal, glycine-rich domain. RRMs are involved in DNA- and RNA-binding (20–22). The glycine-rich C-terminus in TDP-43 has been found to mediate protein-to-protein interaction by binding members of the hnRNP protein family (23–25). The TDP-43–positive inclusions are typically found in the neuronal cytoplasm in frontotemporal lobar degeneration with ubiquitin-positive inclusions (FTLD-U) and amyotrophic lateral sclerosis (ALS) (23). Under disease conditions, TDP-43 is truncated, phosphorylated, ubiquitinated, and aggregated in both the nucleus and the cytoplasm, in which cytosolic aggregation of TDP-43 coincides with the depletion of nuclear TDP-43 (23,26).
In the present study, we found that TDP-43 promoted tau mRNA instability via binding to two (UG)n elements on the 3΄-untranslated region (3΄-UTR) of tau mRNA, resulting in suppression of tau expression. The C-terminal region of TDP-43 was required for its activity to promote tau mRNA instability. TDP-43 was downregulated in AD brain and negatively correlated to tau level.
MATERIALS AND METHODS
Plasmids, antibodies, lentiviruses and other reagents
pCI/TDP-43 tagged with haemagglutinin (HA) at the C-terminal and the C-terminus truncations (pCI/TDP-431–179, TDP-431–275, TDP-431–306) were generated by PCR from pCAG/TDP-43 (Origene, Rockville, MD, USA). pEGFP/tau 3΄-UTR was constructed by PCR amplification from genomic DNA and confirmed by DNA sequence analysis. The ALS/FTLD-U–related mutations of TDP-43 (TDP-43A90V, TDP-43K263E, TDP-43G295R, TDP-43G295S, TDP-43M311V and TDP-43A315T) and two deletion mutants of tau 3΄-UTR (3΄-UTRΔ1, deletion of 724–732 base pairs; 3΄-UTRΔ2, deletion of 1436–1442 base pairs) were achieved by using the QuikChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA) and confirmed by DNA sequence analysis. Lentivirus/GV365-TDP-43 (Ubi-TDP-43-3FLAG-CMV-EGFP) and Lentivirus/GV248-shTDP-43 (hU6-shTDP-43-Ubi- EGFP-IRES-puromycin) and their corresponding control lentiviruses, lentivirus/GV365, and lentivirus/GV248, respectively, were obtained from Genechem (Shanghai, China). Two shTDP-43s target 1201-TTTGGCTCGAGCATGGATT-1219 and 535-AACTCTAAGCAAAGCCCAG-553 of TDP-43 cDNA, respectively. Monoclonal anti-GFP, anti-HA, and anti-actin were bought from Sigma (St. Louis, MO, USA). Polyclonal anti-TDP-43 (A260) was obtained from Cell Signaling Technology (Danvers, MA, USA). R134d, a pan-tau polyclonal antibody was produced in our laboratory by using the longest human recombinant tau isoform to immune rabbit (27). The tau knockout mice do not show any immunoreactivity with this antibody. Polyclonal anti-GAPDH, monoclonal anti-TDP-43 (H-8) and siTDP-43 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). The enhanced chemiluminescence (ECL) kit was provided by Thermo Fisher Scientific (Rockford, IL, USA).
Animals
C57BL/6 mice and Wistar rats were obtained from Nanjing Animal Research Center of Nanjing University (Nanjing, China). Non-transgenic C57BL/6 mice (NTg mice) and C57BL/6-Tg(Prnp-TARDBP*M337V)4Ptrc/J (hTDP-43M337V transgenic mice, stock no. 017604) were purchased from The Jackson Laboratory and maintained at Case Western Reserve University. All mice were weaned on post-natal day 30 and genotyped by PCR analysis of DNA extracted from a punched ear. The animals were housed in cages at 24 ± 1°C, 50–60% humidity with a 12-h light/dark cycle and ad libitum access to food and water. The housing, breeding, and animal experiments were in accordance with the approved protocols from our Institutional Animal Care and Use Committees, according to the United States PHS Policy on Humane Care and Use of Laboratory Animals.
Human brain tissue
Medial frontal cortices and cerebellums of histopathologically confirmed AD and age-matched normal human brains used in this study (Table 1) were obtained without identification of donors from the Sun Health Research Institute Donation Program (Sun City, AZ, USA). Brain samples were stored at −80°C until used. The use of frozen human brain tissue was in accordance with the National Institutes of Health guidelines. The tissue was homogenized in cold buffer consisting of 50 mM Tris–HCl, pH 7.4, 8.5% sucrose, 2.0 mM EDTA, 10 mM β-mercaptoethanol, 1.0 mM orthovanadate, 50 mM NaF, 1.0 mM 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), and 10 μg/ml each of aprotinin, leupeptin and pepstatin and stored at −80°C.
Table 1. Human brain tissue of Alzheimer's disease (AD) and control (Con) cases used in this study.
| Case | Age at death (years) | Gender | PMI (h) | Braak stagea | Tangle scoresb |
|---|---|---|---|---|---|
| AD1c | 89 | F | 3.0 | V | 14.5 |
| AD2c | 80 | F | 2.25 | VI | 14.5 |
| AD3c | 85 | F | 1.66 | V | 12.0 |
| AD4c | 78 | F | 1.83 | VI | 15.0 |
| AD5c | 95 | F | 3.16 | VI | 10.0 |
| AD6c | 86 | M | 2.25 | VI | 13.5 |
| AD7c | 91 | F | 3.0 | V | 8.5 |
| AD8d | 83 | F | 3.0 | VI | 12.4 |
| AD9d | 74 | M | 2.75 | VI | 14.66 |
| AD10d | 79 | F | 1.5 | VI | 14.66 |
| AD11d | 73 | F | 2.0 | V | 15.00 |
| AD12d | 81 | M | 3.0 | V | 11.00 |
| AD13d | 76 | M | 2.33 | VI | 15.00 |
| AD14d | 72 | M | 2.5 | VI | 15.00 |
| AD15d | 74 | F | 2.83 | VI | 15.00 |
| AD16d | 76 | M | 4.0 | V | 15.00 |
| AD17d | 78 | M | 1.83 | VI | 15.00 |
| Mean ± S.D. | 80.59 ± 6.70 | 2.52 ± 0.65 | 13.57 ± 2.05 | ||
| Con1d | 85 | F | 2.75 | II | 5.0 |
| Con2d | 82 | F | 2.0 | II | 4.25 |
| Con3d | 70 | F | 2.0 | I | 0.00 |
| Con4d | 73 | M | 2.0 | III | 2.75 |
| Con5d | 78 | M | 1.66 | I | 0.00 |
| Con6d | 80 | M | 3.25 | I | 2.75 |
| Con7d | 80 | M | 2.16 | II | 1.00 |
| Con8d | 83 | F | 3.25 | I | 0.75 |
| Con9d | 82 | F | 2.25 | II | 3.50 |
| Con10d | 85 | M | 2.5 | II | 4.25 |
| Con11c | 85 | M | 2.5 | II | 4.25 |
| Con12c | 86 | F | 2.5 | III | 5.00 |
| Con13c | 81 | M | 2.75 | III | 6.41 |
| Con14c | 88 | F | 3.0 | II | 2.00 |
| Con15c | 90 | F | 3.0 | III | 4.50 |
| Con16c | 88 | F | 3.5 | III | 2.50 |
| Con17c | 88 | F | 3.0 | IV | 4.50 |
| Mean ± S.D. | 82.59 ± 5.50 | 2.59 ± 0.53 | 3.14 ± 1.89 |
Abbreviation: PMI, postmortem interval.
aNeurofibrillary pathology was staged according to Braak and Braak (1995).
bTangle score was a density estimate and was designated none, sparse, moderate, or frequent (0, 1, 2 or 3 for statistics), as defined according to CERADAD criteria. Five areas (frontal, temporal, parietal, hippocampal, and entorhinal) were examined, and the scores were added up for a maximum of 15.
cCases were used for measurement of tau and TDP-43 in the frontal cortex and cerebellum.
dCases were used for tau measurement by immune-dot-blots.
Rat and mouse brain tissue
Adult Wistar rats (5–6 months old) were euthanized in a CO2 chamber, and frontal cortex and cerebellum were dissected out immediately after death and stored at −80°C until used. Rat brain tissues were homogenized as described above.
Adult male mice (3 months old) were euthanized by cervical dislocation. Brain was dissected and sorted at −80°C until used. Mouse brain tissues were homogenized as described above.
Cell culture and transfection
HEK-293FT, mouse neuroblastoma N2a, or human neuroblastoma SH-SY5Y cells were maintained in Dulbecco's modified Eagle's medium (DMEM) or DMEM/F12 supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA) at 37°C, 5% CO2. All transfections were performed with Lipofectamine 2000 (Invitrogen) or FuGENE HD (Promega, Madison, WI, USA) according to the manufacturer's instructions.
Knockdown of TDP-43 with RNA interference
For inhibition of TDP-43 expression, N2a cells were transfected with certain amounts of short interfering RNAs, siTDP-43 (Santa Cruz) using Lipofectamine 2000. After 48 h transfection, RNA was extracted as described below. The same amount of scramble siRNA was used as control.
Primary neuron culture and lentiviral infection
Mouse cerebral cortical neurons were isolated and cultured according to a previously described method (28). Briefly, fetal cerebral cortices from C57BL/6 mice at embryonic day 15 were cut into pieces in cold PBS buffer, digested with 0.25% trypsin at 37°C for 15 min, agitated into a cell suspension, and centrifuged at 1000 rpm for 5 min. The pellet was suspended with DMEM/10% FBS. Following 400 mer filtering, cell density was counted and adjusted to 1 × 106 cells/ml. The cells were plated in triplicate onto poly-d-lysine (100 μg/ml) coated 24-well plates and were maintained in a humidified atmosphere at 37°C, 5% CO2. The medium was changed to neurobasal supplemented with 2% B27 (Invitrogen) containing 100 U/ml penicillin and 100 μg/ml streptomycin 4 h later. On the second day of culture, the cells were treated with 10 μM Ara-C for 24 h to inhibit non-neuronal cells. On the fourth day, the cells were infected with 1.5 × 107 TU/ml viral particles of TDP-43 or shTDP-43 containing 5 μg/ml polybrene. Lentivirus/GV248 or lentivirus/GV365 was used in the same approach as a control. The medium was changed to neurobasal medium with 2% B27 12 h later. The culture medium was replaced every 2 days. The neurons were harvested 4 days after infection for measurement of tau mRNA and tau protein.
Hippocampus injection of lentivirus
Three-month-old C57BL/6 mice were anesthetized by intraperitoneal injection of cocktail anesthetic solution (0.886% w/v sodium pentobarbital, 4.25% (w/v) chloral hydrate, 2.12% (w/v) magnesium sulfate, 14.25% (v/v) ethanol, 33.8% (v/v) propylene glycol) at a dosage of 2.5 ml/kg body weight and placed in a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). A midsagittal incision was made to expose the cranium, and a burr hole was drilled with a dental drill over both hippocampi to the following coordinates: anteroposterior, -2.5 mm; lateral, ±2.0 mm; ventral, -1.8 mm; all from the Bregma. Lenti/shTDP-43 (2 μl at 3 × 108 TU/ml) or lenti/TDP-43 (the equivalent molar to lenti/shTDP-43) was bilaterally injected by using a 10 μml Hamilton syringe with a custom made 30-gauge/0.5-in./hypodermic needle (Hamilton Syringe Co., Reno, NV, USA) into the hippocampi of mice. Lentivirus/GV248 or GV365 was injected by using the same approach as a control. Three weeks after injection, mice were sacrificed by cervical dislocation. The left hippocampus of mouse was homogenized as described above for Western blots and the right hippocampus was used for mRNA assay.
Reverse transcription-PCR (RT-PCR) and real-time quantitative PCR (qRT-PCR)
Total RNA was extracted from cultured cells or hippocampus by using the RNeasy mini kit (Qiagen, GmbH, Germany) according to the manufacturer's instructions. One microgram of total RNA was used for first-strand cDNA synthesis with oligo-(dT)18 by using the Omniscript reverse transcription kit (Qiagen). PCR was performed by using Prime STAR™ HS DNA Polymerase (Takara Bio Inc., Otsu, Shiga, Japan) with forward primer 5΄-CCTGAGCAAAGTGACCTCCAAG-3΄ and reverse primer 5΄-CAAGGAGCCAATCTTCGACTGG-3΄ to measure tau levels. The PCR condition was: 98°C for 5 min, 98°C for 10 s, and 68°C for 40 s for 30 cycles and then 68°C for 10 min for extension. The PCR products were resolved on 1.5% agarose gels, visualized by ethidium bromide staining, and quantitated by using the Molecular Imager system (Bio-Rad, Hercules, CA, USA).
The qPCR assay was performed in a final volume of 25 μl containing EvaGreen qPCR master mixture 12.5 μl (2×, Agilent Technologies, USA), DNA template 1 μl, 0.5 μl (10 μM) of forward and reverse primers and 0.375 μl of dye (1:500). The qPCR was performed using these primers: mouse total tau (forward, 5΄-CCTGAGCAAAGTGACCTCCAAG-3΄; reverse, 5΄-CAAGGAGCCAATCTTCGACTGG-3΄), mouse GAPDH (forward, 5΄-AGGTCGGTGTGAACGGATTTG-3΄; reverse, 5΄-TGTAGACCATGTAGTTGAGGTCA-3΄), GFP (forward, 5΄-TGAACCGCATCGAGCTGAAGGG-3΄; reverse, 5΄-ACCTTGATGCCGTTCTTCTGCTTG-3΄), human GAPDH (forward, 5΄-CATGAGAAGTATGACAACAGCCT-3΄; reverse, 5΄-AGTCCTTCCACGATACCAAAGT-3΄). Amplification was conducted in a MX3000P real-time PCR system (Stratagene), and the PCR conditions were: 95°C for 10 min, 95°C for 30 s, 55°C for 1 min, 72°C for 1 min for 40 cycles and then 95°C for 1 min, 55°C for 30 s, and 95°C for 30 s. The fluorescence signals were collected between 72°C and 95°C for melting curve analysis. Cyclic threshold (Ct) value was analyzed by the 2−△△Ct method. The PCR products were resolved on 1.5% agarose gel and were visualized by ethidium bromide staining to observe whether the results were consistent with the expected bands.
Preparation of cytoplasmic and nuclear fractionations
Mouse brain tissue was homogenized in nine volumes of cold buffer containing 50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 2.0 mM EDTA, 10 mM β-mercaptoethanol, 1.0 mM Na3VO4, 50 mM NaF, 1.0 mM AEBSF and 10 μg/ml each of aprotinin, leupeptin and pepstatin. The brain homogenates were centrifuged at 900 x g for 5 min, the pellets were homogenized and centrifuged again. The two supernatants were pooled together as cytoplasm fraction and the pellet was nuclear fraction.
Western blots and immuno-dot blots
Cells or brain homogenates were lysed in 1× Laemmli sample buffer (125 mM Tris–HCl, pH 6.8, 2% SDS, 10% glycerol, 10% 2-mercaptoethanol, 0.004% bromphenol blue) and boiled for 5 min. The protein concentration of cell lysates was measured by using the Pierce™ 660 nm protein assay kit (Thermo Fisher Scientific). Equal amounts of protein were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and were electrically blotted on PVDF membrane. The blot was blocked with 5% fat-free milk in TBS for 30 min and then incubated with primary antibody overnight at room temperature. After washing three times with TBST (50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 0.05% Tween 20), the blot was incubated with horseradish peroxidase–conjugated secondary antibodies for 2 h. After being washed three times with TBST, the blot was incubated with ECL for 1 min and then exposed to X-ray film (Kodak, Rochester, NY, USA).
For immunodot-blots, the brain homogenates in the same protein concentration (5 mg/ml) in 1× Laemmli sample buffer were diluted 40 times with dilution buffer (0.2% BSA in TBS containing 50 mM NaF, 1 mM Na3VO4 and 2 μg/ml each of aprotinin, leupeptin and peptstatin) after denaturing, which was identified as 1. Then the samples were diluted serially with dilution buffer and applied onto nitrocellulose membrane (Schleicher and Schuell, Keene, NH, USA) at 5 μl/grid (7 × 7 mm). The blot was placed in a 37°C oven for 1 h to allow the protein to bind to the membrane and was processed as described above for western blots.
RNA immunoprecipitation
The RNA immunoprecipitation (RIP) experiment was performed with Magna RIP™ kit (Millipore, Billerica, MA, USA). Briefly, HEK-293FT cells co-transfected with pCI/TDP-43·HA and pEGFP/tau 3΄-UTR or SH-SY5Y cells were lysed in lysis buffer on ice for 5 min. The cell lysates were centrifuged at 14 000 rpm at 4°C for 10 min, and the supernatants were stored at −80°C. Anti-HA or anti-TDP-43 (H-8) was coupled onto magnetic beads by incubation for 30 min, and then the supernatant from HEK-293FT cells was incubated with anti-HA-beads and the supernatant of SH-SY5Y cells was incubated with anti-TDP-43-beads for 4 h. The immuno-complex was washed sequentially with RIP washing buffer. After digestion with 0.4 mg/ml Proteinase K at 55°C for 30 min, RNA was extracted by RNeasy Mini Kit (Qiagen) and was subjected to first-strand cDNA synthesis with random primer or Oligo-(dT)18 by using the Omniscript Reverse Transcription Kit (Qiagen). The cDNA was amplified by PrimeSTAR™ HS DNA Polymerase (Takara Bio Inc.) with two sets of primers covering each (TG)n elements: (i) primers covering site 1, GTGTGTGTG (724-732 nt): Forward 5΄-ACAGGGTGCTGCAGCTGCCTGCA-3΄, Reverse 5΄-GTACCACCTGCTGGCTTACCTTC-3΄, and Forward 5΄- GACACTGGCTCCTTGCCAAG-3΄, Reverse 5΄-GGAGGAAGAAGCCAGTGCCCTG-3΄; (ii) primers covering site 2, GTGTGTG (1436-1442 nt): Forward 5΄-AACCAGTGTGCCTCCCACAAGG-3΄, Reverse 5΄-AAGGAGTCAGGCCTAGGAGGC-3΄; and Forward 5΄- GTCCAGGGAAGGCAAAGATTTGG-3΄, Reverse 5΄- GACATTGGCACCTGACTTGGCTG-3΄. An initial denaturation for 5 min at 98°C was followed by 30 cycles with denaturation for 10 s at 98°C, annealing for 30 s at 55°C, polymerization for 30 s at 72°C, and a final extension for 10 min at 72°C. PCR products were separated on a 2% agarose gel and visualized by ethidium bromide staining.
Statistical analysis
The data were presented as mean ± SD. Data points were compared by one-way ANOVA for multiple-group analysis with Bonferroni post-hoc test and by the unpaired two-tailed Student's t test (for data with normal distribution) or Mann–Whitney test (for data with non-normal distribution) for two-group comparison. For analyses of the correlation between TDP-43 and tau, Spearman correlation analysis was performed.
RESULTS
TDP-43 suppresses tau mRNA by promoting its RNA instability
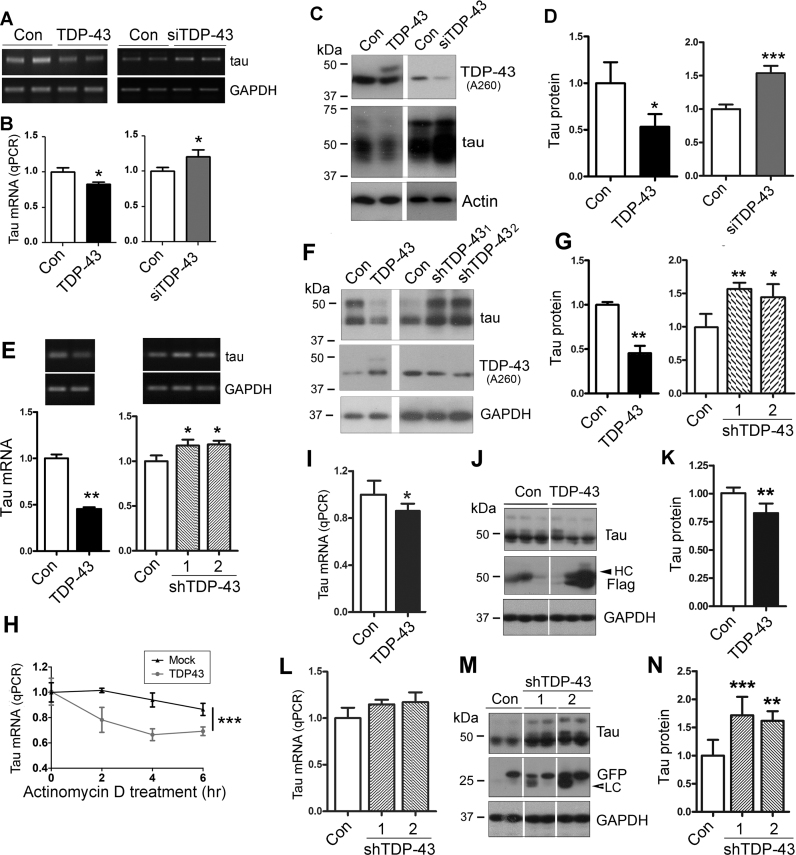
To investigate whether TDP-43 regulates tau mRNA metabolism, we overexpressed or knocked down TDP-43 in N2a cells and measured the tau mRNA level by RT-PCR (Figure 1A) and qRT-PCR (Figure 1B), and tau protein level by Western blots using R134d, a polyclonal pan-tau antibody (Figure 1C). We found that expression of tau was decreased in cells with TDP-43 overexpression and increased by knock-down of TDP-43 with its siRNA at both mRNA (Figure 1A and B) and protein (Figure 1C and D) levels. These data suggest that TDP-43 suppresses tau expression.
Figure 1.
TDP-43 suppresses tau expression by promoting its mRNA instability. (A and B) TDP-43 suppressed tau mRNA expression in N2a cells. N2a cells were transfected with pCI/TDP-43 or siTDP-43 for 48 h. The levels of tau mRNAs were measured by RT-PCR (A) and by qRT-PCR (B). (C and D) TDP-43 suppressed the expression of tau protein in N2a cells. pCI/TDP-43 and siTDP-43 were transfected into N2a cells, and the levels of TDP-43, tau, and GAPDH were determined by Western blots (C). The level of tau was normalized by GAPDH after densitometry (D). (E–G) TDP-43 suppressed tau mRNA expression in primary cultured cortical neurons. Primary cortical neurons from embryonic day 15 were cultured and infected with lentivirus/TDP-43 or lentivirus/shTDP-43. The tau mRNA and protein were measured by RT-PCR (E) and Western blots (F), respectively, four days after viral infection. The level of tau mRNA (E) or tau protein (G) was normalized with GAPDH after densitometry. (H) TDP-43 promoted tau mRNA instability. N2a cells were transfected with pCI/TDP-43, followed by treatment with 5 μg/ml Act D for 2, 4 or 6 h. The cells were harvested 48 h later, after which the level of tau mRNA was measured by qRT-PCR. (I–N) TDP-43 suppressed tau expression in vivo. Lenti/TDP-43 and lenti/shTDP-43s were bilaterally injected into the hippocampi of C57BL/6 mice. The tau level in the hippocampi was measured by qRT-PCR (I, L) and Western blots (J, M) three weeks after viral infection and normalized by GAPDH (K, N). The data are presented as mean ± SD (n = 3–4 for cellular experiments and n = 6 for animals per group for in vivo study). *P < 0.05; **P < 0.01; ***P < 0.001.
To study whether TDP-43 affects tau expression in neurons, we cultured primary cortical neurons isolated from embryonic day 15 mouse brains in vitro for 3–4 days, and then overexpressed or knocked down TDP-43 by using lenti/TDP-43 or two lenti/shTDP-43s. Corresponding lenti/GV365 and lenti/GV248 were used as control. We determined the levels of tau mRNA and protein by RT-PCR (Figure 1E) and Western blots (Figure 1F), respectively, 4 days after viral infection. We found that in accordance with the role of TDP-43 in N2a cells, overexpression of TDP-43 suppressed tau mRNA (Figure 1E) and protein expressions (Figure 1F and G) in primary cultured neurons. Both tau mRNA and tau protein were increased in cultured cortical neurons with lenti/shTDP-431 and lenti/shTDP-432 (Figure 1E–G). These data support that TDP-43 suppresses tau expression at both mRNA and protein levels.
To determine whether the decreased expression of tau mRNA might be due to inhibition of the transcriptional activity or decreased RNA stability, we treated N2a cells with 5 μg/ml transcriptional inhibitor actinomycin D (Act D) for 2, 4, 6 h before harvesting the cells to inhibit mRNA synthesis, and then measured tau mRNA by qRT-PCR. We found that tau mRNA level was decreased in a time-dependent manner by the Act D treatment and that the decrease in the level of tau mRNA was greater in the cells with TDP-43 overexpression (Figure 1H). These results suggest that TDP-43 may suppress tau mRNA stability, leading to a decrease in the level of tau mRNA.
To learn the role of TDP-43 in tau expression in vivo, we injected lenti/TDP-43, lenti/shTDP-43, and their corresponding control viruses into C57BL/6 hippocampus, and then analyzed the tau level by qRT-PCR or western blots 3 weeks after lentivirus injection. The successful infection of lenti/TDP-43 or sh-TDP-43 was confirmed by Western blots with anti-Flag (Figure 1J) or anti-GFP (Figure 1M). We found that tau was reduced in the hippocampi with lenti/TDP-43 injection at mRNA (Figure 1I) and at protein (Figure 1J and K) levels and was slightly increased but not significantly at mRNA level (Figure 1L) and significantly increased at protein level (Figure 1M and N) in the hippocampi with lenti/shTDP-43 injection, suggesting that TDP-43 suppresses tau expression in vivo.
TDP-43 suppresses tau mRNA via its 3΄-UTR
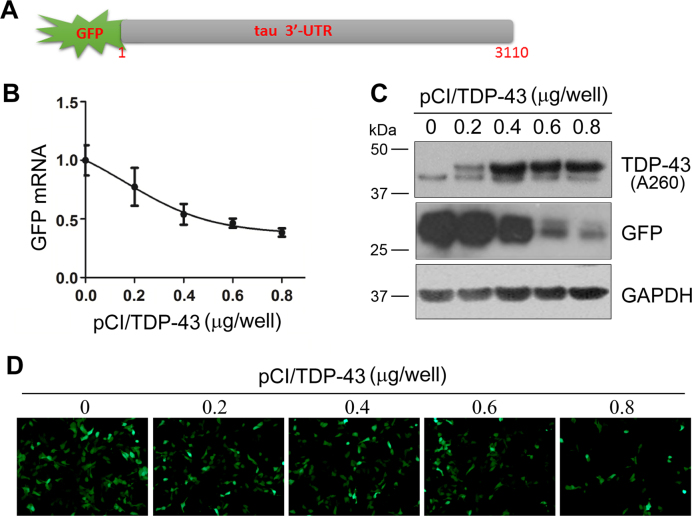
It has been reported that TDP-43 promotes its own mRNA instability via its 3΄-UTR (29). To study whether TDP-43 promoted tau mRNA instability through the 3΄-UTR of the mRNA, we fused tau 3΄-UTR to the C-terminal of green fluorescence protein (GFP) (Figure 2A) to make pEGFP/tau 3΄-UTR plasmid. This plasmid allowed us to study the regulation of tau mRNA through its 3΄-UTR by measuring the expression of GFP. We co-transfected pEGFP/tau 3΄-UTR with different doses of pCI/TDP-43 into HEK-293FT cells and then measured GFP expression by qRT-PCR and Western blots. We found that the levels of GFP mRNA (Figure 2B) or protein (Figure 2C) were decreased in TDP-43 in a dose-dependent manner. By taking advantage of the green fluorescence of GFP, we observed a decrease of fluorescence in a TDP-43 dose-dependent manner (Figure 2D). Thus, the expression of GFP tailed by tau 3΄-UTR is suppressed by TDP-43. These results suggest that TDP-43 suppresses tau expression through the 3΄-UTR of tau mRNA.
Figure 2.
TDP-43 inhibits the expression of GFP tailed with tau 3΄-UTR. (A) The architecture of tau pEGFP/tau 3΄-UTR. (B) TDP-43 suppressed the expression of GFP mRNA dose dependently on pCI/TDP-43. HEK-293FT cells were co-transfected with pCI/TDP-43 and pEGFP/tau 3΄-UTR in triplicate for 48 h. The level of GFP mRNA was detected by qRT-PCR. (C and D) TDP-43 suppressed the expression of GFP protein. The cell lysates of co-transfection of pEGFP/tau 3΄-UTR and pCI/TDP-43 were analyzed by Western blots using antibodies against TDP-43 (A260), GFP, and GAPDH (C). Representative GFP fluorescence of cells after co-transfection of pEGFP/tau 3΄-UTR with the indicated doses of pCI/TDP-43 for 48 h (D).
TDP-43 acts on the (UG)n elements of 3΄-UTR of tau mRNA
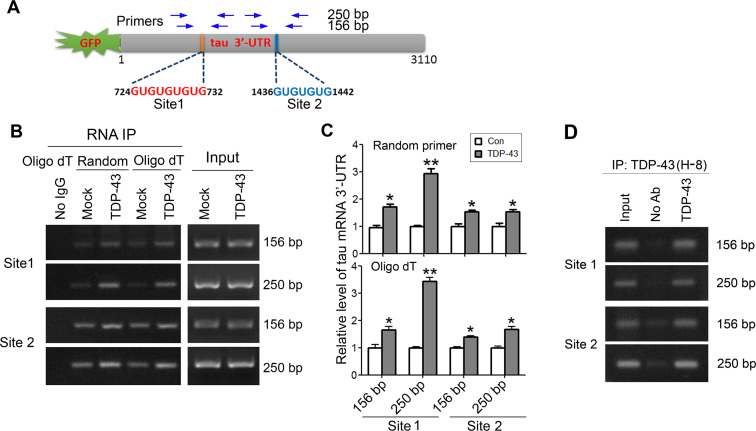
TDP-43 is a highly specific (UG)n-binding protein that binds to UG repeats with high affinity (30–33). There are two (UG)n elements at site 1 (724-732 nt, GUGUGUGUG) and site 2 (1436–1442 nt, GUGUGUG) in tau 3΄-UTR (Figure 3A). To determine whether TDP-43 could bind to the (UG)n elements of tau mRNA 3΄-UTR, we co-transfected pEGFP/tau 3΄-UTR with pCI/TDP-43·HA into HEK-293FT cells and immunoprecipitated TDP-43 with anti-HA. The co-immunoprecipitated pre-mRNA of tau 3΄-UTR with TDP-43 by anti-HA was amplified with RT-PCR by using random or oligo-dT primers for reverse transcription. Then the cDNA was amplified by PCR with two sets of primers covering the (UG)n elements of tau 3΄-UTR to obtain the 156 bp and 250 bp of PCR products, respectively (Figure 3A). We observed that the mRNA covering sites 1 and 2 of tau 3΄-UTR was co-immunoprecipitated with anti-HA, which was determined by random or oligo-dT primers for reverse transcription and followed by PCR (Figure 3B). The tau 3΄-UTR mRNA covering two (UG)n elements—site 1 and site 2—was significantly co-immunoprecipitated with TDP-43 (Figure 3B and C). Amplification by primers corresponding to 250 bp PCR products was more specific than amplification by 156 bp products (Figure 3B). These data suggest that TDP-43 can act on the two (UG)n elements of tau mRNA 3΄-UTR.
Figure 3.
TDP-43 acts on the (UG)n elements of tau mRNA 3΄-UTR. (A) Schematic of (UG)n elements in tau mRNA 3΄-UTR. Two sets of primers specific to (UG)n elements, as indicated for amplifying the cDNA derived from tau mRNA 3΄-UTR. (B and C) TDP-43 acts on two (UG)n elements in tau mRNA 3΄-UTR. pEGFP/tau 3΄-UTR was co-transfected with pCI/TDP-43·HA into HEK-293FT cells. TDP-43 was immunoprecipitated with anti-HA. Co-immunoprecipitated 3΄-UTR of tau mRNA covering (UG)n element 1 (Site 1) or (UG)n element 2 (Site 2) with TDP-43 was reverse-transcribed to cDNA with random primer or oligo-dT18 and amplified by PCR with two sets of primers. The RT-PCR products from RNA-IP or cell lysate (Input) were separated by agarose electrophoresis (B) and quantitated by densitometry (C). (D) Physiological TDP-43 acts on tau mRNA 3΄-UTR. TDP-43 was immunoprecipitated from SH-SY5Y cells with anti-TDP-43 (H-8). Co-immunoprecipitated 3΄-UTR of tau mRNA with TDP-43 was reverse-transcribed to cDNA with oligo-dT18 and amplified by PCR with two sets of primers. The RT-PCR products from RNA-IP or cell lysate (Input) were separated by agarose electrophoresis. The data are presented as mean ± SD (n = 3). *P < 0.05; **P < 0.01.
To learn the binding of TDP-43 on endogenous tau 3΄-UTR under physiological condition, we performed RNA immunoprecipitation in SH-SY5Y cells by using anti-TDP-43 (H-8) as described above. We found that anti-TDP-43 was able to immunoprecipitate tau mRNA 3΄-UTR at the sites 1 and 2 amplified by two sets of primers as described above (Figure 3D), indicating the physiological action of TDP-43 on the 3΄-UTR of tau mRNA.
TDP-43 suppresses GFP expression via two (UG)n elements of tau mRNA 3΄-UTR
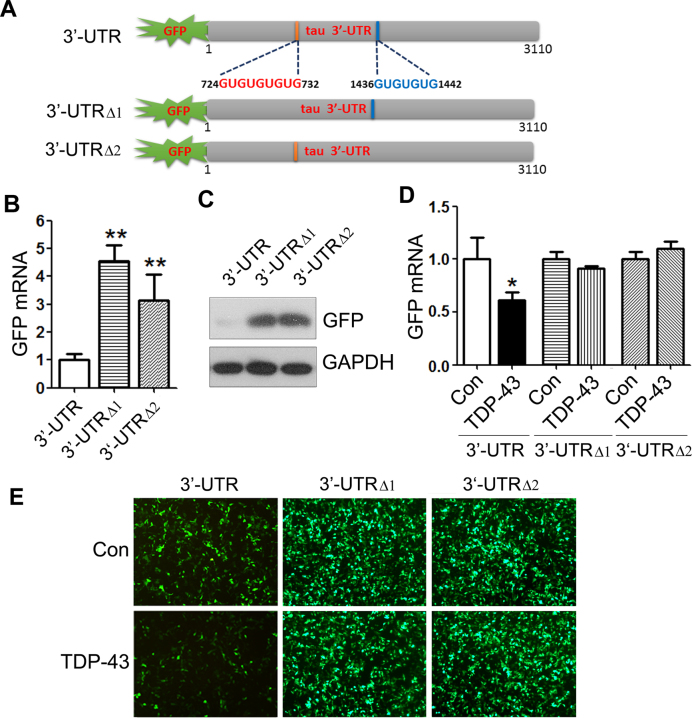
To study the role of the UG repeats on tau mRNA stability, we constructed pEGFP/tau 3΄-UTRΔ1 (724–732 nt deleted) or pEGFP/tau 3΄-UTRΔ2 (1436–1442 nt deleted) (Figure 4A), transfected them into HEK-293FT cells, and measured the levels of GFP mRNA and protein by qRT-PCR (Figure 4B) and Western blots (Figure 4C), respectively. We found that, compared with pEGFP/ tau 3΄-UTR, the levels of GFP mRNA (Figure 4B) and protein (Figure 4C) were dramatically increased in either pEGFP/tau-tailed with 3΄-UTRΔ1 or pEGFP/tau 3΄-UTRΔ2, suggesting that these two (UG)n elements play an important role in promoting the mRNA instability.
Figure 4.
TDP-43 suppresses GFP expression via two (UG)n elements of tau 3΄-UTR. (A) Ideographs of tau 3΄-UTR with two (UG)n elements and the (UG)n deletion mutants. (B and C) Deletion of (UG)n elements enhanced GFP expression. HEK-293FT cells were transfected with pEGFP/tau 3΄-UTR or its deletion mutants for 48 h. GFP mRNA or GFP protein was detected by qRT-PCR or by western blots. (D and E) TDP-43 failed to suppress GFP expression in pEGFP/tau 3΄-UTRΔ1 or pEGFP/tau 3΄-UTRΔ2 transfected cells. HEK-293FT cells were co-transfected with pCI/TDP-43 and pEGFP/tau 3΄-UTR, pEGFP/tau 3΄-UTRΔ1 or pEGFP/tau 3΄-UTRΔ2, in which two (UG)n elements were deleted respectively for 48 h. GFP mRNA was detected by qRT-PCR (D), and GFP protein was shown by fluorescence (E). The data are presented as mean ± SD (n = 3). *P < 0.05, **P < 0.01, versus control.
To confirm that TDP-43 promotes tau mRNA instability through these two (UG)n elements, we co-expressed TDP-43 with pEGFP/tau 3΄-UTR, pEGFP/tau 3΄-UTRΔ1 or pEGFP/tau 3΄-UTRΔ2 in HEK-293FT cells and measured the GFP expression. Consistently, we found that overexpression of TDP-43 suppressed GFP expression in pEGFP/tau 3΄-UTR cells (Figure 4D and E) but did not significantly suppress GFP expression in pEGFP/tau 3΄-UTRΔ1 or pEGFP/tau 3΄-UTRΔ2 transfected cells at mRNA (Figure 4D) or protein levels (Figure 4E). These results confirm that TDP-43 promotes tau mRNA instability through these two (UG)n elements at 3΄-UTR.
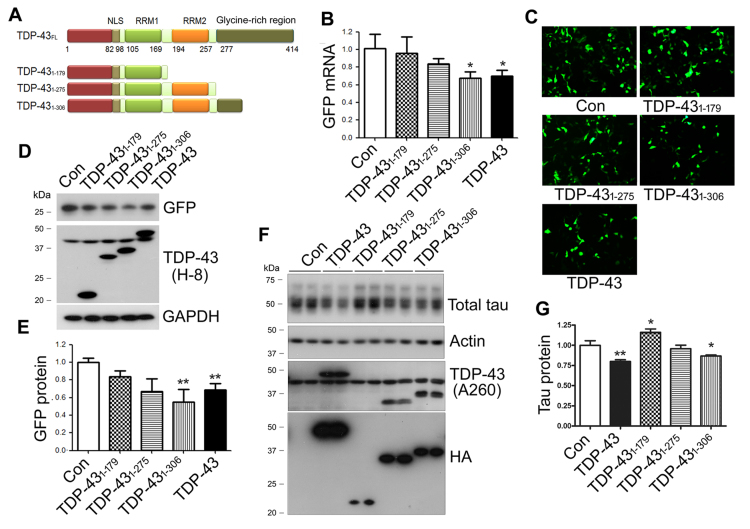
C-terminal glycine-rich region of TDP-43 is required for promoting tau mRNA instability and suppressing tau expression
To determine the key regions of the TDP-43 molecule that regulates tau mRNA stability, we constructed deletion mutants of TDP-43, TDP-431–179, TDP-431–275 and TDP-431–306 (Figure 5A) and co-transfected them with pEGFP/tau 3΄-UTR into HEK-293FT cells. We determined the mRNA expression of GFP by qRT-PCR, and protein expression by green fluorescence and Western blots. We found that inhibition of GFP expression by TDP-43 was abolished by the deletion of RRM2 and/or the glycine-rich region at mRNA (Figure 5B) and protein (Figure 5C–E) levels. TDP-431–306, a deletion of part of the glycine-rich region, inhibited GFP mRNA (Figure 5B) and GFP protein significantly (Figure 5C–E). TDP-431–275 slightly suppressed GFP expression at both mRNA and protein levels, but not reaching statistical significant (Figure 5B and E). TDP-431–179 was unable to suppress GFP expression (Figure 5B and E). Thus, inhibition of GFP expression by TDP-43 was dramatically reduced by the C-terminal deletion. The glycine-rich domain and RRM2 are required for TDP-43 function in the promotion of tau mRNA instability.
Figure 5.
The C-terminal glycine-rich region of TDP-43 is required for promoting tau mRNA instability and for suppression of tau expression. (A) Ideographs of TDP-43 truncations. (B–E) The C-terminal glycine-rich region of TDP-43 was required for promoting tau mRNA instability. HEK-293FT cells were co-transfected with pEGFP/tau 3΄-UTR and TDP-43 or its deletion mutants for 48 h. GFP mRNA was detected by qRT-PCR (B), and GFP and TDP-43 was detected by green fluorescence (C) or by Wstern blots with anti-GFAP and anti-TDP-43 (H-8)(D). The level of GFP protein was normalized with GAPDH after densitometry of blots in panel E. (F and G) The C-terminal glycine-rich region of TDP-43 was required for suppressing tau expression. N2a cells were transfected with TDP-43 and its deletion mutants for 48 h. TDP-43 was detected by Western with anti-TDP-43 (A260) and anti-HA (F). The tau protein was determined by Western blots, normalized by GAPDH after densitometry of blots in panel F. The data are presented as mean ± SD (n = 3 or 4). *P < 0.05; **P < 0.01.
We also determined the effect of C-terminal deletion mutations of TDP-43 on endogenous tau expression by overexpression of TDP-43 and its deletion mutants in N2a cells. We found that the tau level was decreased in cells with the expression of TDP-43 or TDP-431–306, but not of TDP-431–275 and TDP-431–179 (Figure 5F and G). Interestingly, compared with control transfection, the level of tau was slightly increased in cells with TDP-431–179 overexpression (Figure 5F and G). Thus, deletion mutants of TDP-43 at the C-terminus failed to suppress endogenous tau expression, thereby suggesting that the C-terminus of TDP-43 is required for inhibiting tau expression.
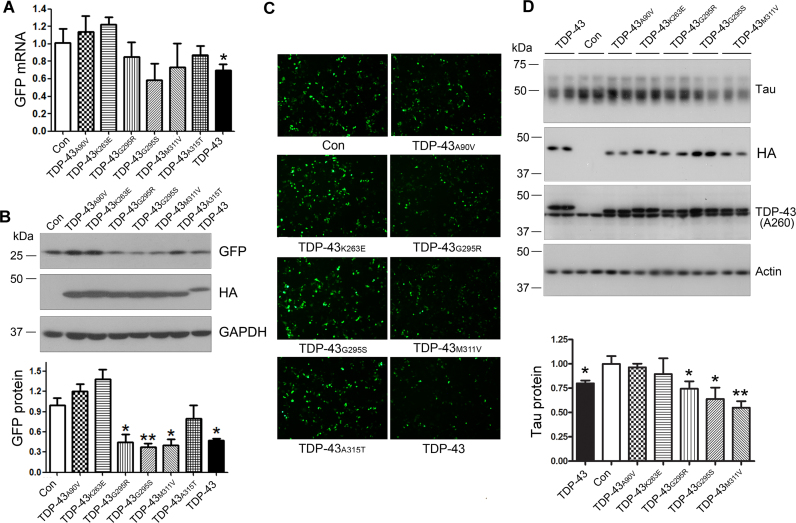
ALS/FTLD-U-causing mutations of TDP-43 affect tau mRNA stability and tau expression differentially
TDP-43 is the major component of neuronal inclusions in ALS and FTLD-U (23), some of which are caused by mutations of the TDP-43 gene (34). To study the effect of the disease-causing mutations on its function in tau mRNA stability, we constructed several ALS/FTLD-U-related mutants of TDP-43 and co-transfected them with pEGFP/tau 3΄-UTR into HEK-293FT cells. We found that ALS/FTLD-U–causing mutations, as indicated in Figure 6A, affected GFP mRNA expression differently (Figure 6A). TDP-43A90V and TDP-43K263E slightly enhanced and TDP-43G295R, TDP-43G295S, TDP-43M311V suppressed GFP mRNA expression, but not reaching statistical significant (Figure 6A). At the protein level, we found that TDP-43G295R, TDP-43G295S, and TDP-43M311V suppressed GFP expression significantly, while TDP-43A90V and TDP-43K263E promoted GFP expression (not statistically significant) (Figure 6B and C). These results suggest that ALS/FTLD-U-related TDP-43 mutants affect tau mRNA instability differentially.
Figure 6.
The ALS/FTLD-U–causing mutations of TDP-43 affect tau mRNA instability and tau expression differentially. (A–C) The ALS/FTLD-U–causing mutations of TDP-43 affected tau mRNA instability differentially. HEK-293FT cells were co-transfected with pEGFP/tau 3΄-UTR and TDP-43 or its ALS/FTLD-U-causing mutations for 48 h. GFP mRNA was detected by qRT-PCR (A) and GFP protein was determined by Western blots (B) or green fluorescence (C). (D) ALS/FTLD-U-causing mutations of TDP-43 affected the expression of tau protein differentially. N2a cells were transfected with TDP-43 or its ALS/FTLD-U-causing mutations for 48 h. Tau protein was determined by Western blots. The data are presented as mean ± SD (n = 3 or 4). *P < 0.05; **P < 0.01.
To learn the effects of these ALS/FTLD-U–related TDP-43 mutations on endogenous tau expression, we overexpressed these TDP-43 mutants in N2a cells and then measured tau protein by Western blots. We found that TDP-43G295R, TDP-43G295S, and TDP-43M311V suppressed tau expression, but TDP-43A90V and TDP-43K263E did not affect tau expression significantly (Figure 6D). These results support that ALS/FTLD-U–related TDP-43 mutations affect the properties to TDP-43 in tau mRNA instability diversely.
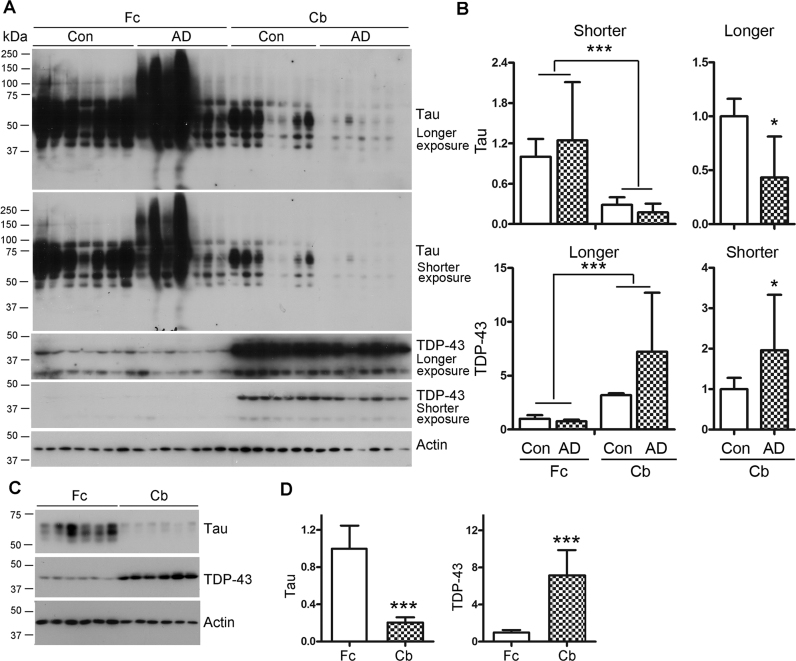
TDP-43 and tau are expressed inversely in the frontal cortex and cerebellum
It is well established that there is no tau pathology in the cerebellum in Alzheimer's disease (35), which led us to determine the expression of TDP-43 and tau in the cerebellum. We compared by Western blots the expressions of TDP-43 and tau in homogenates of cerebellums and frontal cortices from seven AD and seven control cases (Figure 7A). We found two major bands, full-length 43-kDa and truncated 35-kDa forms of TDP-43 detected by TDP-43 antibody A260, which recognizes the region around Ala 260 (Figure 7A). In control brains, the tau level in the cerebellum was ∼29% of that in the frontal cortex, whereas the TDP-43 level was three-fold higher in the cerebellum than in the frontal cortex (Figure 7B). In AD frontal cortex, we observed a smear signal by R134d, a pan-tau antibody, but not in the frontal cortices of control cases or in the cerebellums of control and AD cases (Figure 7A). Interestingly, compared with control cerebellum, TDP-43 was increased and tau was decreased in AD cerebellum (Figure 7B).
Figure 7.
TDP-43 and tau are expressed in the frontal cortex and cerebellum inversely. (A and B) TDP-43 was highly expressed, and tau showed low expression in human cerebellum. Frontal cortices and cerebellums from seven control and seven AD cases were analyzed by Western blots developed with anti-tau (R134d), anti-TDP-43 (A260) and anti-actin under different exposure times (A). The levels of tau and TDP-43 were normalized with actin (B). (C and D) TDP-43 was highly expressed, with a low tau level, in rat cerebellum. Homogenates of frontal cortex and cerebellum from adult Wistar rats were analyzed by Western blots developed with anti-tau, anti-TDP-43 and anti-actin (C). The level of tau or TDP-43 was normalized with actin. The data are presented as mean ± SD (n = 7 or 6). *P < 0.05, ***P < 0.001.
To further confirm that less tau and more TDP-43 are expressed in the cerebellum than in the frontal cortex, we determined the levels of tau and TDP-43 by Western blots in these two regions of rat brain (Figure 7C). Consistently, we found that the tau level in the cerebellum was 20% of, and the TDP-43 level was seven times greater than, that in the frontal cortex. Thus, tau and TDP-43 are expressed in the cerebellum and frontal cortex in an inverse manner. The cerebellum expresses much less tau, but much more TDP-43, than the frontal cortex. Collectively, these data suggest that high expression of TDP-43 may contribute to low expression of tau in the cerebellum.
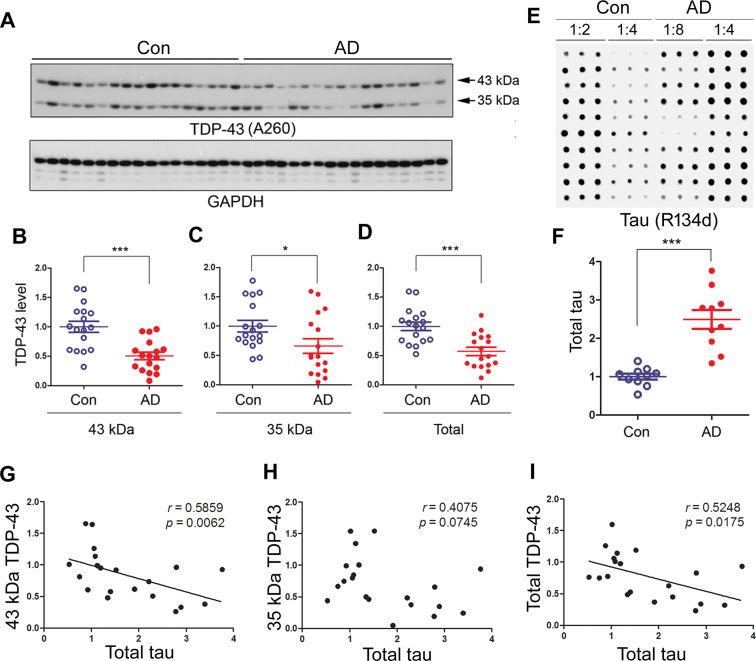
TDP-43 is decreased in AD brain and correlates to tau negatively
To learn the expression of TDP-43 in AD brain, we detected TDP-43 protein level in the frontal cortices by western blots (Figure 8A). We found that the levels of 43-kDa (Figure 8B), 35-kDa (Figure 8C) and total TDP-43 (Figure 8D) all were decreased in AD brain. These results indicate that TDP-43 may be down-regulated in AD brain.
Figure 8.
TDP-43 and truncated TDP-43 are decreased in AD brain and negatively correlated with tau level. Frontal cortices from 17 AD patients and 17 controls were analyzed by Western blots for TDP-43 and GAPDH (A). The levels of the full-length (B), truncated (C) and total TDP-43 (D) were calculated after densitometry. (E and F) Total tau was increased in AD brains. Immuno-dot blots of human frontal cortical homogenates were developed with anti-tau (R134d) (E) and quantified densitometrically (F). The data are represented as mean ± S.D. *P < 0.05; ***P < 0.001. (G–I) TDP-43 is negatively correlated with tau in human brain. The relative level of 43-kDa full-length TDP-43 (G), 35-kDa truncated TDP-43 (H), or total TDP-43 (43 + 35-kDa TDP-43) (I) determined in panel A was plotted against the total tau from panel A (X-axis). The correlation between them was analyzed by Spearman correlation analysis.
To learn the relationship of TDP-43 and tau level, we measured by immuno-dot blots the total tau level in frontal cortex homogenates from 10 AD cases and 10 controls. The tau level was significantly increased in AD brain (Figure 8E and F). Then, we analyzed the correlation between tau and TDP-43 by a linear regression analysis. We found that the total tau level was negatively correlated with full-length 43-kDa TDP-43 (Figure 8G) and total TDP-43 (Figure 8I), but not with the truncated 35-kDa form of TDP-43 (Figure 8H). Thus, decreased TDP-43 levels in AD brain may be associated with increased tau expression.
Expression of tau is increased in TDP-43M337V mouse brain
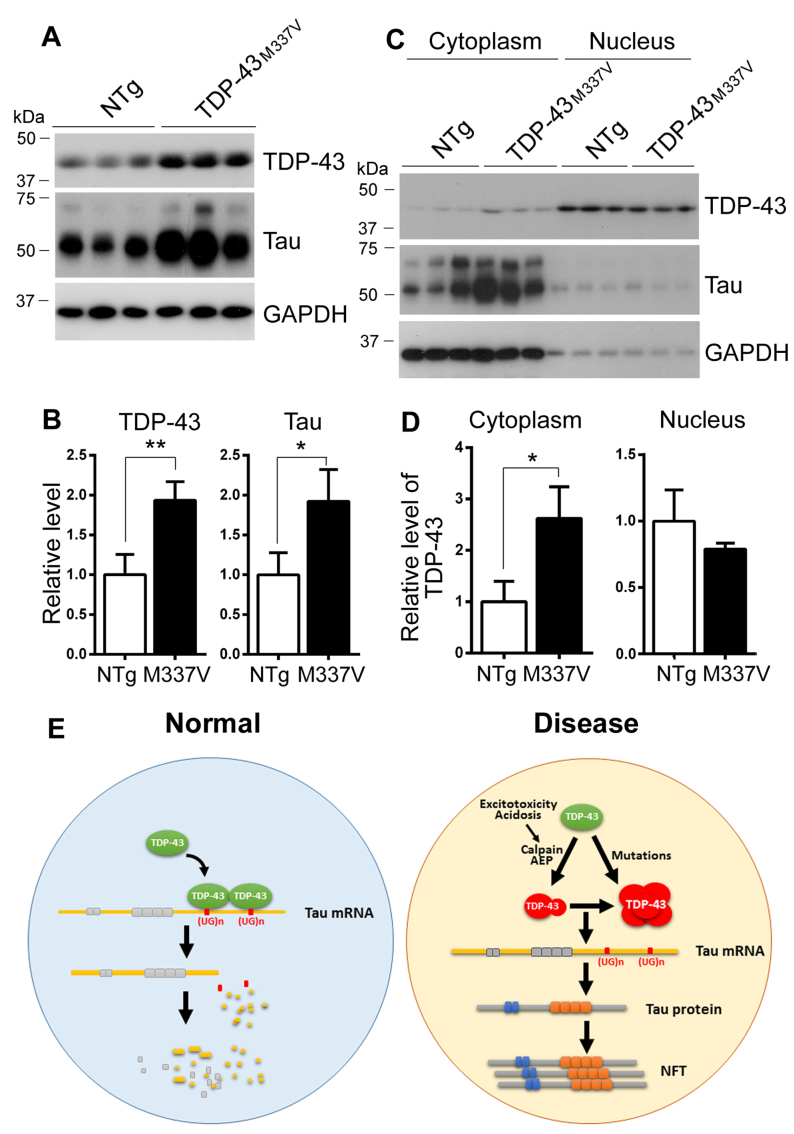
To study the effect of ALS/FTLD-U causing mutation on tau expression in vivo, we analyzed the level of tau by Western blots in the brains of hemizygous TDP-43M337V transgenic mice, in which human TDP-43M337V was directed by a mouse prion protein promoter (36). We found that the levels of TDP-43 and tau both were 2-fold increase in transgenic mouse brains (Figure 9A and B).
Figure 9.
Expression of tau is increased and TDP-43 is dislocated in cytoplasm in TDP-43M337V mouse brain. (A and B) The expression of tau was increased in TDP-43M337V transgenic mouse brains. The levels of TDP-43 and tau in the brain homogenates from hemizygous TDP-43M337V transgenic mice and the littermates were analyzed by Western blots developed with anti-tau (R134d) and anti-TDP-43 (A260) and normalized with GAPDH (B). (C and D) TDP-43 was increased in the cytoplasm in TDP-43M337V mouse brain. The brains from TDP-43M337V transgenic mice and the littermates were homogenized. The cytoplasm and nucleus were separated by centrifugation. The TDP-43, tau and GAPDH in cytoplasmic and nuclear fractions were analyzed by Western blots. The level of TDP-43 in cytoplasmic and nuclear fraction were normalized with GAPDH (D). The data are represented as mean ± S.D. *P < 0.05; **P < 0.01. (E). The proposed mechanism by which TDP-43 regulates tau expression. TDP-43 normally binds to (UG)n of tau mRNA 3΄-UTR and promotes tau mRNA instability, leading to a decrease of tau expression. In AD or CTE, excitotoxicity and /or acidosis in the brain activates calpain and AEP, which cleave TDP-43, leading to loss of its ability and cytoplasmic aggregation. In TDP-43 related ALS/FTLD-U, mutated TDP-43 is aggregated in cytoplasm, resulting in its nuclear depletion. Loss function of TDP-43 as a result of the nuclear depletion leads to an increase of tau expression via promoting its mRNA stability, consequently contribute to the neurofibrillary pathology.
It is well known that pathological TDP-43 is dislocated into the cytoplasm from the nucleus (23,26). Then we separated cytoplasmic and nuclear fractions by centrifugation at 900 ×g for 5 min and analyzed TDP-43 and tau by western blots. We found that TDP-43 mainly expressed in the nucleus and tau and tubulin were predominant in cytoplasm (Figure 9C). Cytoplasmic TDP-43 was 2.6-fold increase in TDP-43M337V transgenic mouse brains as compared with non-transgenic wild type littermates (NTg) (Figure 9C and D). The level of nuclear TDP-43 was slightly decreased in the transgenic mouse brains but not reaching statistical significance (Figure 9C and D). Thus, ALS/FTLD-U causing mutations may promote tau expression via cytoplasmic localization.
DISCUSSION
Intracellular accumulation and aggregation of hyperphosphorylated tau has been linked with several neurofibrillary degenerative disorders, named tauopathies (37). In the present study, we found for the first time that TDP-43 bound to two (UG)n elements at the 3΄-UTR of tau mRNA and promoted its instability, resulting in a negative regulation of tau expression. The C-terminal truncation of TDP-43 leads to the loss of this function. Several ALS/FTLD-U– causing mutations affected TDP-43's activity to promote tau mRNA instability differentially. High expression of TDP-43 in the cerebellum was associated with a low expression of tau. Expression of tau was elevated in TDP-43M337V transgenic mouse brain, in which TDP-43 expression was increased in the cytoplasm, but not in the nucleus. In AD brain, both full-length and truncated TDP-43 were decreased, and TDP-43 was negatively correlated with the tau level in human brain, suggesting that a down-regulation of TDP-43 may contribute to tau accumulation in AD brain by allowing more tau expression through decreased tau mRNA instability (Figure 9E).
Growing evidence suggests that tau pathology plays a critical role in neurodegeneration. Inhibition of tau expression in animal models protects them from cognitive impairment (7,8), neuronal loss and reverses pathological tau deposition (38). The tau protein level is elevated 4 to 5-fold in AD brain (39,40), which may result from impaired degradation or/and overproduction of the protein. Studies have demonstrated the impairment of tau degradation in AD brain (41,42). However, the mRNA level of tau in AD brain has not been well studied. Increased tau mRNA was found in the hippocampus, which is thought to result from neurogenesis; no such increase was found in the visual cortex or cerebellum in AD (43). Interestingly, tau accumulation and tau pathology show neuroanatomical variation. No tau pathology was observed in the cerebellum in AD brain, in which the tau level is ∼25% of that in the frontal cortex, supporting a possible role of tau level in its pathogenesis. Human genomic tau transgenic htau mice, on mouse tau null background, express a 3 to 5-fold higher level of six isoforms of normal human tau than the tau level in wild-type mice and develop tau pathology at 12 months of age (44,45). To date, all transgenic mice that develop tau pathology have expressed a high level of tau in addition to its mutations (46). Therefore, increased tau level could also contribute to tau aggregation and tau pathology.
TDP-43 is a ubiquitously expressed and multi-functional DNA/RNA-binding protein with its main localization in the nucleus, where it has been implicated in several steps of RNA metabolism, such as transcription, pre-mRNA splicing, and microRNA processing (34,47–50). Several studies suggest that TDP-43 may participate in the transport of RNA granules in neurons, and/or it might control mRNA stability and translation (48). The loss of TDP-43 is lethal to embryos. TDP-43 knockout mice die at day 7.5 of embryonic development. Heterozygous TDP-43 mutant mice exhibit signs of motor disturbance and muscle weakness. TDP-43 is essential for viability, and a mild reduction in TDP-43 function is sufficient to cause motor deficits without degeneration of motor neurons (51). Tau is a major neuronal microtubule–associated protein. It stimulates microtubule assembly and stabilizes microtubule structure. Simone et al. reported at AAIC 2011 (The Alzheimer's Association International Conference 2011) that tau promoter contains the putative TDP-43 binding site, and overexpression of TDP-43 promotes tau expression in undifferentiated SK-N-F1 neuroblastoma cells, but in differentiated SK-N-F1 cells TDP-43 suppressed tau expression. In the present study, we found that TDP-43 suppressed tau expression by promoting its mRNA instability.
It was reported that TDP-43 binds to its 3΄-UTR and promotes TDP-43 mRNA instability (29). The C-terminal region of TDP-43 is required for regulation of its mRNA stability (29). Unlike the effect of TDP-43 on its mRNA, TDP-43 interacts directly with the 3΄-UTR of human low molecular weight neurofilament and stabilizes its mRNA (52). CLIP-seq and RIP-seq experiments have shown that TDP-43 binds specifically to the (TG)n element (30–32). There are two (UG)n elements in tau 3΄-UTR mRNA, 724–732 nt (GUGUGUGUG) and 1436–1442 nt (GUGUGUG). TDP-43 can immunoprecipitate the 3΄-UTR of tau mRNA, which contains these two (UG)n elements. Deletion of each (UG)n element significantly increased the GFP expression, which tailed with tau 3΄-UTR, and resulted in loss of the ability of TDP-43 to promote tau mRNA instability. Therefore, these results suggest that TDP-43 binds to the (UG)n elements of tau 3΄-UTR mRNA and suppresses its stability, resulting in decreased tau expression.
TDP-43 is decreased in AD brains and negatively correlated with tau level. High expression of TDP-43 in the cerebellum is accompanied by low expression of tau. Here, we found that TDP-43 promotes tau mRNA instability via acting its 3΄-UTR. Overexpression of TDP-43 inhibits and knockdown of TDP-43 enhances tau expression. Thus, we speculate that in AD brain, decreased TDP-43 may contribute to increased tau expression partially. Tau is a microtubule binding protein and expressed normally in cytoplasm. Whether tau regulates TDP-43 expression remains unclear.
Mutation of TDP-43 has been found to cause FTLD-U and ALS (53–56). The present study has demonstrated that TDP-43 promotes tau mRNA instability. A loss of function of TDP-43 in the brains with TDP-43 pathology could theoretically promote tau pathology under these pathological conditions. This is actually observed in the brains of individuals with Guam ALS/Parkinsonism (57). However, tau pathology is negligible in the brains with ALS or FTLD-U. Most people who develop ALS are between the ages of 40 and 70, with an average age of 55 at the time of diagnosis. Thus, the onset of ALS is much earlier than that of sporadic AD, in which disease starts after the age of 65. It appears that the changes in TDP-43 are insufficient to induce tau pathology under these conditions. The exact reasons remain elusive.
The TDP-43 mutation at the C-terminus, but not at the N-terminus, may affect its ability to promote its mRNA instability (36). In the present study, we found that different FTLD-U/ALS mutations of TDP-43 affected tau mRNA instability and tau expression differentially. TDP-43A90V and TDP-43K263E suppressed tau mRNA instability, whereas TDP-43G295R, TDP-43G295S, and TDP-43M311V promoted tau mRNA instability and suppressed tau expression. Thus, the ALS-linked mutations confer both loss- and gain-of-function properties to TDP-43, which is consistent with the roles of the mutations in alternative splicing (58). Nevertheless, TDP-43 is aggregated and forms inclusions in the neuronal cytoplasm in FTLD-U/ALS, which results in a dramatic depletion of normal nuclear TDP-43 in the affected neurons (23,26). In the present study, we found that TDP-43 was dislocated from the nucleus to the cytoplasm in the brains of 3 months old hemizygous TDP-43M337V transgenic mice, resulting in an increase of tau expression in these transgenic mice. Homozygous TDP-43M337V mice show body tremors, hind limb clasping, and gait abnormality at 1 month of age, but these phenotypes are absent in hemizygous transgenic mice until 12 months of age (36). Hemizygous TDP-43M337V mice develop motor-coordinative and cognitive deficits by 8 months of age and neuronal loss by 12 months of age (59). Moreover, pathological tau deposition in motor neuron disease and FTLD-associated TDP-43 proteopathy was reported (60–62), suggesting that TDP-43 pathology may affect tau pathology. However, it will not be an ideal strategy to suppress tau expression through overexpression of TDP-43 because such an approach may promote TDP-43 pathology (63). In addition, TDP-43 also suppresses its own expression (29).
In the diseased brain or spinal cord, TDP-43 is cleaved N-terminally to generate C-terminal fragments (23). N-terminal truncation enhances aggregation and cellular toxicity (64). Expression of TDP-43 C-terminal fragments in vitro recapitulates pathological features of TDP-43 proteinopathies (65). The C-terminal region is required for TDP-43 to promote its mRNA instability (29). Here, similar to the effect of TDP-43 on its mRNA, we found that deletion of the C-terminal region of TDP-43 failed to promote tau mRNA instability. TDP-43 is cleaved by caspase (66), calpain (67) or asparaginyl endopeptidase (AEP) (68) at the C-terminus, which may play a critical role in TDP-43 pathogenesis (67,69). In AD brain, calpain I and AEP are overactivated as a result of calcium overload, which may be responsible for the decreased TDP-43 and may consequently lead to an increase in tau mRNA stability and tau expression.
Pathological tau and TDP-43 share similar features—phosphorylation, ubiquitination, truncation, and acetylation—which affect their function and promote their aggregation (70). Normally, TDP-43 is located predominantly in the nucleus. In FTLD-U and ALS brain and spinal cord, TDP-43 is abnormally aggregated primarily in the cytoplasm, leading to the depletion of nuclear TDP-43 and the loss of normal nuclear TDP-43 functions (71). Abnormal phosphorylation of TDP-43 on C-terminal serine residues promotes its aggregation (72). Moreover, acetylation of TDP-43 at its RRM impairs RNA binding and promotes accumulation of insoluble, hyper-phosphorylated TDP-43 species (73). N-terminal truncation of TDP-43 also enhances its aggregation (64). However, how these modifications affect TDP-43 function in mRNA metabolism remains unclear. Thus, future studies are required to elucidate the contributions of these post-translational modifications to TDP-43 function in the regulation of tau expression.
In addition to AD, TDP-43 pathology together with tau pathology has been observed in the brains of individuals with CTE (14,15,74). In the present study, we found that various deletion mutations of TDP-43 lost its ability to suppress the expression of tau. Thus, cleavage of TDP-43 due to activation of calpain (67,75) and AEP (68) caused by traumatic brain injury may contribute to tau pathology in CTE.
In summary, in the present study, we found that TDP-43 promotes tau mRNA instability through two (UG)n elements at its 3΄-UTR. The C-terminal region of TDP-43 is required for this function. In AD brain, levels of TDP-43 are decreased, which may contribute to increased tau expression and, consequently, tau pathogenesis.
ACKNOWLEDGEMENTS
We thank M. Marlow for editorial suggestions and J. Murphy for secretarial assistance.
FUNDING
National Natural Science Foundation of China [81030059 and 31671046 to F.L.]; U.S. Alzheimer's Association [DSAD-15-363172 to F.L.]; Neural Regeneration Co-Innovation Center of Jiangsu Province; Priority Academic Program Development of Jiangsu Higher Education institutions (PAPD). Funding for open access charge: National Natural Science Foundation of China.
Conflict of interest statement. None declared.
REFERENCES
- 1. Grundke-Iqbal I., Iqbal K., Quinlan M., Tung Y.C., Zaidi M.S., Wisniewski H.M.. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J. Biol. Chem. 1986; 261:6084–6089. [PubMed] [Google Scholar]
- 2. Grundke-Iqbal I., Iqbal K., Tung Y.C., Quinlan M., Wisniewski H.M., Binder L.I.. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. U.S.A. 1986; 83:4913–4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arriagada P.V., Growdon J.H., Hedley-Whyte E.T., Hyman B.T.. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992; 42:631–639. [DOI] [PubMed] [Google Scholar]
- 4. Alafuzoff I., Iqbal K., Friden H., Adolfsson R., Winblad B.. Histopathological criteria for progressive dementia disorders: clinical-pathological correlation and classification by multivariate data analysis. Acta Neuropathol. 1987; 74:209–225. [DOI] [PubMed] [Google Scholar]
- 5. Tomlinson B.E., Blessed G., Roth M.. Observations on the brains of demented old people. J. Neurol. Sci. 1970; 11:205–242. [DOI] [PubMed] [Google Scholar]
- 6. Moussaud S., Jones D.R., Moussaud-Lamodiere E.L., Delenclos M., Ross O.A., McLean P.J.. Alpha-synuclein and tau: teammates in neurodegeneration. Mol. Neurodegener. 2014; 9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van der Jeugd A., Hochgrafe K., Ahmed T., Decker J.M., Sydow A., Hofmann A., Wu D., Messing L., Balschun D., D’Hooge R. et al. . Cognitive defects are reversible in inducible mice expressing pro-aggregant full-length human Tau. Acta Neuropathol. 2012; 123:787–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roberson E.D., Scearce-Levie K., Palop J.J., Yan F., Cheng I.H., Wu T., Gerstein H., Yu G.Q., Mucke L.. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007; 316:750–754. [DOI] [PubMed] [Google Scholar]
- 9. Santacruz K., Lewis J., Spires T., Paulson J., Kotilinek L., Ingelsson M., Guimaraes A., DeTure M., Ramsden M., McGowan E. et al. . Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005; 309:476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., Mann D., Tsuchiya K., Yoshida M., Hashizume Y. et al. . TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 2006; 351:602–611. [DOI] [PubMed] [Google Scholar]
- 11. Higashi S., Iseki E., Yamamoto R., Minegishi M., Hino H., Fujisawa K., Togo T., Katsuse O., Uchikado H., Furukawa Y. et al. . Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer's disease and dementia with Lewy bodies. Brain Res. 2007; 1184:284–294. [DOI] [PubMed] [Google Scholar]
- 12. Uryu K., Nakashima-Yasuda H., Forman M.S., Kwong L.K., Clark C.M., Grossman M., Miller B.L., Kretzschmar H.A., Lee V.M., Trojanowski J.Q. et al. . Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J. Neuropathol. Exp. Neurol. 2008; 67:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Josephs K.A., Whitwell J.L., Weigand S.D., Murray M.E., Tosakulwong N., Liesinger A.M., Petrucelli L., Senjem M.L., Knopman D.S., Boeve B.F. et al. . TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta Neuropathol. 2014; 127:811–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McKee A.C., Stern R.A., Nowinski C.J., Stein T.D., Alvarez V.E., Daneshvar D.H., Lee H.S., Wojtowicz S.M., Hall G., Baugh C.M. et al. . The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013; 136:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McKee A.C., Gavett B.E., Stern R.A., Nowinski C.J., Cantu R.C., Kowall N.W., Perl D.P., Hedley-Whyte E.T., Price B., Sullivan C. et al. . TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J. Neuropathol. Exp. Neurol. 2010; 69:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Josephs K.A., Murray M.E., Whitwell J.L., Parisi J.E., Petrucelli L., Jack C.R., Petersen R.C., Dickson D.W.. Staging TDP-43 pathology in Alzheimer's disease. Acta Neuropathol. 2014; 127:441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Youmans K.L., Wolozin B.. TDP-43: a new player on the AD field. Exp. Neurol. 2012; 237:90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Caccamo A., Magri A., Oddo S.. Age-dependent changes in TDP-43 levels in a mouse model of Alzheimer disease are linked to Abeta oligomers accumulation. Mol. Neurodegener. 2010; 5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buratti E., Baralle F.E.. TDP-43: gumming up neurons through protein-protein and protein-RNA interactions. Trends Biochem. Sci. 2012; 37:237–247. [DOI] [PubMed] [Google Scholar]
- 20. Shiina Y., Arima K., Tabunoki H., Satoh J.. TDP-43 dimerizes in human cells in culture. Cell. Mol. Neurobiol. 2010; 30:641–652. [DOI] [PubMed] [Google Scholar]
- 21. Buratti E., Baralle F.E.. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J. Biol. Chem. 2001; 276:36337–36343. [DOI] [PubMed] [Google Scholar]
- 22. Kuo P.H., Doudeva L.G., Wang Y.T., Shen C.K., Yuan H.S.. Structural insights into TDP-43 in nucleic-acid binding and domain interactions. Nucleic Acids Res. 2009; 37:1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M. et al. . Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006; 314:130–133. [DOI] [PubMed] [Google Scholar]
- 24. Buratti E., Brindisi A., Giombi M., Tisminetzky S., Ayala Y.M., Baralle F.E.. TDP-43 binds heterogeneous nuclear ribonucleoprotein A/B through its C-terminal tail: an important region for the inhibition of cystic fibrosis transmembrane conductance regulator exon 9 splicing. J. Biol. Chem. 2005; 280:37572–37584. [DOI] [PubMed] [Google Scholar]
- 25. Dewey C.M., Cenik B., Sephton C.F., Dries D.R., Mayer P. 3rd, Good S.K., Johnson B.A., Herz J., Yu G.. TDP-43 is directed to stress granules by sorbitol, a novel physiological osmotic and oxidative stressor. Mol. Cell. Biol. 2011; 31:1098–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cairns N.J., Neumann M., Bigio E.H., Holm I.E., Troost D., Hatanpaa K.J., Foong C., White C.L. 3rd, Schneider J.A., Kretzschmar H.A. et al. . TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am. J. Pathol. 2007; 171:227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gong C.X., Lidsky T., Wegiel J., Zuck L., Grundke-Iqbal I., Iqbal K.. Phosphorylation of microtubule-associated protein tau is regulated by protein phosphatase 2A in mammalian brain. Implications for neurofibrillary degeneration in Alzheimer's disease. J. Biol. Chem. 2000; 275:5535–5544. [DOI] [PubMed] [Google Scholar]
- 28. Choi D.W., Maulucci-Gedde M., Kriegstein A.R.. Glutamate neurotoxicity in cortical cell culture. J. Neurosci. 1987; 7:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ayala Y.M., De Conti L., Avendano-Vazquez S.E., Dhir A., Romano M., D’Ambrogio A., Tollervey J., Ule J., Baralle M., Buratti E. et al. . TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J. 2011; 30:277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Passoni M., De Conti L., Baralle M., Buratti E.. UG repeats/TDP-43 interactions near 5΄ splice sites exert unpredictable effects on splicing modulation. J. Mol. Biol. 2012; 415:46–60. [DOI] [PubMed] [Google Scholar]
- 31. Polymenidou M., Lagier-Tourenne C., Hutt K.R., Huelga S.C., Moran J., Liang T.Y., Ling S.C., Sun E., Wancewicz E., Mazur C. et al. . Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat. Neurosci. 2011; 14:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tollervey J.R., Curk T., Rogelj B., Briese M., Cereda M., Kayikci M., Konig J., Hortobagyi T., Nishimura A.L., Zupunski V. et al. . Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat. Neurosci. 2011; 14:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sephton C.F., Cenik C., Kucukural A., Dammer E.B., Cenik B., Han Y., Dewey C.M., Roth F.P., Herz J., Peng J. et al. . Identification of neuronal RNA targets of TDP-43-containing ribonucleoprotein complexes. J. Biol. Chem. 2011; 286:1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lagier-Tourenne C., Polymenidou M., Cleveland D.W.. TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum. Mol. Genet. 2010; 19:R46–R64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Larner A.J. The cerebellum in Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 1997; 8:203–209. [DOI] [PubMed] [Google Scholar]
- 36. Xu Y.F., Zhang Y.J., Lin W.L., Cao X., Stetler C., Dickson D.W., Lewis J., Petrucelli L.. Expression of mutant TDP-43 induces neuronal dysfunction in transgenic mice. Mol. Neurodegener. 2011; 6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Spillantini M.G., Goedert M., Crowther R.A., Murrell J.R., Farlow M.R., Ghetti B.. Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc. Natl. Acad. Sci. U.S.A. 1997; 94:4113–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DeVos S.L., Miller R.L., Schoch K.M., Holmes B.B., Kebodeaux C.S., Wegener A.J., Chen G., Shen T., Tran H., Nichols B. et al. . Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci. Transl. Med. 2017; 9:eaag0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi J., Qian W., Yin X., Iqbal K., Grundke-Iqbal I., Gu X., Ding F., Gong C.X., Liu F.. Cyclic AMP-dependent protein kinase regulates the alternative splicing of tau exon 10: a mechanism involved in tau pathology of Alzheimer disease. J. Biol. Chem. 2011; 286:14639–14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khatoon S., Grundke-Iqbal I., Iqbal K.. Brain levels of microtubule-associated protein tau are elevated in Alzheimer's disease: a radioimmuno-slot-blot assay for nanograms of the protein. J. Neurochem. 1992; 59:750–753. [DOI] [PubMed] [Google Scholar]
- 41. Lee M.J., Lee J.H., Rubinsztein D.C.. Tau degradation: the ubiquitin-proteasome system versus the autophagy-lysosome system. Prog. Neurobiol. 2013; 105:49–59. [DOI] [PubMed] [Google Scholar]
- 42. Nixon R.A. The role of autophagy in neurodegenerative disease. Nat. Med. 2013; 19:983–997. [DOI] [PubMed] [Google Scholar]
- 43. Barton A.J., Harrison P.J., Najlerahim A., Heffernan J., McDonald B., Robinson J.R., Davies D.C., Harrison W.J., Mitra P., Hardy J.A. et al. . Increased tau messenger RNA in Alzheimer's disease hippocampus. Am. J. Pathol. 1990; 137:497–502. [PMC free article] [PubMed] [Google Scholar]
- 44. Yuan A., Kumar A., Sasaki T., Duff K., Nixon R.A.. Global axonal transport rates are unaltered in htau mice in vivo. J. Alzheimers Dis. 2013; 37:579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Andorfer C., Kress Y., Espinoza M., de Silva R., Tucker K.L., Barde Y.A., Duff K., Davies P.. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J. Neurochem. 2003; 86:582–590. [DOI] [PubMed] [Google Scholar]
- 46. Gotz J., Ittner L.M.. Animal models of Alzheimer's disease and frontotemporal dementia. Nat. Rev. Neurosci. 2008; 9:532–544. [DOI] [PubMed] [Google Scholar]
- 47. van Blitterswijk M., Landers J.E.. RNA processing pathways in amyotrophic lateral sclerosis. Neurogenetics. 2010; 11:275–290. [DOI] [PubMed] [Google Scholar]
- 48. Colombrita C., Onesto E., Megiorni F., Pizzuti A., Baralle F.E., Buratti E., Silani V., Ratti A.. TDP-43 and FUS RNA-binding proteins bind distinct sets of cytoplasmic messenger RNAs and differently regulate their post-transcriptional fate in motoneuron-like cells. J. Biol. Chem. 2012; 287:15635–15647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Buratti E., Baralle F.E.. The multiple roles of TDP-43 in pre-mRNA processing and gene expression regulation. RNA Biol. 2010; 7:420–429. [DOI] [PubMed] [Google Scholar]
- 50. Colombrita C., Onesto E., Tiloca C., Ticozzi N., Silani V., Ratti A.. RNA-binding proteins and RNA metabolism: a new scenario in the pathogenesis of Amyotrophic lateral sclerosis. Arch. Ital. Biol. 2011; 149:83–99. [DOI] [PubMed] [Google Scholar]
- 51. Kraemer B.C., Schuck T., Wheeler J.M., Robinson L.C., Trojanowski J.Q., Lee V.M., Schellenberg G.D.. Loss of murine TDP-43 disrupts motor function and plays an essential role in embryogenesis. Acta Neuropathol. 2010; 119:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Strong M.J., Volkening K., Hammond R., Yang W., Strong W., Leystra-Lantz C., Shoesmith C.. TDP43 is a human low molecular weight neurofilament (hNFL) mRNA-binding protein. Mol. Cell. Neurosci. 2007; 35:320–327. [DOI] [PubMed] [Google Scholar]
- 53. Rutherford N.J., Zhang Y.J., Baker M., Gass J.M., Finch N.A., Xu Y.F., Stewart H., Kelley B.J., Kuntz K., Crook R.J. et al. . Novel mutations in TARDBP (TDP-43) in patients with familial amyotrophic lateral sclerosis. PLoS Genet. 2008; 4:e1000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sreedharan J., Blair I.P., Tripathi V.B., Hu X., Vance C., Rogelj B., Ackerley S., Durnall J.C., Williams K.L., Buratti E. et al. . TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008; 319:1668–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liscic R.M., Grinberg L.T., Zidar J., Gitcho M.A., Cairns N.J.. ALS and FTLD: two faces of TDP-43 proteinopathy. Eur. J. Neurol. 2008; 15:772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yokoseki A., Shiga A., Tan C.F., Tagawa A., Kaneko H., Koyama A., Eguchi H., Tsujino A., Ikeuchi T., Kakita A. et al. . TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann. Neurol. 2008; 63:538–542. [DOI] [PubMed] [Google Scholar]
- 57. Toledo J.B., Brettschneider J., Grossman M., Arnold S.E., Hu W.T., Xie S.X., Lee V.M., Shaw L.M., Trojanowski J.Q.. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol. 2012; 124:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Arnold E.S., Ling S.C., Huelga S.C., Lagier-Tourenne C., Polymenidou M., Ditsworth D., Kordasiewicz H.B., McAlonis-Downes M., Platoshyn O., Parone P.A. et al. . ALS-linked TDP-43 mutations produce aberrant RNA splicing and adult-onset motor neuron disease without aggregation or loss of nuclear TDP-43. Proc. Natl. Acad. Sci. U.S.A. 2013; 110:E736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang W., Arakawa H., Wang L., Okolo O., Siedlak S.L., Jiang Y., Gao J., Xie F., Petersen R.B., Wang X.. Motor-coordinative and cognitive dysfunction caused by mutant TDP-43 could be reversed by inhibiting Its mitochondrial localization. Mol. Ther. 2017; 25:127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yang W., Strong M.J.. Widespread neuronal and glial hyperphosphorylated tau deposition in ALS with cognitive impairment. Amyotroph. Lateral Scler. 2012; 13:178–193. [DOI] [PubMed] [Google Scholar]
- 61. Bieniek K.F., Murray M.E., Rutherford N.J., Castanedes-Casey M., DeJesus-Hernandez M., Liesinger A.M., Baker M.C., Boylan K.B., Rademakers R., Dickson D.W.. Tau pathology in frontotemporal lobar degeneration with C9ORF72 hexanucleotide repeat expansion. Acta Neuropathol. 2013; 125:289–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Behrouzi R., Liu X., Wu D., Robinson A.C., Tanaguchi-Watanabe S., Rollinson S., Shi J., Tian J., Hamdalla H.H., Ealing J. et al. . Pathological tau deposition in Motor Neurone Disease and frontotemporal lobar degeneration associated with TDP-43 proteinopathy. Acta Neuropathol. Commun. 2016; 4:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xu Y.F., Gendron T.F., Zhang Y.J., Lin W.L., D’Alton S., Sheng H., Casey M.C., Tong J., Knight J., Yu X. et al. . Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J. Neurosci. 2010; 30:10851–10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang Y.J., Xu Y.F., Cook C., Gendron T.F., Roettges P., Link C.D., Lin W.L., Tong J., Castanedes-Casey M., Ash P. et al. . Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc. Natl. Acad. Sci. U.S.A. 2009; 106:7607–7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Igaz L.M., Kwong L.K., Chen-Plotkin A., Winton M.J., Unger T.L., Xu Y., Neumann M., Trojanowski J.Q., Lee V.M.. Expression of TDP-43 C-terminal fragments in vitro recapitulates pathological features of TDP-43 proteinopathies. J. Biol. Chem. 2009; 284:8516–8524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang Y.J., Xu Y.F., Dickey C.A., Buratti E., Baralle F., Bailey R., Pickering-Brown S., Dickson D., Petrucelli L.. Progranulin mediates caspase-dependent cleavage of TAR DNA binding protein-43. J. Neurosci. 2007; 27:10530–10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yamashita T., Hideyama T., Hachiga K., Teramoto S., Takano J., Iwata N., Saido T.C., Kwak S.. A role for calpain-dependent cleavage of TDP-43 in amyotrophic lateral sclerosis pathology. Nat. Commun. 2012; 3:1307. [DOI] [PubMed] [Google Scholar]
- 68. Herskowitz J.H., Gozal Y.M., Duong D.M., Dammer E.B., Gearing M., Ye K., Lah J.J., Peng J., Levey A.I., Seyfried N.T.. Asparaginyl endopeptidase cleaves TDP-43 in brain. Proteomics. 2012; 12:2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nonaka T., Kametani F., Arai T., Akiyama H., Hasegawa M.. Truncation and pathogenic mutations facilitate the formation of intracellular aggregates of TDP-43. Hum. Mol. Genet. 2009; 18:3353–3364. [DOI] [PubMed] [Google Scholar]
- 70. Irwin D.J., Cairns N.J., Grossman M., McMillan C.T., Lee E.B., Van Deerlin V.M., Lee V.M., Trojanowski J.Q.. Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol. 2015; 129:469–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lee E.B., Lee V.M., Trojanowski J.Q.. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nat. Rev. Neurosci. 2012; 13:38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Choksi D.K., Roy B., Chatterjee S., Yusuff T., Bakhoum M.F., Sengupta U., Ambegaokar S., Kayed R., Jackson G.R.. TDP-43 Phosphorylation by casein kinase Iepsilon promotes oligomerization and enhances toxicity in vivo. Hum. Mol. Genet. 2014; 23:1025–1035. [DOI] [PubMed] [Google Scholar]
- 73. Cohen T.J., Hwang A.W., Restrepo C.R., Yuan C.X., Trojanowski J.Q., Lee V.M.. An acetylation switch controls TDP-43 function and aggregation propensity. Nat. Commun. 2015; 6:5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E.T., Gavett B.E., Budson A.E., Santini V.E., Lee H.S., Kubilus C.A., Stern R.A.. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 2009; 68:709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yang Z., Lin F., Robertson C.S., Wang K.K.. Dual vulnerability of TDP-43 to calpain and caspase-3 proteolysis after neurotoxic conditions and traumatic brain injury. J. Cereb. Blood Flow Metab. 2014; 34:1444–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]