Abstract
Thymic stromal lymphopoietin (TSLP) and interleukin (IL)-7 are related cytokines that mediate growth and differentiation events in the immune system. They signal through IL-7Rα-containing receptors. Target cells of TSLP in Th2 responses include CD4 T cells and dendritic cells (DC's). While it has been reported that expression of TSLPR on CD4 T cells is required for OVA-induced lung inflammation, DC's have also been shown to be target cells of TSLP. We show here that murine ex vivo splenic DC's are unresponsive to TSLP, as they fail to phosphorylate STAT5, but in vitro overnight culture especially in presence of IL-4 renders DC's responsive to both TSLP and IL-7. This induced responsiveness is accompanied by dramatic upregulation of IL-7Rα on DC's with little change in expression of TSLPR or of γc. In splenic DC's, the induction of IL-7Rα occurs mainly in CD8 negative DC's. In vivo, we found that IL-4 has differential regulatory role on expression of IL-7Rα depending on the cell type; IL-4 decreases IL-7Rα expression on CD4 T cells while upregulating the expression on DC's. Our results indicate that the induction of IL-7Rα expression on DC's is critical for TSLP responsiveness and that IL-4 can upregulate IL-7Rα on DC's.
Keywords: Dendritic cells, Cytokines, Cytokine receptors, Transcription factors, Allergy, Signal transduction, Spleen and Lymph Nodes
Introduction
Signaling by TSLP and IL-7 depends upon ligand-mediated assemblage of a receptor complex consisting of a cytokine-specific binding chain and an “auxiliary” chain. The TSLP and IL-7 receptors share IL-7Rα, although they utilize this chain distinctly. IL-7 binds with high affinity to IL-7Rα this complex then binds γc, activating the Jak1/Jak3 kinases, followed by phosphorylation of critical tyrosine residues on IL-7Rα(1). Docking of STAT5 to the phosphorylated tyrosine residues of IL-7Rα results in Jak-mediated phosphorylation and subsequent nuclear translocation and DNA-binding of STAT5 resulting in regulation of STAT5-mediated transcription. By contrast, TSLP binds TSLPR, a close homolog of γc, followed by IL-7Rα recruitment to the complex. The affinity constant (KA) for TSLP binding to TSLPR is ∼ 7×109(2), while KA for IL-7 binding to IL-7Rα is 1×1010(3), indicating that initial cytokine binding to ligand binding receptor chain occurs with higher affinity by IL-7 than by TSLP. As opposed to IL-7, the Jak1 and Jak2 kinases are activated by TSLP in human and mouse primary CD4 T cells with the consequent phosphorylation of STAT5(1). In human myeloid DC's, activation of STAT6 by TSLP has also been reported(4).
Functionally, IL-7 is a critical growth factor for B and T-cell development and induces survival of memory T-cells(5). Recently, the role of IL-7 in development of innate lymphoid cells (ILC's) has also been established(6). It is of interest, that epithelial cells possess the capacity to produce IL-7, which might be important for survival of tissue lymphocytes locally(7). Memory cell competition for IL-7 has been suggested to be the mechanism of regulation of IL-7-mediated growth(8).
Though mast cells(9), basophils(10) and dendritic cells(11) have been reported to produce TSLP, the main TSLP-producing cells appear to be the epithelial cells in human and particularly the keratinocytes in mice(12-14). Thus, TSLP appears to be principally expressed at skin and mucosal barriers; by contrast, IL-7-production seems more a property of stromal cells in primary and secondary lymphoid organs(15). Effectively, this suggests a specialization of the functions of the two cytokines and particularly emphasizes the potential importance of TSLP in action at mucosal surfaces, be it in the induction of antigen-presenting competence in dendritic cells or in the differentiation/expansion of memory/effector T cells in tissues.
To respond to TSLP, target cells need to express both IL-7Rα and TSLPR while those responsive to IL-7 require expression of IL-7Rα and γc(16). Of conventional or plasmacytoid DC's from un-manipulated mice, only migratory and skin DC's express IL-7Rα at a low level although IL-7Rα expression appears to be important for DC development(17). Human myeloid DC's have been reported to alter their behavior in response to TSLP, by directly upregulating CD40 and CD80 and by enhancing their capacity to induce T-cell proliferation and chemotaxis, the latter through expression of the CCL17 and CCL22(12, 18). Freshly isolated human myeloid DC's express low levels of both IL-7Rα and TSLPR and o/n culture of these cells strongly upregulates expression of both receptor chains(19). Recently, basophils were shown to be responsive to TSLP and it has been reported that such basophils acquire a “Th2”-phenotype in that they produce more IL-4 in response to cytokine stimulation than do basophils cultured in IL-3(20). IL-4 itself is a critical regulator of Th2 responses and cytokine binding receptor chain for IL-4 (IL-4Rα) is found virtually on all cells and thus, basically all cells have capability to respond to IL-4, though with different sensitivity(21).
Since IL-7Rα appears to be expressed at very low levels in murine DC's, we became interested in how could they be a direct target of TSLP? We reasoned that upregulation of IL-7Rα on DC's might be required. Indeed, we observed that neither TSLP nor IL-7 can cause freshly isolated splenic DC's to phosphorylate significant amounts of STAT5 but that, overnight culture of splenic DC's especially in presence of IL-4 results in their responsiveness to both cytokines. We observed that TSLPR and γc were expressed on freshly isolated DC's and showed only modest enhancement as a result of overnight culture but that IL-7Rα expression was dramatically upregulated by overnight culture and more prominently so, in the presence of IL-4. In spleen, IL-7Rα upregulation was less prominent in CD8+ DC's, while CD4+ DC's showed significant upregulation. This suggests that “tuning” of different DC subclasses to TSLP and IL-7 occurs via differential regulation of receptor chains for these cytokines and prepares these cells for differential responsiveness. We also discovered that IL-4 further upregulated IL-7Rα expression and cytokine responsiveness of DC's suggesting yet another regulatory mechanism for IL-4 in orchestrating allergic responses. Further, our results suggest that TSLP responses require both the induction of TSLP production and the induction of IL-7Rα on DC, presumably both events occurring in the same microanatomic location.
Materials and Methods
Mice and Cell cultures
C57BL/6 mice (The Jackson Laboratory) were housed either in the NIAID pathogen-free animal facility or at the Animal facility of School of Medicine, University of Tampere. Experiments were performed under a protocol approved by the NIAID Animal Care and Use Committee. TSLPR-/- mice were provided by Dr W. J. Leonard (NHLBI, NIH). The spleens and lymph nodes from 6-10 week old mice were minced and incubated for 30 minutes at 37°C in HBSS containing 75 μg/ml Liberase DL and 10U/ml DNase (both Roche, Basel, Switzerland). The cells were filtered through a 40 μM cell strainer and red blood cells were lysed with 30 second ACK (Lonza, Basel, Switzerland) treatment. Where indicated, dendritic cells were isolated using MACS Pan Dendritic Cell Isolation Kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were either cultured overnight in RPMI with 10% FBS, l-glutamine and penicillin-streptomycin (Lonza) or used immediately. Where indicated, spleens were mechanically disrupted using a cell culture strainer to release DC's. In the in vivo experiment, mice were injected intraperitoneally twice with either 6 μg of IL-4 (Peprotech, Rocky Hill, NJ) in PBS, complexed with 30 μg of anti-IL-4 antibody, clone BVD4-1D11 (Thermo Fisher Scientific, Carlsbad, CA) as described(22), or 30 μg of anti-IL-4 alone in PBS. The injections were given 8 hours apart. The mice were euthanized 12 hours after the last injection. Human DC's were purified from peripheral blood of 5 healthy donors under Ethical permission R21002 from Ethics Committee of Pirkanmaa Hospital District using pan human DC purification Kit (Miltenyi Biotec). All reagents used were provided endotoxin free by the manufacturers and where applicable they were dissolved into sterile PBS containing BSA (low endotoxin, Sigma Aldrich, St. Louis, MO). Strict aseptic techniques in Biosafety level 2 sterile tissue culture hoods were used in all experiments.
Antibodies, cytokines, flow cytometry and statistics
Mouse antibodies and isotype controls were either from BD (Franklin Lakes NJ; pSTAT5, B220), eBioscience (Santa Clara, CA; CD11c, CD49/Dx5, MHCII, CD3, CD4, CD8, F4/80, IL-7Rα, γc, CD80, CD86, isotype controls for IL-7Rα and γc) or R&D (Minneapolis, MN; TSLPR and polyclonal goat Ig as a control). Human antibodies were from eBiosicence (CD3, CD4, CD8, CD11c, CD14, CD19, CD56, HLA-DR), BD (CD80) or Thermo Fisher Scientific (IL-7Ra, and isotype control for IL-7Ra). Murine cytokines were from Peprotech (GM-CSF, IL-4, IL-7) or R&D (TSLP). Human cytokines (IL-4, TSLP) were from Peprotech. Where indicated, 1 μg/ml LPS (Invivogen, San Diego, CA), 20 ng/ml IL-4 with or without LPS or 40 ng/ml IL-7 was added to the o/n culture. For pSTAT5 assays, cultured cells were starved for 2 hours in RPMI (with l-glutamine, penicillin-streptomycin and 1% FBS) at 37°C and stimulated for 15 minutes with 100 ng/ml GM-CSF, IL-7 or TSLP. Intracellular pSTAT5 staining was done after surface staining by permeabilizing the cells with 90% ice-cold methanol(23). For surface staining, 0.1% BSA, 0.1% mouse serum and CD64 and CD32 blocking 4G2 antibodies was used. Cells were analyzed with FACSCanto II (BD), and data analyzed with FlowJo (Tree Star, Ashland, OR) analysis program. Prism program (GraphPad Software, Inc., La Jolla, CA) was used for statistics. Geometrical means and standard errors of mean (±) are indicated. The p-values were calculated using two-tailed, paired Student's t-test.
Results
Splenic DC's respond to TSLP after in vitro culture
The TSLP and IL-7 receptors share IL-7Rα(Fig 1A). We became interested in how TSLP might regulate DC function particularly because of a recent report showing that splenic or lymph node DC's of mice had no detectable IL-7Rα with the exception of lymph node migratory DC's and their dermal and epidermal counterparts that showed low level of IL-7Rα expression(17). Since several reports suggest a role for TSLP in the regulation of DC activities(24), the low level of expression of IL-7Rα in murine DC's seemed enigmatic. We chose to determine whether DC's were responsive to TSLP and IL-7 by measuring the appearance of an immediate signaling intermediate elicited by these cytokines, STAT5 tyrosine phosphorylated at Y594 (pSTAT5). We prepared single cell suspensions from spleens of C57BL/6 mice by using Dnase/Liberase treatment, followed by enrichment of DC's as described in materials and methods. The cells were incubated with the indicated cytokines for 15 minutes or left untreated, surface stained to allow their phenotyping and permeabilized to allow the detection of pSTAT5. We analyzed the degree of pSTAT5 in CD11cbright, MHCIIbright cells (Fig 1B). As a positive control for pSTAT5 in DC's, we stimulated the cells with GM-CSF.
Figure 1. Splenic DC IL-7 and TSLP responsiveness requires overnight culture of the cells.
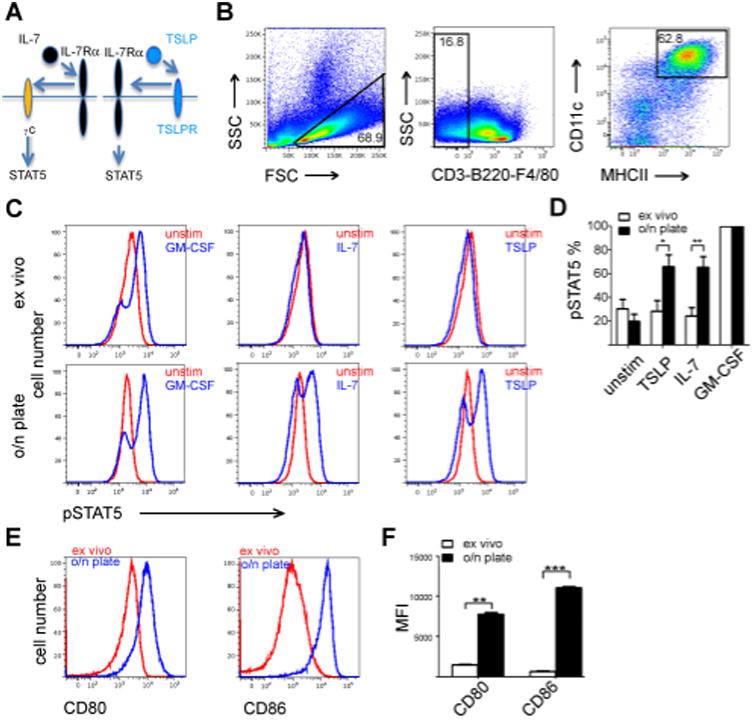
(A) Schematic presentation of assemblage of functional IL-7 and TSLP receptor complexes. (B) Gating of enriched CD11cbright/MHCIIbright splenic DC's. (C) STAT5 phosphorylation of DC's stimulated as indicated for 15 minutes either immediately after harvesting the spleens (ex vivo), or after 16 hours of in vitro culture. Representative flow cytometer plots are shown.(D) Quantitation of pSTAT5 in DC's in response to cytokines. The number of pSTAT5+ cells after GM-CSF stimulation was given the value of 100%. The bars represent the average percent (with +SEM indicated) of pSTAT5+ cells among CD11cbright/MHCIIbright cells after cytokine stimulation in six independent experiments. (*p,0.05,**p<0.01 two-tailed, unpaired t-tests.) (E) The expression of CD80 and CD86 activation markers was measured from CD11cbright/MHCIIbright cells either immediately after harvesting spleens or after 16 hour culture. Representative result is shown. (F) Quantitation of CD80 and CD86 expression. Statistical analysis of three independent experiments. The bars represent the average of MFI, +SEM indicated. (**p<0.01, ***p<0.001, two-tailed, paired t-tests.)
As expected, stimulation of splenic DC's with GM-CSF resulted in robust phosphorylation of STAT5 (Fig 1C) in majority of these cells. TSLP and IL-7 stimulated modest or non-existing response in the freshly isolated splenic DC's (Fig 1C and D). We then asked whether “spontaneous” (i.e. DC-damage-independent, microbial product-independent), DC activation caused by overnight culture altered TSLP or IL-7 responsiveness by these cells. We cultured splenocytes overnight on regular tissue culture plates(25) and stimulated them with GM-CSF, TSLP or IL-7 for 15 minutes. GM-CSF induced STAT5 phosphorylation on the majority of the cultured DC's as it had on freshly isolated DC's. In contrast to freshly isolated splenic DC's, a substantial proportion of the overnight-cultured DC's showed robust phosphorylation of STAT5 in response to TSLP or to IL-7 (Fig 1C, lower panel). Quantitatively, when per cent of GM-CSF-induced pSTAT5 was set to 100, stimulation of splenic DC's with IL-7 or TSLP after overnight culture showed a significant enhancement in the proportion of pSTAT5+ DC's (Fig 1D). The increase in the proportion of DC's that exhibited STAT5 phosphorylation in response to TSLP or IL-7 was highly significant (p= < 0.05 and p=<0.01 respectively, two-tailed unpaired t-test Fig 1D).
To establish that the Dnase/Liberase treatment had not impaired the cytokine response, we compared IL-7 stimulation on ex vivo splenocytes prepared either mechanically or with Dnase/Liberase treatment; IL-7 induced pSTAT5 to a similar degree on splenic CD4 T cells from both preparations (Supplementary Figure 1, upper panel) indicating IL-7Rα was not degraded by Dnase/Liberase. As the DC yields were higher when using Dnase/Liberase treatment, we chose to use this approach. Also, enrichment of DC's did not effect on expression of IL-7Rα on freshly isolated DC's (Supplementary Figure 1, lower panel). Overnight culture activated DC's; both CD80 and CD86 expression were significantly upregulated on DC's after 16 hours of culturing the cells (Fig 1E), the p-values for upregulation were p<0.01 for CD80 and p<0.001for CD86 (two-tailed paired t-test Fig 1F).
Expression of receptors for IL-7 and TSLP on DC's
As IL-7Rα is a component of the receptors for both IL-7 and TSLP (Fig 1A), the induction of responsiveness to these cytokines might reflect an upregulation of IL-7Rα expression on cultured DC's. As reported(17), we found that the great majority of ex vivo splenic DC's had no detectable IL-7Rα since isotype control and monoclonal antibody against IL-7Rα stained same amount of CD11cbright/MHCIIbright cells (Supplementary Fig 2). O/n culture of splenocytes resulted in no change in the binding of the isotype control antibody to CD11cbright/MHCIIbright cells (Supplementary Fig 2) whereas anti-IL-7Rα staining showed a dramatic upregulation of this receptor chain (Fig 2A). Antibodies to γc and to TSLPR stained essentially all freshly isolated DC's; overnight culturing resulted in only a modest enhancement in the intensity of staining for each receptor chain. Quantitatively, ∼ 1% of the total ex vivo DC's expressed IL-7Rα while ∼ 20% of the cells were positive for IL-7Rα after o/n culture (Fig 2B). The upregulation of IL-7Rα on DC's was statistically highly significant (p-value <0.001, two-tailed, paired t-test).
Figure 2. The increased responsiveness of splenic DC's to TSLP and IL-7 is due to robust increase in IL-7Rα expression upon overnight culture.
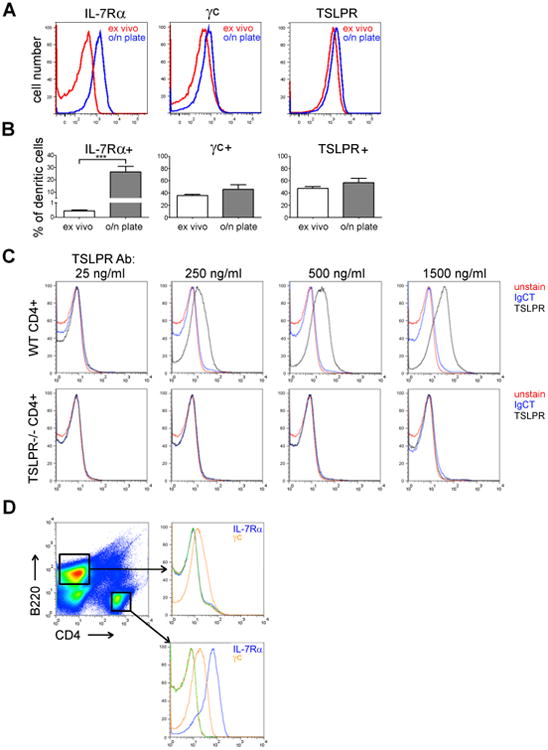
(A) IL-7Rα, γc and TSLPR expression of splenic DC's (CD11cbright/MHCIIbright cells) was measured either directly after harvesting the spleens (ex vivo) or after overnight culture. A representative example is shown. (B) Statistical analysis of 5-8 independent experiments. Bars represent the average per cent of receptor chain positive cells, +SEM is indicated (***p<0.001, two-tailed, paired t-tests). (C) TSLPR antibody specificity was tested using splenic CD4 T cells from WT or TSLPR-/- mice. The experiment was done twice (one WT and one TSLPR-/- mice per experiment). (D) The specificity of IL-7Rα and γc antibodies were tested on splenic CD4 and B220 cells, as indicated with antibody specific isotype controls (green and red lines). The staining was done twice with two different WT B6 mice.
We confirmed the specificity of the polyclonal anti-TSLPR antibody by using various amounts of anti-TSLPR antibody or control Ig on cell type known to express TSLPR (splenic CD4 T cells) from wild-type and TSLPR-deficient mice (Fig 2C). To test the specificity of IL-7Rα and γc antibodies, we compared their staining profiles on B220+ cells and CD4 T cells. As expected, anti-IL-7Rα showed strong staining on CD4 T cells, but no staining on B-cells, that are known to lack surface IL-7Rα, whereas anti-γc staining was positive on both cell types (Fig 2D).
Induction of IL-7Rα in different splenic DC subsets
The CD11cbright/MHCIIbright splenic DC's are actually a group of subtypes characterized by differential expression of surface markers. Differential expression of CD4 and CD8 has been used to classify CD11cbright DC's as CD4+/CD8-, CD4-/CD8+ or CD4-/CD8-subclasses(26). The different subclasses of splenic DC's might have a different capacity to upregulate IL-7Rα. We stained splenic cells for IL-7Rα immediately ex vivo or after overnight culture as in Fig 2, but we analyzed the expression of CD4 and CD8 simultaneously(26). We identified three major populations among the cells in the CD11c+/MHCII+ gate based on CD4 and CD8 expression (Fig 3A). None expressed detectable IL-7Rα when tested immediately after harvest. Strikingly, the upregulation of IL-7Rα in o/n cultured cells was most prominent on CD8- DC populations (Fig 3B). While overnight cultured CD8+ DC's showed levels of IL-7Rα that were modestly changed when compared to CD8+ DC's ex vivo (less than 10 % of the cells became IL-7Ra positive), ∼25% of CD4+ DC's became positive for IL-7Rα (Fig 3C). CD4-/CD8-DC's fell between CD4+ and CD8+ DC's; less than 20% of the cells became IL-7Rα+ during o/n culture. Quantitatively, the difference of IL-7Rα expression on CD8+ DC's was significant ex vivo and after o/n culture (p<0.01 and p<0.001, respectively, Fig 3C).
Figure 3. IL-7Rα upregulation occurs mainly in CD8- splenic DC's.
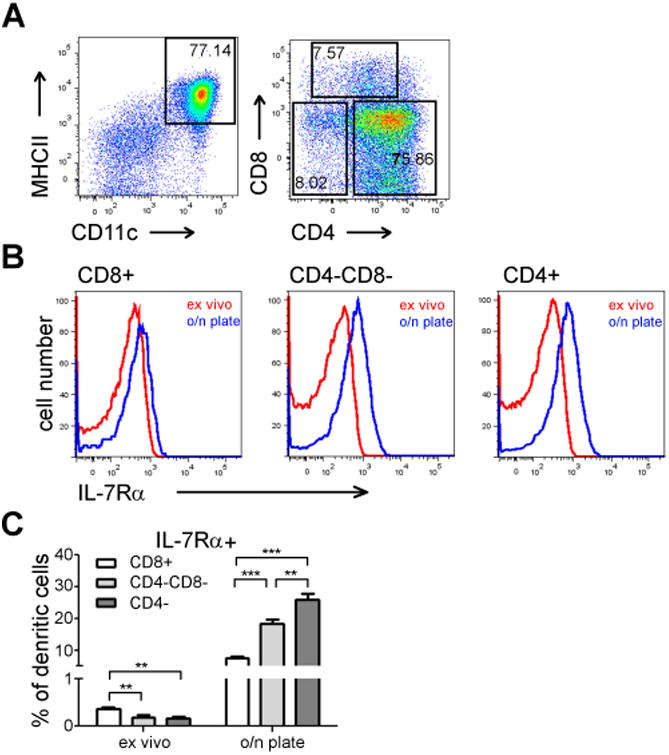
(A) Gating the o/n cultured splenic DC's on the basis of CD4 and CD8 expression. Numbers indicate percent of cells of total CD11c/MHCII positive cells. (B) IL-7Rα expression was studied in different splenic DC subpopulations ex vivo or after o/n culture. Representative result from one experiment is shown. (C) Statistical analysis of IL-7Rα expression on splenic DC subpopulations from three independent experiments. The bars indicate the percent of IL-7Rα positive cells in the indicated subpopulation (the mean and +SEM are indicated, **p<0.01, ***p<0.001, two-tailed, paired t-tests.)
IL-4 and LPS enhance IL-7Rα expression in cultured DC's
Next, we studied how various soluble factors might regulate the IL-7Rα expression in cultured DC's. In T-cells, IL-7 itself downregulates IL-7Rα transcription and IL-4 has also been associated in downregulation of IL-7Rα(27). In addition, LPS is a potent activator of DC's via TLR4-Myd88-nfκB pathway and could control the cytokine sensitivity by regulating the expression of cytokine receptors on DC's. For these experiments, the cells were left overnight in either 10% serum containing culture medium alone, or with IL-4 (20 ng/ml), IL-7 (40 ng/ml), LPS (1 μg/ml) or IL-4+LPS and then measured IL-7Rα in CD11cbright, MHCIIbright cells, that were negative for CD3, B220 and F4/80 (Fig 4A).
Figure 4. IL-4 induces IL-7Rα expression and TSLP responsiveness of splenic DC's.
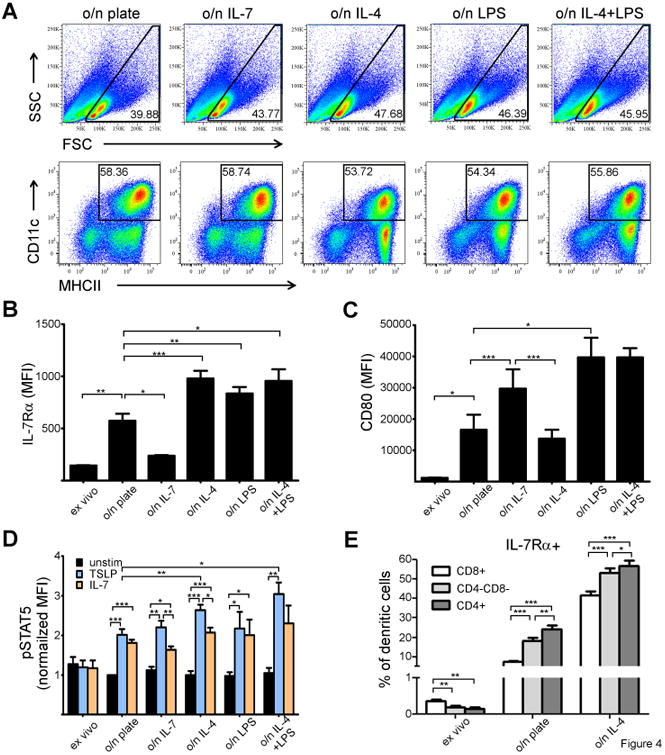
(A) Comparison of splenic DC populations after different o/n culturing conditions. (B-C) Statistical analysis of IL-7Rα (B) and CD80 (C) expression on splenic DC's, Bars indicate MFI (with +SEM). IL-7Rα and CD80 staining data is from three independent experiments (3 animals used in each experiment). (D) STAT5 phosphorylation in splenic DC's (either ex vivo or o/n cultured with indicated stimulations) in response to either vehicle (PBS,) or IL-7 or TSLP. The bars indicate normalized MFI of pSTAT5 (o/n cultured unstimulated sample was given the value 1). Data is from three independent experiments (three animals used in each experiment). (E) IL-7Rα upregulation in different DC subpopulations in response to IL-4 was measured. MFI and +SEM are indicated. *p<0.05, **p<0.01,***p<0.001, two-tailed, paired t-test was used.
In splenic DC's, o/n culturing alone increased IL-7Rα expression significantly as compared to ex vivo purified DC's (p= 0.0003). Strikingly, as compared to o/n alone cultured DC's, adding IL-7 into the culture appeared to strongly downregulate IL-7Rα expression in DC's (p=0.019), while adding IL-4 significantly increased IL-7Rα expression (p=0.0001, Fig 4B). LPS alone also induced IL-7Rα expression significantly, when compared to DC's cultured in medium alone (p=0.0076), while adding IL-4 to LPS did not further increase the expression of IL-7Rα in DC's. The induction of IL-7Rα was not associated with further “activation” of DC's as judged by CD80 expression. CD80 was upregulated in o/n cultured cells but both IL-7 and LPS further induced CD80 expression, while IL-4 did not (Fig 4C).
In line with changes observed in the expression of IL-7Rα, the induction of intracellular pSTAT5 by 15 minutes stimulation with TSLP was enhanced in splenic DC's that had been cultured in IL-4 when compared to DC's cultured in culture medium alone (p=0.0037, Fig 4D). Strikingly, no difference in TSLP responsiveness was observed between DC's that been cultured either in medium alone or in medium containing IL-7 (p=0.5008), while IL-7 responses were slightly, but not statistically significantly lower in IL-7 cultured cells when compared to medium alone (p=0.0998). Albeit LPS upregulated IL-7Rα in splenic DC's (Fig 4B), culturing these cells in LPS did not enhance their cytokine responsiveness (Fig 4D). Taken together TSLP-induced STAT5 phosphorylation was significantly enhanced only in DC's cultured in IL-4. The slight decrease in pSTAT5 responsiveness to IL-7 by DC's cultured in IL-7 was not due to decreased expression of γc by IL-7 stimulation (data not shown).
In DC's purified from lymph nodes (Supplementary Figure 3), o/n culture significantly increased expression of IL-7Rα (p=0.0010), while IL-4 appeared to enhance IL-7Rα but this difference was not statistically significant (p=0.0871), while IL-7 clearly down regulated IL-7Rα (p=0.0118) in these cells (Supplementary Figure 3). For CD80 expression in lymph node DC's, IL-7 induced CD80 expression while IL-4 had no effect on CD80 expression (Supplementary Figure 3).
Human DC's upregulate IL-7Rα during overnight culture
In human peripheral blood myeloid DC's, not only IL-7Rα but also TSLPR are upregulated during o/n culture(19), while in human airway mucosal myeloid DC's TSLPR is constitutively activated(28). This could suggest differential regulation of the TSLP receptor system between two anatomic locations. To learn if the observed IL-7Rα upregulation we discovered in murine DC's would occur in human DC's and would be linked particularly to stimulation with IL-4, we studied human peripheral blood DC's from healthy donors (n=5) that were enriched as described in Materials and methods. We analyzed HLA-DRbright/CD11cbright cells either directly ex vivo or after o/n culture on tissue culture plates (Fig 5A). For o/n culture, we either left the cells unstimulated or stimulated the cells with human IL-4 or human TSLP. Quite differently from murine DC's, CD80 expression in human DC's was only modestly upregulated during o/n culture, but IL-4 and particularly TSLP strongly upregulated CD80 expression (Fig 5B). For IL-7Rα expression, low expression ex vivo, was strongly upregulated during o/n culture (p=0.0012), the upregulation was biphasic, indicating that IL-7Rα upregulation was not a feature of all HLA-DRbright/CD11cbright cells or vice versa, only portion of the cells was able to overcome possible inhibitory factors keeping IL-7Rα expression low on these cells in the overnight culture. IL-4 stimulation itself did not induce IL-7Rα expression in human DC's (p o/n culture vs o/n culture with IL-4 = 0.0933) while combining IL-4 stimulation to TSLP, strikingly downregulated IL-7Rα expression (p=0.0024) in human DC's. Thus, for human total HLA-DRbright/CD11cbright DC's, IL-4 did not upregulate IL-7Rα. It will be of interest to characterize the DC subpopulations that clearly upregulate IL-7Rα and if IL-4 specifically regulates IL-7/TSLP sensitivity in these subpopulations.
Figure 5. IL-7Rα expression is upregulated in human peripheral blood DC's during overnight culture.
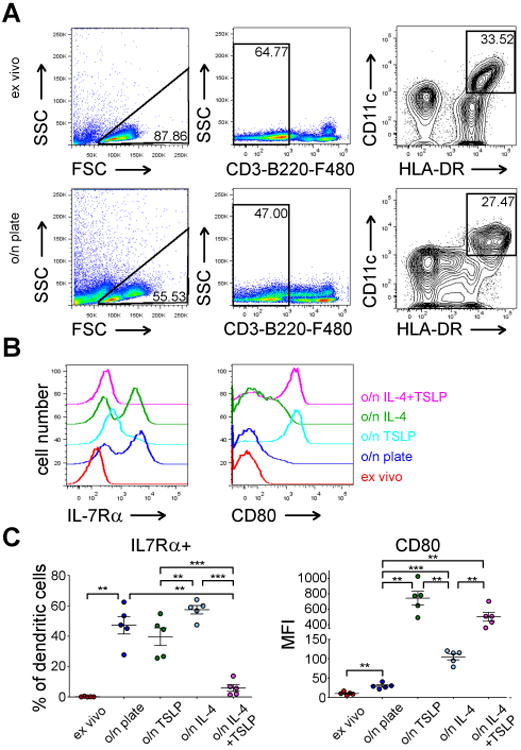
(A) Gating strategy of enriched human peripheral DC's ex vivo (upper panel) or after overnight culture (lower panel). (B) A representative experiment of expression of IL-7Rα (left panel) or CD80 in human DC's from one healthy donor after indicated culture conditions. (C) Statistical analysis of IL-7Rα and CD80 expression in peripheral blood DC's from five healthy donors after indicated culture conditions/stimulations. The mean and ±SEM are indicated, **p<0.01, ***p<0.001, two-tailed, paired t-tests.
IL-4 increases IL-7Rα expression on mouse splenic DC's in vivo
The notion that IL-4 appeared to further upregulate IL-7Rα on o/n cultured DC's in mice prompted us to ask if IL-4 might have the same effect on DC's in vivo. Since the half-life of IL-4 in biologic fluids is rather short, we utilized method described by Morris et al(22), where complexing IL-4 to a soluble anti-IL-4 antibody prolongs the cytokine half life. WT B6 mice (n=5 per treatment) were either injected with anti-IL-4 (aIL-4) or anti-IL-4 complexed to IL-4 (complex). To verify, that the complex administration resulted in IL-4-mediated responses, we measured IL-7Rα and IL-4Rα on T cells. As expected(27), the expression of IL-7Rα was decreased in CD4 T cells in response to the complex (p<0.0001) whereas complex significantly upregulated IL-4Rα (p=0.0092) in these cells (Fig 6A, gating strategy for T-cells is shown in Supplementary Fig 4). For CD8 T cells, we found the results were similar; IL-4 downregulated IL-7Rα (p=0.0013) and upregulated IL-4Rα (p<0.0001) expression (Fig 6B). Since IL-4 upregulated IL-7Rα on DC's in vitro (Fig 4B) we next asked, if the same occurs in vivo. As we measured IL-7Rα expression from mice treated with aIL-4, no effect on IL-7Rα expression was observed. However, mice treated with complex we noticed consistent and statistically significant induction in IL-7Rα on DC's (Fig 6C left panel, p=0.0005). In line with observations from in vitro cultures, CD80 was not induced by IL-4 in vivo (Fig 6C right panel).
Figure 6. IL-4 affects surface expression of IL-7Rα in T cells and dendritic cells differently in vivo.
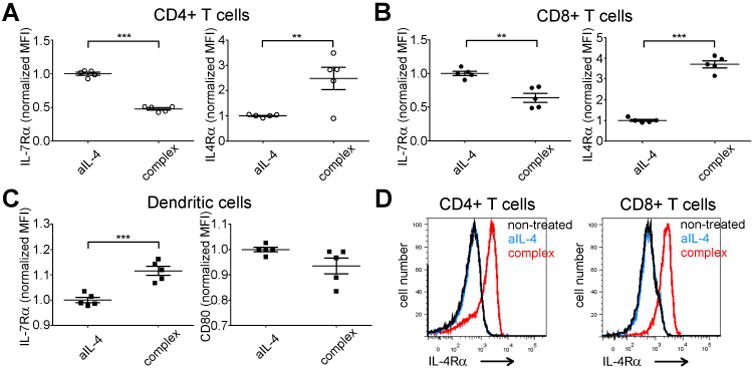
. Anti-IL-4 antibody alone (aIL-4) or mixed with IL-4 (complex) were administrated intraperitoneally to B6 mice (5 per group) twice 8 hours apart. 16 hours later, mice were euthanized, splenocytes were harvested and analyzed based on CD3+/CD4+/CD8+ (A-B, D) or CD11c+/MHCII+ (C) expression. (A) IL-7Rα (left panel) and IL-4Rα (right panel) expression in CD4 T cells. In all experiments dot represents expression level in one mouse (+ and - SEM indicated). (B) IL-7Rα (left panel) and IL-4Rα (right panel) expression in CD8 T cells. (C) Expression of IL-7Rα (left panel) and CD80 (right panel) in splenic DC's. (D) Comparison of IL-4Rα expression in CD4 (left panel) and CD8 (right panel) T cells of untreated, aIL-4- and complex-treated B6 mice. **p<0.01, *** p<0.001 two-tailed, paired t-test.
To confirm that a-IL-4 was an appropriate control for these experiments, we compared IL-4Rα expression in CD4 and CD8 T cells. We found that IL-4Rα expression in either cell type was similar in untreated or anti-IL-4-treated mice whereas complex-treated mice showed dramatic upregulation of this receptor chain (Fig 6D).
Discussion
The requirement of IL-7Rα for both IL-7- and TSLP-induced signaling coupled with the distinct anatomical distribution of the two cytokines prompted us to study how they act on DC's since these cells have been implicated as direct targets of TSLP. In accordance with previous studies, we found that only a small minority of freshly isolated splenic or lymph node DC's expressed IL-7Rα(17, 19) and that the great majority of these cells failed to phosphorylate STAT5 in response to either TSLP or IL-7. Overnight culture of these cells, which is known to activate DC's(25), resulted in upregulation of IL-7Rα and rendered a substantial proportion of the cells responsive to both IL-7 and TSLP. Evidence has been presented indicating that TSLP can cause skin or mucosal DC;s to acquire Th2-inducing potential. The mechanism by which this occurs is still uncertain but one possibility - at least in the human DC's - is the TSLP-mediated upregulation of OX40L expression(29).
The failure of freshly isolated splenic DC's to express IL-7Rα and to show responsiveness to TSLP implies that if TSLP truly plays a major physiologic role in Th2 differentiation through its effects on skin and/or mucosal DC's, then a dual induction process is required - the induction of TSLP synthesis/secretion by skin/mucosal epithelial cells and the induction of TSLP responsiveness by the local DC's. Induction of TSLP expression has been studied to some degree. Among the stimulants known to cause induction of TSLP are vitamin D3, TNFα, IL-4 and IL-13(12, 13). Less is known about the mechanism through which IL-7Rα is induced on tissue DC's. Our study of splenic DC's implies that IL-4 and the LPS pathways regulate its in vitro induction. The notion that IL-7 appeared to inhibit IL-7Rα expression on DC's is interesting but it may also be a technical artifact. It is feasible that IL-7 in the culture media blocks the binding of the antibody recognizing IL-7Rα. This conclusion is supported by the fact that overnight IL-7 stimulation of DC's has no inhibitory effect on TSLP-induced STAT5 phosphorylation (Fig 4C), indicating that the building blocks of functional receptor for TSLP are available on the cell surface. The notion that addition of IL-4 into the o/n DC culture induced IL-7Rα expression but only modestly enhanced the responsiveness of the splenic DC's to IL-7 might be explained by the fact that IL-4 in the culture medium could keep the γc chain constantly occupied to the type I IL-4 receptor thus limiting the increase in IL-7 signaling. Related to receptor bioavailability, increased expression of IL-7Rα on DC's could have another important consequence namely reducing the bioavailability of IL-7 in biologic fluids, which plays a major role in survival of T cells(27).
Our studies imply that a coordinated induction of TSLP and IL-7Rα is essential for TSLP-mediated activation/differentiation of DC's. It should be noted here, that recent work indicated that physiologically TSLP executes some of it functions via non-hematopoietic cells(30). This calls for further studies of TSLP-signaling and the possibility that an alternative receptor may still exist since IL-7Rα is considered to be expressed solely by hematopoietic cells(31). Furthermore, comparison of murine B-cells from WT or TSLPR-/- mice indicated that WT B cells express substantial amount of TSLPR, while they are negative for IL-7Rα. Would WT B-cells from IL-7Rα deficient mice respond to TSLP, and would this occur via Stat5 or independently of Stat5? Comparing genome-wide transcription analysis combined with cellular phosphorylation analysis in WT and IL-7Rα deficient mice would clarify if TSLP truly could signal without IL-7Rα.
The notion that IL-4, which itself induces TSLP production(14), also regulates a critical component of TSLP receptor complex on DC's in our experiments is interesting. A possibility remains, that in the early induction phase of allergic inflammation, basophil-derived IL-4(32) acts locally in barrier tissues on both keratinocytes to produce TSLP and simultaneously on DC's to upregulate the receptor for TSLP and thus, IL-4 could orchestrate locally both production and responsiveness to TSLP on specific cell types. Further studies on functional characteristics of IL-4/TSLP cytokine axis on IL-4 deficient mice will reveal how TSLP signaling and receptor expression (particularly IL-7Rα) are regulated by IL-4. Experiments following IL-7Rα expression on DC's utilizing mice genetically modified to lack the expression of IL-4, IL-4Rα, Insulin receptor substrate(IRS)2 and Stat6 should answer to question how IL-4 signaling regulates the expression of IL-7Rα on DC's.
Our observation, that CD8+/CD4- splenic DC's showed less induction of IL-7Rα as compared to CD8-/CD4+ or CD8-/CD4- DC's implies, that professional IL-12-producing DC's respond poorly to TSLP and IL-7 or that TSLP stimulation may divert the cells from acquiring or maintaining IL-12 producing capacity, at least in this experimental setting. As CD8+/CD4- DC's are specific producers of IL-12, the failure of these cells to acquire IL-7Rα expression would be rational.
The benefit of the local regulation of IL-7Rα could be explained by locally regulated TSLP production depending on the signals that alveolar, bronchial and skin epithelial cells receive. While migratory DC's in lymph node and their skin counterparts(17) do express IL-7Rα at low levels, possibly allowing some cytokine signaling, the induction of IL-7Rα (and ensuing cytokine responsiveness) we observed here is quite drastic. The notion that in human freshly isolated DC's both TSLPR and IL-7Rα are expressed at low levels(19) indicate that human and mouse DC's may differ in the steady-state expression of TSLPR. Alternatively, TSLPR expression in human DC's may be regulated by infectious and genetic background differences that are absent in inbred mice lines housed in standardized animal facilities. We found that in human peripheral blood DCs from small group of individuals (n=5), the expression of IL-7Rα was induced during o/n culture as expected(19) but the additive IL-4-mediated effect seen in mice was not observed. One explanation could be that human DC's are continuously encountering antigens and are thus more activated than inbred mouse DC's from sterile housing, this is supported by the finding that CD80 expression was only modestly up-regulated in human peripheral blood DC's as compared to splenic murine DC's. Thus, the phenotype of “resting” human DC's is quite different than corresponding mouse cells. Another point to consider is the fact that myeloid human DC's from different anatomical locations differ in their expression of TSLPR(19, 28) which could be explained by the fact that TSLP is expressed closer to epithelial and mucosal barriers and thus “sensing” of the cytokine is restricted to the cells closer these structures. It should also be noted, that both IL-7Rα and TSLPR expression on DC's could be under active inhibitory regulation particularly while in circulation. In murine CD4 T cells, IL-7Rα is downregulated by various cytokines(27), which could be the case for human DC's too. If this was the case, peripheral blood DC's would inherently express low levels of one or both of these receptor chains and only upon appropriate activation signal induce receptor chain expression resulting in increased responsiveness to cytokine (TSLP/IL-7). Such a system would protect DC's against inappropriate activation by type2-polarizing TSLP in the peripheral blood. Future work characterizing anatomy and activation status of various human DC populations in context of TSLP (and IL-7) responses as well as expression of TSLPR and IL-7Rα will be a fruitful endeavor.
Another point to be considered is how much of the action of TSLP in Th2 responses is on CD4 T cells. While there is the possibility that the STAT5 activation known to be critical to in vitro Th2 differentiation is mediated by TSLP in vivo, TSLP seems an unlikely cytokine to mediate its function in secondary lymphoid organs since its principal sites of production appear to be at mucosal and skin surfaces. If STAT5 activation is as essential for in vivo Th2 differentiation as it is for in vitro differentiation, IL-2 or IL-7 would appear to be far more likely to mediate this function during the initial phases of T-cell priming. However, once activated CD4 T cells migrate to the tissues, TSLP-induced STAT5 phosphorylation could be important in completing or sustaining Th2 differentiation and/or survival.
Overall, our results provide a mechanistic explanation how DC responsiveness to TSLP in mouse can be modified simply by induction of IL-7Rα, the receptor chain required for both IL-7 and TSLP response.
Supplementary Material
Acknowledgments
Animal facilities both at NIAID and at School of Medicine, University of Tampere are thanked for support in animal experimentation.
Supported by: the National Institute of Allergy and Infectious Diseases Intramural Research Program (XC, SC, RY, WEP, JZ, ISJ), the Finnish Medical Foundation (ISJ), the Sigrid Juselius Foundation (ISJ, MP), the Tampere Tuberculosis Foundation (ISJ, MP) and the Competitive State Research Financing of the Expert Responsibility areas of Tampere University Hospital (Grants 9M080, 9N056, 9S051 (MP)/Fimlab Laboratories (Grant X51409, ISJ), the Academy of Finland (projects 263955, 135980 (MP)), and the Emil Aaltonen Foundation (LK,MP).
Abbreviations
- DC
dendritic cell
- Gfi-1
Growth Factor Independent-1
- MFI
Mean Fluorescence Intensity
- TSLP
Thymic Stromal Lymphopoietin
Footnotes
The online version of this article contains supplemental material.
References
- 1.Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez-Rodriguez J, Wagner KU, Leonard WJ. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A. 2010;107:19455–19460. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, Ziegler SF, Morrissey PJ, Paxton R, Sims JE. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park LS, Friend DJ, Schmierer AE, Dower SK, Namen AE. Murine interleukin 7 (IL-7) receptor. Characterization on an IL-7-dependent cell line. J Exp Med. 1990;171:1073–1089. doi: 10.1084/jem.171.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arima K, Watanabe N, Hanabuchi S, Chang M, Sun SC, Liu YJ. Distinct signal codes generate dendritic cell functional plasticity. Sci Signal. 2010;3:ra4. doi: 10.1126/scisignal.2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 6.Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 7.Oshima S, Nakamura T, Namiki S, Okada E, Tsuchiya K, Okamoto R, Yamazaki M, Yokota T, Aida M, Yamaguchi Y, Kanai T, Handa H, Watanabe M. Interferon regulatory factor 1 (IRF-1) and IRF-2 distinctively up-regulate gene expression and production of interleukin-7 in human intestinal epithelial cells. Mol Cell Biol. 2004;24:6298–6310. doi: 10.1128/MCB.24.14.6298-6310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 9.Moon PD, Kim HM. Thymic stromal lymphopoietin is expressed and produced by caspase-1/NF-kappaB pathway in mast cells. Cytokine. 2011;54:239–243. doi: 10.1016/j.cyto.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol. 2011;187:1207–1211. doi: 10.4049/jimmunol.1100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, Mc Clanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 13.Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A. 2006;103:11736–11741. doi: 10.1073/pnas.0604575103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewas C, Chen X, Honda T, Junttila I, Linton J, Udey MC, Porcella SF, Sturdevant DE, Feigenbaum L, Koo L, Williams J, Paul WE. TSLP Expression: Analysis with a ZsGreen TSLP Reporter Mouse. J Immunol. 2014 doi: 10.4049/jimmunol.1400519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzucchelli RI, Warming S, Lawrence SM, Ishii M, Abshari M, Washington AV, Feigenbaum L, Warner AC, Sims DJ, Li WQ, Hixon JA, Gray DH, Rich BE, Morrow M, Anver MR, Cherry J, Naf D, Sternberg LR, McVicar DW, Farr AG, Germain RN, Rogers K, Jenkins NA, Copeland NG, Durum SK. Visualization and identification of IL-7 producing cells in reporter mice. PLoS One. 2009;4:e7637. doi: 10.1371/journal.pone.0007637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogt TK, Link A, Perrin J, Finke D, Luther SA. Novel function for interleukin-7 in dendritic cell development. Blood. 2009;113:3961–3968. doi: 10.1182/blood-2008-08-176321. [DOI] [PubMed] [Google Scholar]
- 18.Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, Zurawski SM, Johnston J, Liu YJ, Spits H, de Waal Malefyt R, Kastelein RA, Bazan JF. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 19.Lu N, Wang YH, Wang YH, Arima K, Hanabuchi S, Liu YJ. TSLP and IL-7 use two different mechanisms to regulate human CD4+ T cell homeostasis. J Exp Med. 2009;206:2111–2119. doi: 10.1084/jem.20090153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, Wherry EJ, Jessup HK, Siegel LA, Kambayashi T, Dudek EC, Kubo M, Cianferoni A, Spergel JM, Ziegler SF, Comeau MR, Artis D. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Junttila IS, Mizukami K, Dickensheets H, Meier-Schellersheim M, Yamane H, Donnelly RP, Paul WE. Tuning sensitivity to IL-4 and IL-13: differential expression of IL-4Ralpha, IL-13Ralpha1, and gammac regulates relative cytokine sensitivity. J Exp Med. 2008;205:2595–2608. doi: 10.1084/jem.20080452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris SC, Orekhova T, Meadows MJ, Heidorn SM, Yang J, Finkelman FD. IL-4 induces in vivo production of IFN-gamma by NK and NKT cells. J Immunol. 2006;176:5299–5305. doi: 10.4049/jimmunol.176.9.5299. [DOI] [PubMed] [Google Scholar]
- 23.Krutzik PO, Nolan GP. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- 24.Saenz SA, Taylor BC, Artis D. Welcome to the neighborhood: epithelial cell-derived cytokines license innate and adaptive immune responses at mucosal sites. Immunol Rev. 2008;226:172–190. doi: 10.1111/j.1600-065X.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vremec D, O'Keeffe M, Wilson A, Ferrero I, Koch U, Radtke F, Scott B, Hertzog P, Villadangos J, Shortman K. Factors determining the spontaneous activation of splenic dendritic cells in culture. Innate Immun. 2011;17:338–352. doi: 10.1177/1753425910371396. [DOI] [PubMed] [Google Scholar]
- 26.Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- 27.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Melum GR, Farkas L, Scheel C, Van Dieren B, Gran E, Liu YJ, Johansen FE, Jahnsen FL, Baekkevold ES. A thymic stromal lymphopoietin-responsive dendritic cell subset mediates allergic responses in the upper airway mucosa. J Allergy Clin Immunol. 2014;134:613–621.e.7. doi: 10.1016/j.jaci.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reardon C, Lechmann M, Brustle A, Gareau MG, Shuman N, Philpott D, Ziegler SF, Mak TW. Thymic stromal lymphopoetin-induced expression of the endogenous inhibitory enzyme SLPI mediates recovery from colonic inflammation. Immunity. 2011;35:223–235. doi: 10.1016/j.immuni.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


