Abstract
The optimal management of resectable oesophageal adenocarcinoma is controversial, with many centres using neoadjuvant chemotherapy following the Medical Research Council (MRC) oesophageal working group (OE02) trial and the MRC Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial. The more intensive MAGIC regimen is used primarily in gastric cancer but some also use it for oesophageal cancer.
A database of cancer resections (2001–2013) provided information on survival of patients following either OE02 or MAGIC-type treatment. The data were compared using Kaplan–Meier analysis. Straight-to-surgery patients were also reviewed and divided into an ‘early’ cohort (2001–2006, OE02 era) and a ‘late’ cohort (2006–2013, MAGIC era) to estimate changes in survival over time. Subgroup analysis was performed for responders (tumour regression grade [TRG] 1–3) versus non-responders (TRG 4 and 5) and for anatomical site (gastro-oesophageal junction [GOJ] vs oesophagus).
An OE02 regimen was used for 97 patients and 275 received a MAGIC regimen. Those in the MAGIC group were of a similar age to those undergoing OE02 chemotherapy but the proportion of oesophageal cancers was higher among MAGIC patients than among those receiving OE02 treatment.
MAGIC patients had a significantly lower stage following chemotherapy than OE02 patients and a higher median overall survival although TRG was similar. On subgroup analysis, this survival benefit was maintained for GOJ and oesophageal cancer patients as well as non-responders. Analysis of responders showed no difference between regimens.
‘Late’ group straight-to-surgery patients were significantly older than those in the ‘early’ group. Survival, however, was not significantly different for these two cohorts.
Although the original MAGIC trial comprised few oesophageal cancer cases, our patients had better survival with MAGIC than with OE02 chemotherapy in all anatomical subgroups, even though there was no significant change in operative survival over the time period in which these patients were treated. The use of the MAGIC regimen should therefore be encouraged in cases of operable oesophagogastric adenocarcinoma.
Keywords: Oesophageal neoplasm, Adenocarcinoma, Neoadjuvant chemotherapy, Kaplan–Meier estimate
Oesophageal adenocarcinoma is an aggressive malignancy that has been shown to be best treated with multimodal therapy rather than surgery alone. Various treatment regimens have been proposed, including combinations of pre and postoperative chemotherapy and/or radiotherapy.1,2
Preoperative chemotherapy may downstage tumours and allow greater success from surgical resection as well as treating micrometastatic disease that may be a source of distant recurrence following the proinflammatory insult of surgery.3 However, the disease is frequently resistant to preoperative treatment, which may delay potentially curative surgery, rendering it no longer effective.
Debate continues regarding the optimal preoperative chemotherapy for oesophageal adenocarcinomas. In 2002 the Medical Research Council (MRC) oesophageal working group trial (OE02) reported benefit from two cycles of cisplatin and fluorouracil therapy preoperatively when compared with surgery alone in oesophageal cancer.4 This practice was therefore employed for cases at our trust until 2006, when results were published for the MAGIC (MRC Adjuvant Gastric Infusional Chemotherapy) trial, which treated a group of patients using three cycles of epirubicin, cisplatin and fluorouracil pre and postoperatively.2 Although the trial comprised mainly gastric tumours, some oesophageal adenocarcinoma patients were included in the analysis and so our practice changed to treat all oesophagogastric adenocarcinomas with a MAGIC-type regimen from late 2006. There remains wide variation in clinical practice with different centres preferring either type of neoadjuvant therapy for oesophageal adenocarcinoma.
Nottingham University Hospitals NHS Trust is one of the largest volume centres for the treatment of oesophageal cancer in the UK.5 It maintains a database of sufficient size to report on the outcomes of both OE02 and MAGIC patients, and to determine survival (including anatomical site specific outcomes) in order to assess whether MAGIC-type therapy is useful in oesophageal adenocarcinoma. So as to minimise bias relating to changes in selection and outcome over time, the outcomes of patients treated with surgery alone during the same time periods are also reviewed for comparison. These patients are not compared here with the chemotherapy cohorts because they were not matched demographically or in terms of co-morbidity and there is no expectation of equal survival to the contemporaneous chemotherapy groups. Indeed, the difference in survival between chemotherapy and non-chemotherapy patients has been reported by our unit previously.6
Response (or lack ofthere) may be determined using tumour regression grade (TRG), which has been shown to correlate with survival in oesophagogastric cancer.7,8 In many drug trials, the difference between study arms may be small and a lengthy follow-up period can be required to confirm any difference. TRG as a surrogate marker of survival might be useful in the research field to detect the success of novel drugs in clinical trials without the need for mature survival data but only if it can be shown to be sensitive enough to detect the relatively small benefits that might be expected when comparing two neoadjuvant regimens. As a secondary outcome, we hoped to learn more about the interpretation of TRG by studying it in this context.
Methods
Details of patients undergoing surgery at our trust for oesophageal and gastric cancer were collected prospectively for audit and research purposes between 2001 and 2013. The reporting of TRG was increasingly adopted by local pathologists and TRG was included routinely in pathology reports by 2009. All specimens were examined by a consultant pathologist experienced in the assessment of oesophageal cancer who was blinded with regard to the treatment regimen. All representative sections were examined in a systematic fashion as described by Mandard et al.9
All adenocarcinoma patients with disease located in the oesophagus or gastro-oesophageal junction (GOJ) and who received preoperative chemotherapy were reviewed. Only those historical cases that had no recoverable slides were excluded from the study. For comparison, the patients during this period who were not suitable for chemotherapy were also identified. Patients had been selected for these treatments following discussion at a multidisciplinary team meeting with review of staging investigations. These investigations evolved over time but all included assessment by endoscopy and computed tomography of the thorax, abdomen and pelvis, with increasing numbers of patients receiving endoscopic ultrasonography and positron emission tomography.
Patients who progressed to surgery with curative intent underwent resection using an approach appropriate to the tumour site. In most cases, this was a two-stage Ivor Lewis oesophagectomy although single stage procedures were also carried out (eg transhiatal oesophagogastrectomy and left thoracoabdominal oesophagectomy, which was used particularly in the earlier years of the period studied). A small number of patients underwent a three-stage procedure with cervical anastomosis, either as open surgery owing to higher tumours or as part of a minimally invasive oesophagectomy. Local lymph nodes were included in the resection to complete a thoracic and upper abdominal two-field lymphadenectomy.
Although the original OE02 and MAGIC trials did not use capecitabine (oral equivalent of fluorouracil), this has since been shown to provide equivalent results10 and so regimens including this drug were also included in the analysis. Consequently, patients who had received chemotherapy using either cisplatin and fluorouracil/capecitabine (OE02-type) or epirubicin, cisplatin and fluorouracil/capecitabine (MAGIC-type) were included in the study. TRG was scored using the criteria of Mandard et al,9 and patients were then divided into responders (TRG 1–3) and non-responders (TRG 4 and 5). Other data on the database were taken from the hospital’s electronic records. Survival was updated yearly using mortality data compiled by the hospital data services department.
Statistical analysis
Data were analysed with SPSS® version 22 (IBM, New York, US). Survival differences between groups were compared using Kaplan–Meier analysis and the logrank test. Other differences were compared with the Mann–Whitney U test, Fisher’s exact test, chi-squared test and t-test as appropriate. A p-value of <0.05 was considered statistically significant.
Results
During the study period, a total of 372 patients received preoperative OE02 or MAGIC-type regimens for adenocarcinoma and had complete survival data. OE02 chemotherapy was used in 97 patients and 275 were treated with MAGIC therapy. During the same time period, 343 patients proceeded straight to surgery because they were unfit for neoadjuvant chemotherapy. These cases were divided into two groups: an ‘early’ cohort (treated during the same time period in which the OE02 regimen was used) and a ‘late’ cohort (treated after MAGIC therapy was put in place). These ‘straight to surgery’ groups provide a comparison in order to detect changing survival over the timeframe of this study.
The demographics for all groups in the study are summarised in Table 1. Patients given MAGIC chemotherapy were not significantly older than those selected for OE02 treatment. In the straight-to-surgery cohort, however, the ‘late’ group was significantly older than the ‘early’ group. The OE02 group contained a higher proportion of GOJ cancer cases than the MAGIC group. The ratios were similar for the ‘early’ and ‘late’ cohorts in the straight-to-surgery group, there being proportionately more GOJ cancer cases among patients treated in the OE02 era.
Table 1.
Demographics of patients undergoing preoperative chemotherapy with either MAGIC or OE02-type drugs as well as of those receiving surgery alone during the matching time periods
| Chemotherapy | Surgery alone | |||||
| OE02 (n=97) | MAGIC (n=275) | p-value | OE02 era (n=144) | MAGIC era (n=196) | p-value | |
| Median age in years (IQR) | 63.0 (57.0–69.0) | 64.0 (58.2–70.1) | 0.233* | 73.0 (64.5–77.5) | 75.0 (67.0–79.0) | 0.027* |
| >70 years | 23.7% | 28.0% | – | 59.7% | 67.8% | – |
| Male | 87.6% | 85.8% | – | 86.8% | 79.9% | – |
| 30-day mortality | 3.1% | 2.2% | 0.702** | 7.2% | 6.9% | 0.536** |
| Oesophageal cancer | 25 (25.8%) | 109 (39.6%) | 0.009** | 30 (20.8%) | 80 (40.8%) | <0.001** |
| GOJ cancer | 72 (74.2%) | 166 (60.4%) | 114 (79.2%) | 116 (59.2%) | ||
| Stage 0 | 1 (1.0%) | 9 (3.3%) | 0.022 † | 0 (0%) | 3 (1.5%) | 0.032† |
| Stage 1 | 9 (9.3%) | 54 (19.6%) | 23 (16.0%) | 42 (21.4%) | ||
| Stage 2 | 25 (25.8%) | 53 (19.3%) | 57 (39.6%) | 50 (25.5%) | ||
| Stage 3 | 57 (58.8%) | 155 (56.4%) | 62 (43.1%) | 100 (51.0%) | ||
| Stage 4 | 5 (5.2%) | 4 (1.5%) | 2 (1.4%) | 1 (0.5%) | ||
| TRG 1–3 | 20 (28.2%) | 82 (30.9%) | 0.384† | N/A | – | |
| TRG 4 and 5 | 51 (71.8%) | 183 (69.1%) | ||||
| TRG not recorded | 26 (26.8%) | 10 (3.6%) | – | N/A | – | |
| Mean cycles of preoperative chemotherapy | 1.95 | 2.86 | <0.001 ‡ | N/A | – | |
| Mean cycles of postoperative chemotherapy | 0 | 1.37 | – | N/A | – | |
| Mean total cycles of chemotherapy | 1.95 | 4.24 | <0.001‡ | N/A | – | |
GOJ = gastro-oesophageal junction; IQR = interquartile range; TRG = tumour regression grade
*Mann–Whitney U test; **Fisher’s exact test; † chi-squared test; ‡t-test
The median overall survival was significantly longer in the MAGIC group (3.00 years, 95% confidence interval [CI]: 2.21–3.79 years) than for OE02 patients (1.97 years, 95% CI: 1.47–2.47 years) (p=0.001, hazard ratio [HR]: 1.60, 95% CI: 1.22–2.09) (Fig 1). Stage was significantly reduced in the MAGIC group following treatment (Table 1).
Figure 1.
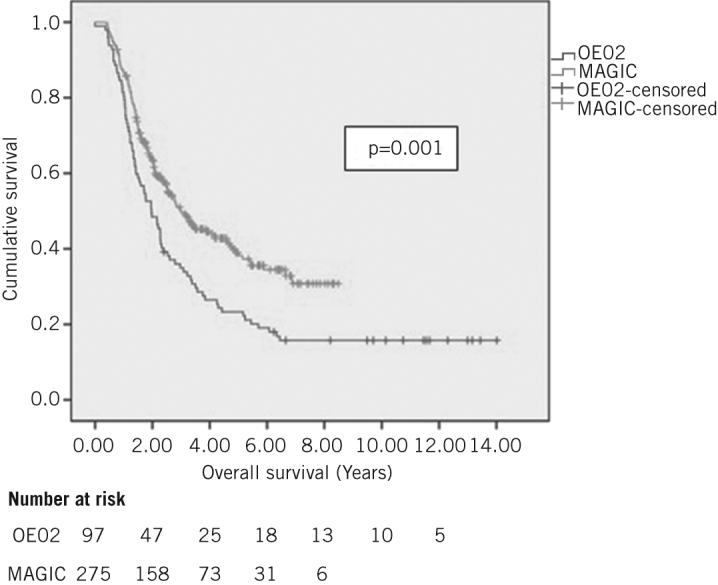
Kaplan–Meier survival analysis of all patients with oesophageal and gastro-oesophageal junction adenocarcinomas who received either OE02 or MAGIC-type chemotherapy prior to surgery
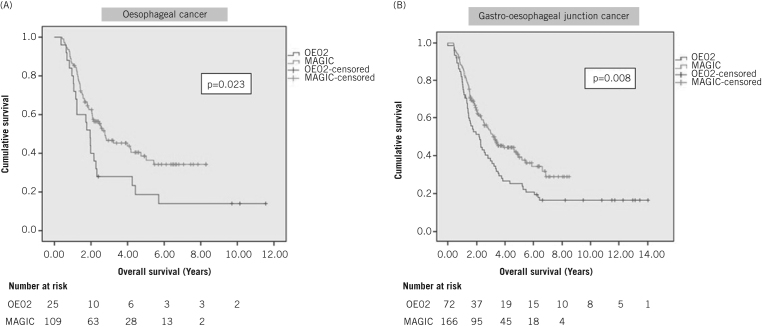
Anatomical subgroup analysis was undertaken to determine whether oesophageal cancer patients were also benefiting from MAGIC chemotherapy. Survival in both anatomical subgroups was longer with the MAGIC regimen (Fig 2). The oesophageal cancer subgroup demonstrated a median survival of 1.95 years (95% CI: 1.54–2.36 years) with OE02 and 2.76 years (95% CI: 1.45–4.06 years) with MAGIC (p=0.023, HR: 1.77, 95% CI: 1.08–2.90). For GOJ cancer patients, the median survival was 2.17 years (95% CI: 1.44–2.90 years) with OE02 and 3.25 years (95% CI: 2.39–4.12 years) with MAGIC (p=0.008, HR: 1.55, 95% CI: 1.12–2.14).
Figure 2.
Kaplan–Meier subgroup analysis of patients with oesophageal cancer (A) and gastro-oesophageal junction cancer (B) who received either OE02 or MAGIC-type chemotherapy prior to surgery
For the straight-to-surgery patients, the median survival in the ‘early’ cohort (1.70 years, 95% CI: 1.27–2.13 years) was similar to that in the ‘late’ cohort (1.92 years, 95% CI: 1.30–2.54 years) (p=0.542). However, as mentioned above, those in the ‘late’ group were significantly older (Table 1).
Of the 275 patients who underwent MAGIC chemotherapy, 149 (54.2%) received some postoperative chemotherapy; these patients had an average of 2.54 cycles following surgery. In total, only 100 patients (36.4%) completed the full 6 cycles of the MAGIC regimen and the median survival in this group was 3.23 years, which was not significantly different to that in the MAGIC cohort as a whole (p=0.479).
Tumour regression grading
A total of 372 patients were identified (median follow-up duration 5.50 years) who underwent resection for oesophageal or GOJ adenocarcinoma and had received preoperative chemotherapy using a standard regimen. Of these, the TRG was calculated for 336 patients. In this cohort, there was a survival benefit for those who had a good histological response to chemotherapy compared with those who did not (median survival for TRG 1–3: 5.41 years, 95% CI: 3.89–6.93 years; median survival for TRG 4 and 5: 2.29 years, 95% CI: 1.80–2.78 years; p<0.001, HR: 0.53, 95% CI: 0.39–0.74).
Table 2 shows the numbers of OE02 and MAGIC patients with different TRG scores. The proportion of responders was not significantly higher among MAGIC patients (31.7%) than among OE02 patients (28.1%) (p=0.500) even though the MAGIC regimen was shown to be associated with improved survival.
Table 2.
Frequency of TRG response (1–3) and non-response (4 and 5) of oesophageal and gastro-oesophageal junction cancer to either OE02 or MAGIC-type preoperative chemotherapy
| Regimen | TRG 1 | TRG 2 | TRG 3 | TRG 4 | TRG 5 | Total |
| OE02 | 1 (1.4%) | 3 (4.2%) | 16 (22.5%) | 24 (33.8%) | 27 (38.0%) | 71 |
| MAGIC | 9 (3.4%) | 25 (9.4%) | 50 (18.9%) | 93 (35.1%) | 88 (33.2%) | 265 |
| Total | 10 | 28 | 66 | 117 | 115 | 336 |
TRG = tumour regression grade
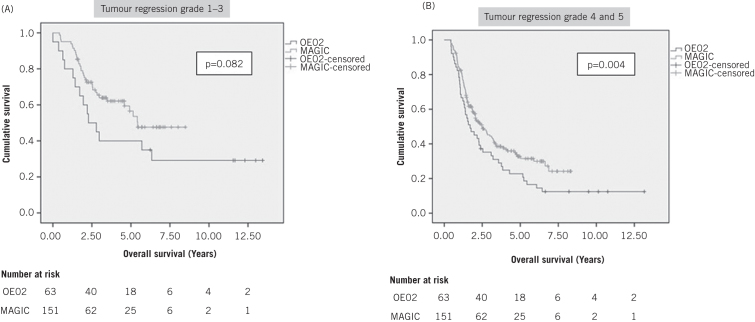
However, the benefits of MAGIC therapy seemed to exist for both responders and non-responders. Non-responders had better survival following the MAGIC regimen than following the OE02 regimen (OE02 median survival: 1.97 years, 95% CI: 1.34–2.60 years; MAGIC median survival: 3.17 years, 95% CI: 2.30–4.04 years; p=0.004, HR: 1.55, 95% CI: 1.15–2.11) (Fig 3). The survival benefit of MAGIC versus OE02 chemotherapy in responders was not proven statistically with the small number of responders, particularly in the OE02 group (OE02 median survival: 5.43 years, 95% CI not calculated; p=0.082, HR: 1.74, 95% CI: 0.93–3.26; MAGIC median survival: 2.27 years, 95% CI: 1.00–3.55 years).
Figure 3.
Kaplan–Meier survival analysis of patients undergoing resection who had a good response (A) or a poor response (B) following either OE02 or MAGIC-type chemotherapy
Discussion
No trial exists that directly compares the OE02 and MAGIC regimens. However, provisional data of the MRC’s OE05 trial show improvement in surrogate markers of survival when four cycles of epirubicin, cisplatin and capecitabine are used preoperatively compared with patients given cisplatin and fluorouracil (OE02 regimen).11 On the other hand, there was no difference in overall survival at the current follow-up review.
The results of the OE05 trial demonstrated better survival than expected in both study arms11 (presumably owing to improvements in staging and perioperative care) and a longer follow-up period may be required than the three-year survival outcomes currently reported to determine the final outcome. With prolonged follow-up review, it is possible that the surrogate markers of survival may yet translate into improved overall survival and our data would complement this understanding of neoadjuvant treatment. The MAGIC regimen does differ from the OE05 regimen and the impact of the fourth neoadjuvant cycle in OE05 chemotherapy as well as the adjuvant portion of MAGIC therapy remain poorly defined. As a result, the data of the present study remain important in defining the outcomes of these specific regimens.
Analysis of our cohort also addresses the absence of evidence for using MAGIC chemotherapy in cases of oesophageal cancer. While the original trial grouped gastric with oesophageal cancer patients, the reporting of differences between anatomical sites is crucial as the behaviour of tumours at these sites can vary greatly owing to underlying biological differences,12,13 which are likely to be of great importance in the design of future clinical trials that incorporate novel targeted chemotherapeutic agents. Our research has demonstrated that these agents can achieve good survival outcomes in oesophageal cancer.
There are, of course, certain limitations of this analysis. The OE02 group predates the MAGIC cohort and would not have benefited from improvements in care that were implemented later in the study period, creating a bias that is difficult to quantify. However, the benefit from MAGIC-type chemotherapy is unlikely to just be related to general improvements in perioperative care as there was no significant difference in survival in the corresponding straight-to-surgery groups.
On the other hand, average age increased between the groups and this may have masked improvements in age matched patients. Changes in case selection have meant that surgery is offered to increasingly frail individuals (as implied by the rising age). As a result, the static survival does seem to indicate some improvements in perioperative care as more frail patients survived surgery. The age of those receiving neoadjuvant treatment changed less over time and so although advances have allowed surgery to be offered to older patients, the same does not apply to chemotherapy.
The OE02 and CROSS (chemoradiotherapy for oesophageal cancer followed by surgery) trials recruited approximately ten years apart (closing in 1998 and 2008); both included straight-to-surgery arms, albeit from different countries in Europe.4,14 The median survival in each straight-to-surgery trial arm was approximately 13 months in the OE02 study and 24 months in the CROSS study, suggesting an improvement in survival related to improved perioperative care and selection over the time period between the two trials, although a small age difference may have also contributed to this. Changes in service provision in the UK have led to centralisation of services during this time, which could also account for the poor survival in the OE02 trial. Our data come from a single high volume specialist centre and are therefore not subject to this aspect of the improvements occurring over this time period.
This study showed survival differences between the chemotherapy cohorts of mostly greater than one year (both overall and for anatomical subgroup analysis) over a much smaller time gap than that between the OE02 and CROSS trials (approximately 6 years). For this reason, it can be concluded that although some of the improvement will relate to better staging and perioperative care, this cannot be of a magnitude to account for all the improvement. Unfortunately, further data regarding confounding factors that may also have also affected outcomes are missing for patients treated in the earlier part of our study. Consequently, although an association cannot be proven, it can be hypothesised that part of the improvement in survival is related to the differing chemotherapy regimens.
Another important problem when comparing OE02 and MAGIC groups is that the analysis only includes patients who underwent surgery following chemotherapy and therefore excludes patients dropping out of neoadjuvant treatment. The original trials suggest that the proportion of patients unable to have surgery were similar for those undergoing OE02 and MAGIC therapy (7.0% and 6.1% respectively).2,4 Conversely, an examination of 100 patients treated with MAGIC chemotherapy in Nottingham revealed that as many as 19% of those commencing MAGIC treatment with curative intent failed to proceed to surgery.15
Although not demonstrated in the original trials, the longer and more aggressive chemotherapy given in a MAGIC regimen would intuitively be expected to result in greater toxicity and disease progression prior to surgery in resistant cases. As such, an intention-to-treat analysis (that included the survival outcomes of those who started treatment but did not undergo surgery) would be a more accurate comparison. However, it was not possible to satisfactorily identify and determine which historical patients started treatment with curative intent.
The fact that cancer stage was generally lower following the MAGIC regimen may reflect the increased frequency of response to chemotherapy although there was no statistically significant increase in the proportion of responders as determined by TRG. Furthermore, in the straight-to-surgery cohort, disease stage also differed between the ‘early’ and ‘late’ groups; the ‘early’ group had a higher proportion of stage 2 cases while the ‘late’ group had a higher proportion of stage 1 and 3 patients (Table 1). This could partly be due to a change in practice, with the ‘early’ period being defined by preoperatively low stage disease (mostly stage 2 at resection) and the ‘late’ period containing more elderly patients, known to have stage 3 disease but offered surgery owing to a lack of fitness for chemotherapy.
This hypothesis may not account entirely for the changes seen. Nevertheless, there is no evidence of a decrease in stage in our population over time due to earlier detection of disease or due to the impact of improvements in preoperative staging,16 which would account for the lower stage disease of the MAGIC group.
The higher ratio of GOJ to oesophageal cancer cases seen in the OE02 group could create bias as oesophageal cancer may have a different survival expectation to that for GOJ cancer. However, among the patients in our study treated with OE02 therapy, survival was similar in the oesophageal (1.97 years) and GOJ (1.95 years) subgroups, suggesting that each type of cancer carried a similar prognosis (at least in this study cohort).
The survival advantage of MAGIC chemotherapy appeared to exist for non-responders as well as responders (Fig 3). As the amount of regression was not statistically different between the OE02 and MAGIC groups (p=0.384), there must be tumour responses other than regression that offer a survival advantage that are not apparent using histological techniques. This hypothesis is supported by the fact that non-responders had significantly better survival with MAGIC than with OE02 therapy (p=0.004). Although the survival difference between the responder cohorts failed to reach statistical significance (p=0.082) despite the widely differing median survival, this can be explained by the small number of responders to the OE02 regimen.
It is known that cells may undergo various responses when stressed by drug treatment, and this may include necrosis, apoptosis, autophagy and cellular senescence.17,18 MAGIC chemotherapy may be inducing some of these non-fibrotic changes. Alternatively, the MAGIC regimen may be more effective in treating micrometastatic disease than is evident in primary tumours while response to OE02 therapy is similar across heterogeneous sites.
Clearly, markers of response are useful and may help in guiding treatment choices in the future. It must be remembered, however, that TRG is a surrogate marker of response and therefore may not be accurately detecting some aspects of response. It remains to be shown whether the benefit of MAGIC chemotherapy is due to the extra treatment given pre or postoperatively, or whether both treatment phases are of equal importance.
Conclusions
Although definitive evidence for using MAGIC-type chemotherapy to treat oesophageal cancer was not provided by the original trial data, our study has added support for the view that good results can be achieved using this regimen in oesophageal and GOJ cancer patients. MAGIC therapy may represent one of the major improvements in care over the last ten years that have promoted improved survival compared with OE02 treatment in previous years.
References
- 1.van Hagen P, Hulshof MC, van Lanschot JJ et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012; : 2,074–2,084. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; : 11–20. [DOI] [PubMed] [Google Scholar]
- 3.Hyngstrom JR, Posner MC. Neoadjuvant strategies for the treatment of locally advanced esophageal cancer. J Surg Oncol 2010; : 299–304. [DOI] [PubMed] [Google Scholar]
- 4.Medical Research Council Oesophageal Cancer Working Party. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002; : 1,727–1,733. [DOI] [PubMed] [Google Scholar]
- 5.National Oesophago-Gastric Cancer Audit 2010. Leeds: NHS Information Centre; 2010.
- 6.Reece-Smith AM, Madhusudan S, Duffy JP et al. Survival outcomes following the introduction of MAGIC style chemotherapy. Br J Surg 2011; (Suppl 3): 13. [Google Scholar]
- 7.Becker K, Langer R, Reim D et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg 2011; : 934–939. [DOI] [PubMed] [Google Scholar]
- 8.Fareed KR, Ilyas M, Kaye PV et al. Tumour regression grade (TRG) analyses in patients with resectable gastro-oesophageal adenocarcinomas treated with platinum-based neoadjuvant chemotherapy. Histopathology 2009; : 399–406. [DOI] [PubMed] [Google Scholar]
- 9.Mandard AM, Dalibard F, Mandard JC et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994; : 2,680–2,686. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham D, Starling N, Rao S et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008; : 36–46. [DOI] [PubMed] [Google Scholar]
- 11.Alderson D, Langley RE, Nankivell MG et al. Neoadjuvant chemotherapy for resectable oesophageal and junctional adenocarcinoma: results from the UK Medical Research Council randomised OEO5 trial. J Clin Oncol 2015; (Suppl): 4,002.25366687 [Google Scholar]
- 12.Huang D, Lu N, Fan Q et al. HER2 status in gastric and gastroesophageal junction cancer assessed by local and central laboratories: Chinese results of the HER-EAGLE study. PLoS One 2013; : e80290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buskens CJ, Sivula A, van Rees BP et al. Comparison of cyclooxygenase 2 expression in adenocarcinomas of the gastric cardia and distal oesophagus. Gut 2003; : 1,678–1,683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro J, van Lanschot JJ, Hulshof MC et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015; : 1,090–1,098. [DOI] [PubMed] [Google Scholar]
- 15.Reece-Smith AM, Saha S, Cunnell ML et al. MAGIC in practice: experience of peri-operative ECF/X chemotherapy in gastro-esophageal adenocarcinomas. J Surg Oncol 2012; : 748–752. [DOI] [PubMed] [Google Scholar]
- 16.Blencowe NS, Whistance RN, Strong S et al. Evaluating the role of fluorodeoxyglucose positron emission tomography-computed tomography in multi-disciplinary team recommendations for oesophago-gastric cancer. Br J Cancer 2013; : 1,445–1,450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galluzzi L, Vitale I, Abrams JM et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 2012; : 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez-Outschoorn UE, Trimmer C, Lin Z et al. Autophagy in cancer associated fibroblasts promotes tumor cell survival: role of hypoxia, HIF1 induction and NFκB activation in the tumor stromal microenvironment. Cell Cycle 2010; : 3,515–3,533. [DOI] [PMC free article] [PubMed] [Google Scholar]