Abstract
Introduction
The aim of this study was to identify patient factors including serum biomarkers that may predict response to neoadjuvant chemoradiotherapy (CRT) in patients with locally advanced rectal cancer staged on magnetic resonance imaging. Prediction of response may be helpful when selecting patients for a non-operative programme.
Methods
A retrospective review was carried out of patients undergoing neoadjuvant CRT for rectal cancer, conducted at the Royal Devon and Exeter Hospital. All patients were managed through the multidisciplinary team. Receiver operating characteristic (ROC) curve analysis was undertaken to assess the ability of biomarkers to predict response to neoadjuvant CRT. The biomarkers assessed included neutrophils, lymphocytes, monocytes, haemoglobin, platelets, C-reactive protein and carcinoembryonic antigen.
Results
Seventy-three patients underwent neoadjuvant CRT between January 2006 and December 2011. Nine (12.3%) of these experienced a clinical complete response and were managed with a ‘watch and wait’ approach. An additional ten patients (13.7%) had a pathological complete response following surgery. Using ROC curve analysis, the biomarkers with the largest area under the curve (AUC) were pre-CRT haemoglobin and post-CRT lymphocyte concentrations, producing AUC values of 0.673 and 0.618 respectively for clinical complete response. Pre-CRT haemoglobin and neutrophil concentrations produced the highest AUC values for pathological complete response at 0.591 and 0.614 respectively.
Conclusions
None of the assessed biomarkers offer the ability to predict response to neoadjuvant CRT in patients with rectal cancer. They cannot therefore assist in identifying complete clinical or pathological responders who could be considered for a non-operative, observational approach.
Keywords: Rectal cancer, Chemoradiotherapy, Surgery, Survival, Recurrence
Recent advances in magnetic resonance imaging (MRI) staging have led to progress in preoperative prediction of circumferential resection margin involvement.1–3 The use of preoperative (neoadjuvant) chemoradiotherapy (CRT) is advocated in patients with locally advanced disease to induce clear surgical resection margins,4 defined as the absence of tumour cells in the margin of healthy tissue.5
A patient’s response to CRT can be classified in four ways. The first of these is a pathological complete response (pCR), demonstrated by the absence of viable tumour cells in a tissue specimen.6 Second, there can be a partial tumour response to CRT, assessed histologically by a variety of regression grading scores7 or radiologically.8 Third, there may be no response or even disease progression.9 Finally, some patients have what is known as a clinical complete response (cCR). This category is used if there is absence of clinically and radiologically detectable tumour for a certain period of time, increasingly defined as one year following completion of therapy.6
The ‘watch and wait’ or ‘active surveillance’ approach is a method that is being used in some patients who have evidenced a cCR to CRT.10 As a result of a cCR, patients are offered the option not to undergo surgery, instead being monitored intensively. A retrospective study by our unit in 2012 demonstrated that 12% of patients treated with CRT had a cCR that had been managed without surgery.11 In addition, an additional 12% of patients who underwent surgery were found to have a pCR, meaning the surgical procedure and subsequent reduction in patient quality of life could have been avoided. This approach was advocated initially by Habr-Gama et al, who demonstrated a cCR rate of 26.8% in patients with rectal adenocarcinoma undergoing CRT.12
Attitudes to cCR among surgeons are changing rapidly. A 2007 survey of members of the Association of Coloproctology of Great Britain and Ireland found that 69% of colorectal surgeons were not comfortable offering a non-surgical treatment plan for rectal cancer patients13 but a repeat survey in 2013 demonstrated wider acceptance of this strategy in selected patients.14 Prediction of response to CRT would help with preselecting those patients in whom surgery could potentially be avoided. The aim of this study was therefore to identify serum biomarkers that could predict a complete response to neoadjuvant CRT.
Methods
A retrospective review of data from our local cancer database was carried out. Tumour and patient characteristics were retrieved from a database of patients undergoing neoadjuvant CRT for rectal cancer with curative intent between January 2006 and December 2011. The protocol for investigation and treatment of rectal cancer in our unit has been described in detail previously.11 Following multidisciplinary team discussion, patients are considered for neoadjuvant CRT if the circumferential resection margin is threatened (≤1mm) or involved by tumour, or if there is an involved lymph node. All patients received 45Gy in 25 fractions over 5 weeks with concurrent capecitabine (825mg/m2). Outcomes (recurrence, local or distant metastasis or death from another cause) were determined from patient records. Full haematological data were available for 58 patients from hospital computer records.
Statistical analysis
Pretreatment patient demographics and clinical factors were assessed in the context of cCR and pCR to neoadjuvant CRT. In order to determine the predictive qualities of biomarkers, receiver operator characteristic curve analysis was undertaken to generate an area under the curve (AUC) value. An AUC of 0.75 is considered a ‘good’ predictor of outcome.15 In addition, so as to ascertain correlations between biomarkers, R2 values were generated using linear correlation analysis. Adjusted R2 values can range from -1 to +1, with +1 indicating a 100% correlation. Likelihood ratios were calculated from these R2 values.16
The Mann–Whitney U test was employed to establish the extent to which biomarkers could predict response to neoadjuvant CRT. In order to determine the effects of age and sex on response to CRT, Fisher’s exact test was used. All statistical analysis was performed using SPSS® (IBM, New York, US), StatsDirect (StatsDirect, Altrincham, UK), MATLAB® (MathWorks, Natick, MA, US) and Excel® (Microsoft, Redmond, WA, US). A p-value of <0.05 was considered statistically significant.
Results
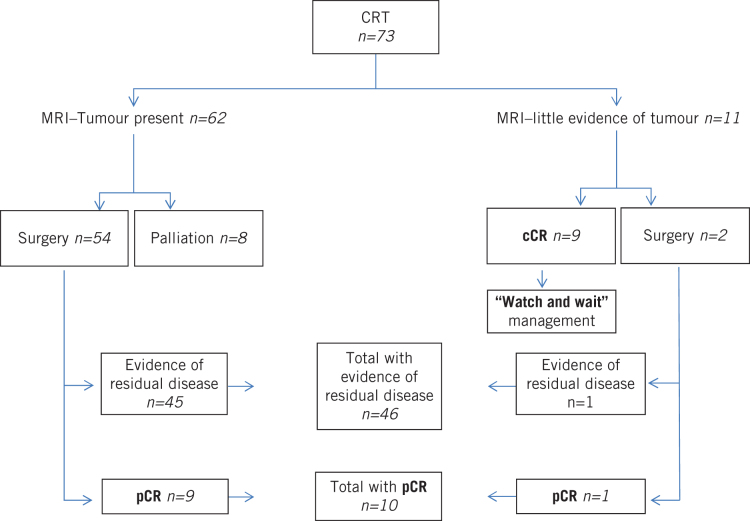
Between January 2006 and December 2011, 73 patients with a rectal adenocarcinoma underwent neoadjuvant CRT at the Royal Devon and Exeter Hospital. Of these, 56 (77%) proceeded to surgery. Eight patients (11%) did not undergo surgery as a result of disease progression despite therapy. A further nine patients (12%) avoided surgery, having experienced a cCR to CRT, and were managed with a ‘watch and wait’ approach. Of the 56 patients who underwent surgery, 10 (18%) had a pCR. Overall, therefore, a quarter of the patients who had neoadjuvant CRT (19/73, 26%) experienced a complete response, following either surgical or non-surgical management (Fig 1). After treatment with neoadjuvant CRT, there was a downstaging of MRI T stage and the number of potentially node positive tumours (Table 1).
Figure 1.
Response to chemoradiotherapy in patients with rectal adenocarcinoma
Table 1.
Comparison of T stage and node positivity among patients who underwent neoadjuvant chemoradiotherapy, prior to and following treatment
| MRI T stage | n | Node positive | Histological T stage | n | Node positive | |
| T1 | 0 | 0 | T1 | 2 | 0 | |
| T2 | 5 | 3 | T2 | 11 | 4 | |
| T3 | 48 | 38 | T3 | 27 | 15 | |
| T4 | 14 | 10 | T4 | 6 | 2 | |
| pCR | 10 | – | ||||
| cCR** | 9 | – | ||||
| Total | 67* | 51 | Total | 65 | 21 |
cCR = clinical complete response; MRI = magnetic resonance imaging; pCR = pathological complete response
*MRI T stage data were unavailable for 6 patients, who were excluded from this analysis.
**Based on MRI following chemoradiotherapy
From the initial AUC analysis, two biomarkers, post-CRT lymphocytes and pre-CRT haemoglobin (Hb), were identified as potential predictors of cCR, albeit both with an AUC of <0.75 (Table 2). Post-CRT lymphocyte concentration produced an AUC of 0.618. Nevertheless, this biomarker had a positive likelihood ratio (LR) of 1 and a negative LR of 1, indicating very little diagnostic or predictive use.
Table 2.
Ability of biomarkers to predict cCR and pCR
| Biomarker | cCR AUC | pCR AUC |
| Pre-CRT neutrophils | 0.421 | 0.591 |
| Pre-CRT lymphocytes | 0.432 | 0.588 |
| Pre-CRT NLR | 0.521 | 0.503 |
| Post-CRT neutrophils | 0.536 | 0.506 |
| Post-CRT lymphocytes | 0.618 | 0.506 |
| Post-CRT NLR | 0.436 | 0.567 |
| Pre-CRT monocytes | 0.439 | 0.506 |
| Pre-CRT haemoglobin | 0.673 | 0.614 |
| Pre-CRT PLR | 0.436 | 0.387 |
| Pre-CRT CEA | 0.304 | – |
AUC = area under the curve; cCR = clinical complete response; CEA = carcinoembryonic antigen; CRT = chemoradiotherapy; NLR = neutrophil-to-lymphocyte ratio; pCR = pathological complete response; PLR = platelet-to-lymphocyte ratio
Hb was analysed separately to understand its effectiveness in predicting a cCR following CRT. Pre-CRT Hb concentration produced an AUC of 0.673, suggesting that higher Hb offers a greater chance of achieving a cCR to CRT. A positive LR of 1.258 and a negative LR of 0.578 further support the idea that Hb may have some predictive value, albeit not at the level that would be considered clinically relevant.
AUC analysis was also undertaken to elucidate whether any biomarkers would predict a pCR to CRT. A number were identified as potentially offering a predictive insight with an AUC of >0.5 (Table 2). These included pre-CRT neutrophil, lymphocyte and Hb concentration, and post-CRT neutrophil-to-lymphocyte ratio (NLR). Pre-CRT neutrophil and Hb concentration yielded the strongest AUC values. For that reason, these biomarkers were analysed individually.
Pre-CRT neutrophil concentration produced an AUC of 0.591. However, a positive LR of 0.152 and a negative LR of 0.934 indcates that it has no predictive value.
Pre-CRT Hb concentration was again suggestive of a positive response to CRT. Specifically, in the context of pCR, pre-Hb levels produced an AUC of 0.614 and this was therefore the biomarker offering the best predictive value for a pCR.
The ability of the change in NLR to predict response to CRT was evaluated by comparing pre and post-CRT values. For cCR, the change in NLR produced an AUC of 0.390 while for pCR, the AUC was 0.560. Consequently, there appears to be little predictive value with regard to NLR change during treatment with CRT determining tumour response.
The medians of the different biomarkers were compared for complete and incomplete responders to CRT (Table 3). These data yielded no statistically significant results. Nevertheless, the difference between pre-CRT Hb concentration in complete responders and that in incomplete responders had a p-value of 0.064, suggesting that a larger sample size could potentially demonstrate a significant difference. Pre-CRT carcinoembryonic antigen (CEA) and C-reactive protein yielded p-values of 0.401 and 0.970 respectively. Our data also showed that neither age nor sex were associated with a significant difference in response to CRT (Table 4) and the same was true for pretreatment clinical T stage (Table 5).
Table 3.
Comparison of median biomarker levels for patients with complete and incomplete responses to CRT
| Biomarker | Complete response | Incomplete response | p -value |
| Pre-CRT neutrophils | 5.09 × 109/l | 4.87 × 109/l | 0.585 |
| Pre-CRT lymphocytes | 1.91 × 109/l | 1.57 × 109/l | 0.290 |
| Pre-CRT NLR | 3.00 | 3.32 | 0.774 |
| Post-CRT neutrophils | 3.54 × 109/l | 3.98 × 109/l | 0.423 |
| Post-CRT lymphocytes | 0.83 × 109/l | 0.68 × 109/l | 0.408 |
| Post-CRT NLR | 4.37 | 4.81 | 0.542 |
| Pre-CRT monocytes | 0.58 × 109/l | 0.61 × 109/l | 0.713 |
| Pre-CRT haemoglobin | 13.8g/dl | 13.2g/dl | 0.064 |
| Pre-CRT CRP |
9.5mg/l | 7.5mg/l | 0.970 |
| Pre-CRT CEA |
3.1ng/ml | 4.3ng/ml | 0.401 |
CEA = carcinoembryonic antigen; CRP = C-reactive protein; CRT = chemoradiotherapy; NLR = neutrophil-to-lymphocyte ratio
Table 4.
Influence of age and sex on pCR and/or cCR to chemoradiotherapy
| Variable | All patients | cCR | p -value | pCR | p -value | cCR/pCR | p -value |
| Male | 45 | 8 | 0.218 | 9 | 0.290 | 17 | 0.063 |
| Female | 20 | 1 | 1 | 2 | |||
| Age ≤65 | 33 | 7 | 0.159 | 4 | 0.313 | 11 | 0.733 |
| Age >65 | 32 | 2 | 6 | 8 | |||
cCR = clinical complete response; pCR = pathological complete response
Table 5.
Numbers of patients with cCR/pCR and incomplete response to chemoradiotherapy by pretreatment T stage
| MRI T stage | Incomplete response | cCR | p -value |
| T1/T2 | 5 | 0 | 0.910 |
| T3/T4 | 44 | 9 | |
| pCR | |||
| T1/T2 | 5 | 0 | 0.575 |
| T3/T4 | 36 | 8 | |
cCR = clinical complete response; MRI = magnetic resonance imaging; pCR = pathological complete response
Discussion
The ability to accurately predict how individual patients would respond to neoadjuvant CRT would enable the tailoring of treatments to specific subsets of patients. Although non-operative management following cCR remains controversial,17 the possibility of poor functional outcome due to low anterior resection syndrome and the associated reduced quality of life18,19 or a permanent end colostomy means that it remains an attractive proposition for patients who have a cCR. Risk stratification, particularly for those patients unlikely to respond to neoadjuvant CRT, would allow appropriate patient counselling and consideration of alternative treatment strategies. In our series, 11% of patients developed disease progression despite CRT. This aspect of response is not often reported in trials and demonstrates the diversity of response to CRT.
Numerous attempts have been made to identify factors that predict the degree of response to neoadjuvant CRT. Most have focused on genetic, epigenetic or molecular factors on tissue analysis from resected specimens, with a few using pretreatment biopsy samples. Several investigative techniques have been employed, including whole genome, single and multimarker analyses. Translation into routine clinical practice has not yet occurred owing to a combination of conflicting results and the retrospective nature of the studies, meaning there is a lack of independent prospective validation.20 Furthermore, some authors have argued that the whole concept of personalising treatment is deeply flawed because of intratumour heterogeneity and plasticity.21
Serum biomarkers may offer a cheaper and more pragmatic approach to response prediction. Our data suggest that higher levels of Hb prior to the initiation of CRT are associated with cCR (AUC 0.673) or pCR (AUC 0.614) in rectal cancer patients. However, no significant relationships between clinical T stage and response to neoadjuvant CRT were found (Table 2). Previous studies have reported that pretreatment Hb concentration is important in terms of tumour downstaging.22,23 In addition, it has recently been reported that patients with anaemia prior to neoadjuvant CRT are less likely to experience a pCR.24,25 Only one study has previously examined Hb levels in the context of cCR and it failed to demonstrate any relationship.26
Our results have shown that there is a limited role for NLR in predicting response to CRT. Pre and post-CRT NLR did not offer any predictive insight into response to CRT (Tables 2 and 3). Furthermore, the change in NLR from before CRT to after CRT also appears to be limited as an indicative predictor of pCR to CRT as this only produced an AUC of 0.56. In addition, in terms of predicting cCR, the AUC was 0.39, suggesting no predictive value.
This is in contrast to the results of two previous retrospective studies that implied a relationship between lymphocyte ratios and response to neoadjuvant CRT.26,27 The underlying hypothesis postulated by these studies is that the proportion of lymphocytes is an indicator of the adaptive immune response against the cancer while the neutrophil count reflects the innate inflammatory response that may cause the suppression of lymphocyte mediated immunity via the production of reactive oxygen species, nitric oxide and arginase.28 Despite being a plausible hypothesis, our data suggest alternative factors are likely to be as important in determining cCR.
In our study, male patients had a greater response to CRT although this failed to reach statistical significance with the limited number in the series. There was no difference in response between those aged ≤65 years and those aged >65 years (Table 4).
There was no evidence of CEA providing any insight into predicting patient response to CRT. In all of the AUC analyses undertaken, CEA failed to produce any values of clinical relevance. This is surprising given the findings from other studies, which have indicated that circulating CEA levels could offer some prognostic value in determining response to neoadjuvant CRT.29,30 However, one possibility for the difference in results could be related to the accepted notion that CEA is increased in smokers, with suggestions that smokers commonly exhibit CEA levels that are almost double those of non-smokers.31 Unfortunately, in this study, there was limited information on patient smoking status. It is therefore difficult to interpret the results presented here because it is not possible to accurately attribute CEA concentration as the sole consequence of presence of the rectal neoplasm.
Conclusions
None of the serum biomarkers tested in this study reliably predicted either cCR or pCR to neoadjuvant CRT for rectal cancer. Determinants of response to CRT are likely to be multifactorial and it is therefore unlikely that response can be predicted using clinical parameters alone. The impact of genetic, epigenetic and molecular factors on response to CRT needs further investigation, and selection of patients for a ‘watch and wait’ approach cannot therefore be recommended on currently available pretreatment factors in patients with locally advanced rectal cancer staged by MRI.
References
- 1.MERCURY Study Group Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. BMJ 2006; : 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor FG, Quirke P, Heald RJ et al. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg 2011; : 711–719. [DOI] [PubMed] [Google Scholar]
- 3.Taylor FG, Quirke P, Heald RJ et al. One millimetre is the safe cut-off for magnetic resonance imaging prediction of surgical margin status in rectal cancer. Br J Surg 2011; : 872–879. [DOI] [PubMed] [Google Scholar]
- 4.Scott NA, Susnerwala S, Gollins S et al. Preoperative neo-adjuvant therapy for curable rectal cancer – reaching a consensus 2008. Colorectal Dis 2009; : 245–248. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein TE, Endreseth BH, Romundstad P, Wibe A. What is a safe distal resection margin in rectal cancer patients treated by low anterior resection without preoperative radiotherapy? Colorectal Dis 2012; : e48–e55. [DOI] [PubMed] [Google Scholar]
- 6.Dedemadi G, Wexner SD. Complete response after neoadjuvant therapy in rectal cancer: to operate or not to operate? Dig Dis 2012; (Suppl 2): 109–117. [DOI] [PubMed] [Google Scholar]
- 7.MacGregor TP, Maughan TS, Sharma RA. Pathological grading of regression following neoadjuvant chemoradiation therapy: the clinical need is now. J Clin Pathol 2012; : 867–871. [DOI] [PubMed] [Google Scholar]
- 8.Patel UB, Blomqvist LK, Taylor F et al. MRI after treatment of locally advanced rectal cancer: how to report tumor response – the MERCURY experience. Am J Roentgenol 2012; : W486–W495. [DOI] [PubMed] [Google Scholar]
- 9.Oberholzer K, Menig M, Kreft A et al. Rectal cancer: mucinous carcinoma on magnetic resonance imaging indicates poor response to neoadjuvant chemoradiation. Int J Radiat Oncol Biol Phys 2012; : 842–848. [DOI] [PubMed] [Google Scholar]
- 10.Hingorani M, Hartley JE, Greenman J, Macfie J. Avoiding radical surgery after pre-operative chemoradiotherapy: a possible therapeutic option in rectal cancer? Acta Oncol 2012; : 275–284. [DOI] [PubMed] [Google Scholar]
- 11.Dalton RS, Velineni R, Osborne ME et al. A single-centre experience of chemoradiotherapy for rectal cancer: is there potential for nonoperative management? Colorectal Dis 2012; : 567–571. [DOI] [PubMed] [Google Scholar]
- 12.Habr-Gama A, Perez RO, Nadalin W et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy. Ann Surg 2004; : 711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wynn GR, Bhasin N, Macklin CP, George ML. Complete clinical response to neoadjuvant chemoradiotherapy in patients with rectal cancer: opinions of British and Irish specialists. Colorectal Dis 2010; : 327–333. [DOI] [PubMed] [Google Scholar]
- 14.São Julião GP, Smith FM, Macklin CP et al. Opinions have changed on the management of rectal cancer with a complete clinical response to neoadjuvant chemoradiotherapy. Colorectal Dis 2014; : 392–394. [DOI] [PubMed] [Google Scholar]
- 15.Ray P, Le Mannach Y, Riou B, Houle TT. Statistical evaluation of a biomarker. Anesthesiology 2010; : 1023–1040. [DOI] [PubMed] [Google Scholar]
- 16.McGee S. Simplifying likelihood ratios. J Gen Intern Med 2002; : 646–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glynne-Jones R, Hughes R. Critical appraisal of the ‘wait and see’ approach in rectal cancer for clinical complete responders after chemoradiation. Br J Surg 2012; : 897–909. [DOI] [PubMed] [Google Scholar]
- 18.Bregendahl S, Emmertsen KJ, Lous J, Laurberg S. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis 2013; : 1,130–1,139. [DOI] [PubMed] [Google Scholar]
- 19.Emmertsen KJ, Laurberg S. Impact of bowel dysfunction on quality of life after sphincter-preserving resection for rectal cancer. Br J Surg 2013; : 1,377–1,387. [DOI] [PubMed] [Google Scholar]
- 20.Grade M, Wolff HA, Gaedcke J, Ghadimi BM. The molecular basis of chemoradiosensitivity in rectal cancer: implications for personalized therapies. Langenbecks Arch Surg 2012; : 543–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buczacki SJ, Davies RJ. The confounding effects of tumour heterogeneity and cellular plasticity on personalized surgical management of colorectal cancer. Colorectal Dis 2014; : 329–331. [DOI] [PubMed] [Google Scholar]
- 22.Yoon SM, Kim DY, Kim TH et al. Clinical parameters predicting pathologic tumor response after preoperative chemoradiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2007; : 1,167–1,172. [DOI] [PubMed] [Google Scholar]
- 23.Lee SD, Park JW, Park KS et al. Influence of anemia on tumor response to preoperative chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis 2009; : 1,451–1,458. [DOI] [PubMed] [Google Scholar]
- 24.Lee Park HC, Park W et al. Negative impact of pretreatment anemia on local control after neoadjuvant chemoradiotherapy and surgery for rectal cancer. Radiat Oncol J 2012; : 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan AA, Klonizakis M, Shabaan A, Glynne-Jones R. Association between pretreatment haemoglobin levels and morphometric characteristics of the tumour, response to neoadjuvant treatment and long-term outcomes in patients with locally advanced rectal cancers. Colorectal Dis 2013; : 1,232–1,237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitayama J, Yasuda K, Kawai K et al. Circulating lymphocyte number has a positive association with tumor response in neoadjuvant chemoradiotherapy for advanced rectal cancer. Radiat Oncol 2010; : 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dou X, Wang RB, Yan HJ et al. Circulating lymphocytes as predictors of sensitivity to preoperative chemoradiotherapy in rectal cancer cases. Asian Pac J Cancer Prev 2013; : 3,881–3,885. [DOI] [PubMed] [Google Scholar]
- 28.Kitayama J, Yasuda K, Kawai K et al. Circulating lymphocyte is an important determinant of the effectiveness of preoperative radiotherapy in advanced rectal cancer. BMC Cancer 2011; : 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park YA, Sohn SK, Seong J et al. Serum CEA as a predictor for the response to preoperative chemoradiation in rectal cancer. J Surg Oncol 2006; : 145–150. [DOI] [PubMed] [Google Scholar]
- 30.Moreno García V, Cejas P, Blanco Codesido M et al. Prognostic value of carcinoembryonic antigen level in rectal cancer treated with neoadjuvant chemoradiotherapy. Int J Colorectal Dis 2009; : 741–748. [DOI] [PubMed] [Google Scholar]
- 31.Kato T, Ishikawa K, Aragaki M et al. Optimal predictive value of preoperative serum carcinoembryonic antigen for surgical outcomes in stage I non-small cell lung cancer: differences according to histology and smoking status. J Surg Oncol 2013; : 619–624. [DOI] [PubMed] [Google Scholar]