Abstract
INTRODUCTION
There is increasing and conflicting research debating the oncological benefits of extralevator abdominoperineal excision (ELAPE) compared with standard abdominoperineal excision (SAPE). However, there is very little in the literature on the long-term effects on patients’ wellbeing following the two procedures. The aim of this study was to determine the oncological outcomes and long-term quality of life (QoL) of patients at two hospitals having undergone ELAPE or SAPE.
METHODS
Consecutive patients with rectal cancer who underwent either ELAPE or SAPE between January 2009 and June 2015 at a single centre were analysed. Oncological outcomes were determined by histology and follow-up imaging. QoL data were obtained prospectively using the QLQ-C30 and QLQ-CR29 questionnaires.
RESULTS
A total of 48 patients (36 male, 12 female; 27 ELAPE, 21 SAPE) were reviewed. The mean age was 67.4 years and the median follow-up duration was 44 months (range: 6–79 months). Four patients (2 ELAPE, 2 SAPE) developed local recurrence. Rates of distant metastasis were similar (ELAPE: 11%, SAPE: 14%). There was no significant difference in mean global health status score (ELAPE: 77.3, SAPE: 65.3). Impotence was the most frequently reported problem (mean symptom scores of 89.7 and 78.8 for ELAPE and SAPE respectively).
CONCLUSIONS
This is the largest study with the longest follow-up period that compares QoL after ELAPE with that after SAPE. Although more radical in nature, ELAPE did not demonstrate any significant impact on QoL compared with SAPE. There was no significant difference in long-term oncological outcome between the groups. Impotence remains a significant problem for all patients and they should be well informed of this risk prior to surgery.
Keywords: Colorectal neoplasia, Quality of life, Rectal cancer
Anterior resection is the most common surgical treatment for rectal cancers located in the upper two-thirds of the rectum.1 For tumours located in the lower third of the rectum, sphincter excisional surgery can be an oncological necessity.2 Abdominoperineal excisions are performed for cancers within 6cm of the anal verge.2,3 Compared with anterior resection, standard abdominoperineal excision (SAPE) is more invasive, and has higher rates of local recurrence, morbidity and mortality.2,4,5 Over the last decade, attempts have been made to modify SAPE to reduce the rate of local recurrence and improve survival.2,4,6–8 SAPE involves dissection along the mesorectal line, which creates ‘waisting’ of the specimen removed.9 As the mesorectal tissue becomes thinner further into the pelvis, the dissection becomes close to the circumferential resection margin (CRM), resulting in a higher risk of bowel perforation, CRM involvement and local recurrence.8
In order to minimise this risk, extralevator abdominoperineal excision (ELAPE), introduced in 2007, involves a more vertical excision.2 The levator muscles are removed and the specimen excised appears more cylindrical shaped. This excision creates a larger distance between the dissection margin and the bowel containing the tumour, thereby reducing the chance of CRM involvement, bowel perforation and local recurrence.8 For patients undergoing ELAPE, a larger volume of tissue is removed and a larger perineal defect is formed, increasing the likelihood of wound complications.5 Furthermore, the removal of the levators cannot protect the pelvic nerves and vessels along the lateral pelvic wall, with the consequent issues of urinary and/or sexual dysfunction, perineal wound complications, infection and dehiscence.4
Laparoscopic ELAPE has been introduced to reduce the operating time compared with open ELAPE and provide a less invasive alternative for the patient.4 A transabdominal levator transection performed laparoscopically reduces the risk of pelvic nerve and vessel damage. ELAPE has been shown by some authors to reduce rates of recurrence and bowel perforation, thereby improving survival.8 Nevertheless, there is disagreement about the potential oncological superiority of ELAPE over SAPE, with others showing no advantage of ELAPE in terms of oncological outcomes.10 What is not known is whether patients who undergo ELAPE suffer from a poorer quality of life (QoL) because of the more invasive and extensive nature of this procedure. The aim of this study was to compare the oncological outcomes and long-term QoL after ELAPE with those after SAPE at a single centre. Subgroup analysis was also conducted to assess differences between laparoscopic and open surgery.
Methods
Retrospective analysis was carried out of consecutive patients who had undergone abdominoperineal excision for low rectal cancer (ELAPE vs SAPE, laparoscopic vs open) between January 2009 and June 2015 at a single trust. Data capture was performed by analysing the hospital’s operation database. Patient notes (which included clinic letters and operation details) were subsequently collected. Patients with a diagnosis other than adenocarcinoma were excluded. Patients were operated on by eight colorectal consultants across two hospitals (James Cook University Hospital and Friarage Hospital). The decision for abdominoperineal resection was made following a multidisciplinary team meeting for each patient. From 2012 onwards, patients underwent ELAPE as standard, owing to its perceived oncological superiority by our surgeons.
Information collected on patient demographics included age, sex and preoperative details (downstaging treatment, diabetes and length of stay). Histology results (tumour grade, completeness of resection and whether the CRM was intact) were also recorded. In addition, postoperative recurrence rates for individual patients were determined. Subsequent to this analysis, information regarding postoperative QoL was collected prospectively using the QLQ-C30 (version 3.0) and QLQ-CR29 questionnaires (European Organisation for Research and Treatment of Cancer [EORTC], Brussels, Belgium).11 The questionnaires were accompanied by a full set of instructions, and were completed between June 2015 and January 2016. Linear transformation of raw scores was used to give answers a value of 0–100, as described in the EORTC’s scoring manual.12 A higher functional scale score indicates a better level of function whereas a higher symptom score indicates greater symptom severity.
Following completion of an online decision tool developed by the Medical Research Council, it was ascertained that this study did not require research ethics committee approval.
Operative technique
The ELAPE technique performed in this study reflects that described by Holm et al.2 In laparoscopic cases, a standard four-port technique is used for the abdominal phase. Pelvic dissection during the abdominal phase of the operation stops before the mesorectum is dissected off the levator ani muscles. The limit of dissection is bounded by the seminal vesicles or cervix anteriorly, by the upper border of the coccyx posteriorly and at the level of the inferior hypogastric plexus anterolaterally. A small swab is placed behind the rectum to guide the perineal part of the operation. The abdomen is then closed and the stoma formed.
At this point, the patient’s position is changed to the prone jack-knife position. The anus is closed with a purse string suture, and a tear-drop incision is made close to the anus and extended to the coccyx. The coccyx is disarticulated and the levator ani muscles are divided under direct vision as laterally as possible. The resultant perineal defect is closed in the majority of cases with biological mesh. (One patient in our study had a myocutaneous flap.)
The perineal dissection during SAPE is often performed with the patient in the lithotomy position. There is limited external sphincter and levator muscle excision, and the perineal defect is closed primarily with sutures. The abdominal phase is performed using the standard technique according to the principles of total mesorectal excision.13
Statistical analysis
The chi-squared test, Fisher’s exact test and a two-sample t-test were used for categorical variables while the Mann–Whitney U test was employed for quantitative QoL variables. Data were analysed with Minitab® version 17.1.0 (Minitab, Coventry, UK) and a p-value of <0.05 was considered statistically significant.
Results
Overall, 48 patients had an abdominoperineal excision between January 2009 and June 2015: 27 underwent ELAPE and 21 SAPE. Owing to some consultants being newly appointed and one retiring during the study period, the total number of cases performed by each surgeon differed. All but one of the consultants performed both forms of surgery. The total number of cases and median time interval between each case is presented in Table 1.
Table 1.
Number of cases performed by each surgeon
| Surgeon | Total (n=48) | ELAPE (n=27) | SAPE (n=21) | Mean time between cases (range) in months |
| A | 6 | 3 | 3 | 6 (1–13) |
| B | 12 | 6 | 6 | 5 (0–13) |
| C | 2 | 1 | 1 | 14 |
| D | 5 | 1 | 4 | 12 (2–24) |
| E | 8 | 2 | 6 | 7 (2–17) |
| F | 10 | 6 | 4 | 6 (2–18) |
| G | 4 | 2 | 2 | 5 (2–14) |
| H | 1 | 1 | 0 | – |
ELAPE = extralevator abdominoperineal excision; SAPE = standard abdominoperineal excision
There were no significant differences between the ELAPE and SAPE groups in terms of age, sex or histopathology (Table 2). Fifteen patients (31%) underwent open surgery (7 ELAPE vs 8 SAPE). The majority of patients received preoperative chemotherapy or radiotherapy (ELAPE: 22/27 [81%], SAPE: 15/21 [71%]). Three of the SAPE patients (14%) had adjuvant chemotherapy. The decision to proceed with preoperative chemoradiotherapy was made at the multidisciplinary team meeting, and was based on factors such as CRM positivity, a T stage of ≥2 and local lymph node spread. The frequency of ELAPE increased with time although SAPE was still being performed during the final year of data collection (Fig 1). The 30-day mortality rate was 2.1%: one in-hospital death occurred from aspiration pneumonia in the SAPE cohort.
Table 2.
Patient and tumour characteristics for ELAPE and SAPE cases
| Total (n=48) | ELAPE (n=27) | SAPE (n=21) | p-value | |
| Sex | 0.18 | |||
| Male | 36 | 18 | 18 | |
| Female | 12 | 9 | 3 | |
| Mean age | 67.4 yrs | 67.8 yrs | 66.9 yrs | 0.78 |
| T stage | 0.39 | |||
| T0 | 4 | 4 | 0 | |
| T1 | 6 | 4 | 2 | |
| T2 | 11 | 5 | 6 | |
| T3 | 25 | 13 | 12 | |
| T4 | 2 | 1 | 1 | |
| N stage | 0.74 | |||
| N0 | 34 | 18 | 16 | |
| N1 | 9 | 6 | 3 | |
| N2 | 5 | 3 | 2 | |
| CRM | 0.50 | |||
| Positive | 2 | 2 | 0 | |
| Negative | 46 | 25 | 21 | |
| Mean CEA | 5.7μg/l | 7.3μg/l | 4.1μg/l | 0.55 |
| Downstaging treatment | 1.00 | |||
| Therapy given | 40 | 22 | 18 | |
| No therapy | 8 | 5 | 3 | |
| Recurrence | 1.00 | |||
| Local | 1 | 1 | 0 | |
| Distant | 9 | 4 | 5 |
CEA = carcinoembryonic antigen; CRM = circumferential resection margin; ELAPE = extralevator abdominoperineal excision; SAPE = standard abdominoperineal excision
Figure 1.
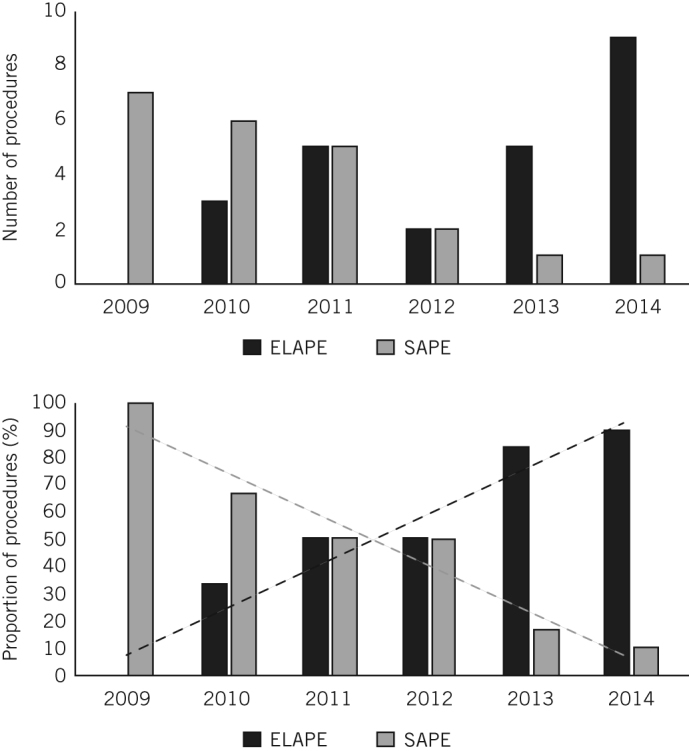
The numbers and proportions of extralevator abdominoperineal excision (ELAPE) and standard abdominoperineal excision (SAPE) procedures performed annually
Disease recurrence
Disease recurrence was determined by surveillance computed tomography (CT) in keeping with National Institute for Health and Care Excellence guidelines.14 Four patients (2 ELAPE, 2 SAPE) had local recurrence. The two ELAPE cases had CRM involvement (R1). Both of these had received preoperative chemoradiotherapy; one had a perforated T3 N0 tumour and the other had a non-perforated T3 N1 tumour. Histology of the two SAPE specimens demonstrated T3 N0 disease with a close but uninvolved CRM with vascular involvement in one patient and T2 N2 disease with an intact CRM in the other. Vascular involvement was not commented on in the report of the latter specimen but it was not standard practice to do so during the earlier stages of this study. Both SAPE patients had received preoperative adjuvant therapy (chemoradiotherapy and radiotherapy only respectively).
Seven patients (3 ELAPE, 4 SAPE) had distant metastases in at least one location (5 intra-abdominal, 4 pulmonary, 1 renal). Two of these had evidence on preoperative CT of either previous metastatic disease (1 had undergone a previous lobectomy) or active metastatic disease (1 had liver and lung metastases). One further patient had a suspicious pulmonary nodule with mediastinal lymphadenopathy. Management of these distant metastases included liver resection (n=2), wedge resection of the lung (n=1), complete colectomy (n=1), endoscopic retrograde cholangiopancreatography and stent placement (n=1), and palliative conservative management (n=1). Ten patients had died at the time of data collection. Of the remaining 38 patients, 79% were disease free at the last follow-up visit. Similar total recurrence rates were seen in both the ELAPE and SAPE groups (19% and 24% respectively). However, two ELAPE patients had not yet undergone postoperative CT.
There was a higher recurrence rate following laparoscopic procedures than following open procedures (8/33 [26%] vs 2/15 [13%]) although the difference was not statistically significant (p=0.47). Among the laparoscopic cases, one patient (alluded to above) had advanced disease, which resulted in an incomplete (R1) primary resection despite preoperative chemoradiotherapy. For the remaining seven cases, the CRM was intact on histology. The earliest documented evidence of metastatic disease was at 8 months (range: 8–46 months). Histology revealed a lack of vascular involvement in all primary resected specimens. Of the ten patients who died during the study period, six had metastatic disease.
Perineal wound breakdown
Ten ELAPE patients (37%) and five SAPE patients (24%) developed a degree of wound breakdown although this difference was not statistically significant. The Clavien–Dindo grades for these cases are presented in Table 3. Of the total study cohort, 83% of patients (n=40) had radiotherapy prior to surgery (ELAPE: 22/27, SAPE: 18/21). Among the cases with wound breakdown, seven of the ten ELAPE patients and all five of the SAPE patients underwent preoperative radiotherapy. Of those who developed dehiscence, one ELAPE and one SAPE patient returned to theatre. One patient in the ELAPE group developed a perineal abscess that required drainage and a vacuum dressing. One patient in the SAPE group had a failed gracilis myocutaneous flap. The remaining cases of perineal wound breakdown were managed conservatively as breakdown was deemed superficial (discharging sinus or dehiscence of skin and subcutaneous tissue only).
Table 3.
Perineal wound classification
| Operation | Neoadjuvant therapy | Complication | Clavien–Dindo grade* | Treatment |
| ELAPE | Chemoradiotherapy | Perineal abscess | IIIb | Incision and drainage with healing by secondary intention |
| ELAPE | Chemoradiotherapy | Superficial dehiscence | II | Antibiotics |
| ELAPE | Chemoradiotherapy | Superficial dehiscence | II | Antibiotics |
| ELAPE | Chemoradiotherapy | Discharging sinus | II | Antibiotics |
| ELAPE | Chemoradiotherapy | Discharging sinus | II | Antibiotics |
| ELAPE | Radiotherapy | Discharging sinus | II | Antibiotics |
| ELAPE | Radiotherapy | Cellulitis | II | Antibiotics |
| ELAPE | None | Perineal abscess | IIIb | Vacuum dressing |
| ELAPE | None | Superficial dehiscence | II | Antibiotics |
| ELAPE | None | Discharging sinus | II | Antibiotics |
| SAPE | Chemoradiotherapy | Failed gracilis myocutaneous flap | IIIb | Antibiotics |
| SAPE | Chemoradiotherapy | Superficial dehiscence | II | Antibiotics |
| SAPE | Radiotherapy | Superficial dehiscence | II | Antibiotics |
| SAPE | Radiotherapy | Discharging sinus | II | Antibiotics |
| SAPE | Radiotherapy | Discharging sinus | II | Antibiotics |
ELAPE = extralevator abdominoperineal excision; SAPE = standard abdominoperineal excision
*Grade II: Requiring pharmacological treatment with drugs other than such allowed for grade I complications. Grade IIIb: Requiring intervention under general anaesthesia.
Review of outpatient clinic letters at the time of data collection confirmed that all wounds had healed by secondary intention. However, the timelines for wound healing were not available. None of the patients had perineal herniation. Radiotherapy was not a significant factor of wound breakdown in either cohort.
Overall quality of life and function
Of the 48 patients in the study, 10 had died before the QLQ-C30 and QLQ-CR29 questionnaires were sent out. One patient was in a palliative hospice and one had metastatic disease prior to the procedure. It was therefore deemed inappropriate to disseminate the questionnaires to these patients. Among the 36 remaining patients, 32 completed the questionnaires (response rate 89%).
QoL was assessed at 6–73 months after surgery (ELAPE: 6–57 months, median 26 months; SAPE: 7–73 months, median 59 months). The mean global health status score for ELAPE patients was higher than for SAPE patients although this did not reach statistical significance (77.3 vs 65.3 respectively, p=0.27) (Fig 2a). On average, the ELAPE group demonstrated higher physical, role, emotional, cognitive and social functioning than the SAPE cohort although these differences were not statistically significant (Fig 2b). There were no significant differences between ELAPE and SAPE patients for body image, anxiety, weight loss or sexual interest (Fig 2c).
Figure 2.
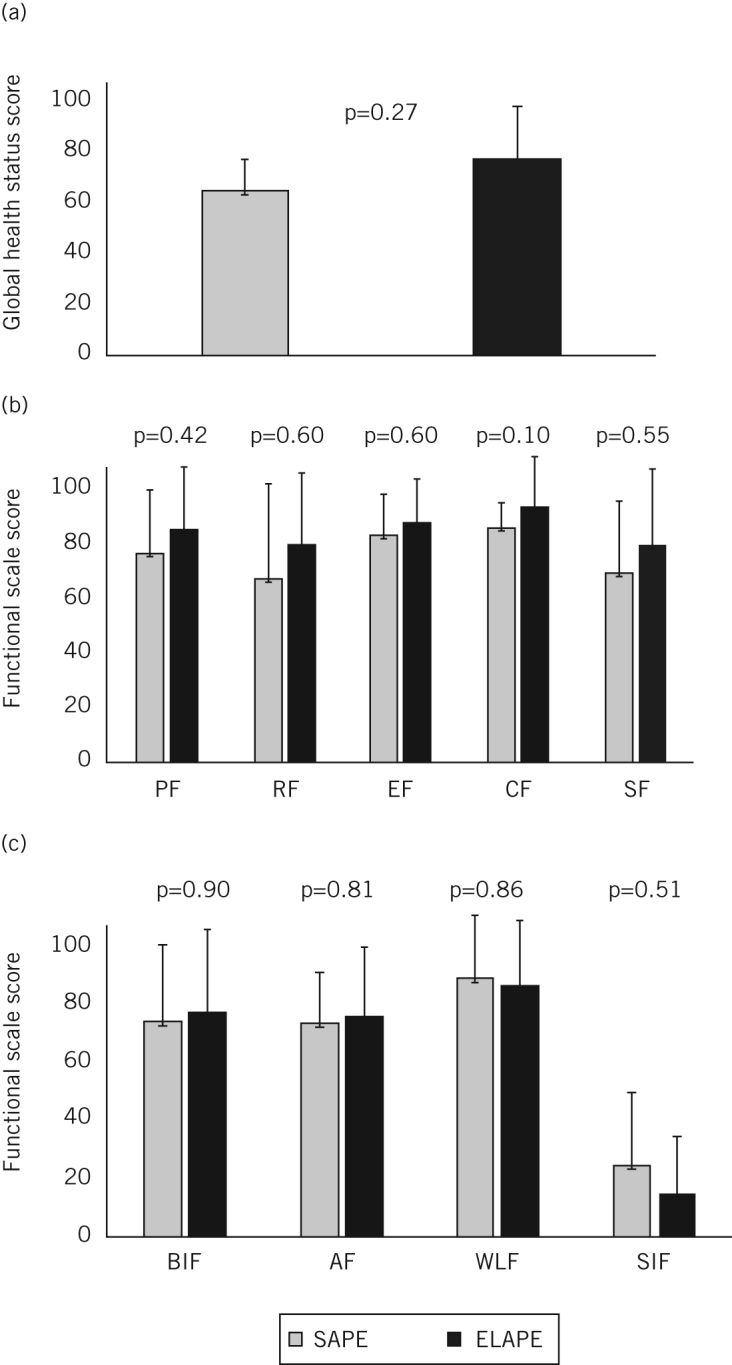
Quality of life after standard abdominoperineal excision (SAPE) and extralevator abdominoperineal excision (ELAPE): mean global health status scores (A), mean functional scale scores from the QLQ-C30 questionnaire (B) and mean functional scale scores from the QLQ-CR29 questionnaire (C) PF = physical functioning; RF = role functioning; EF = emotional functioning; CF = cognitive functioning; SF = social functioning; BIF = body image functioning; AF = anxiety functioning; WLF = weight loss functioning; SIF = sexual interest functioning
Symptoms
There were no significant differences between the ELAPE and SAPE groups in terms of urinary and bowel symptoms, abdominal and buttock pain or complications of living with a colostomy (Table 4). Impotence was a very common adverse effect for both ELAPE and SAPE patients (mean symptom scores of 89.7 and 78.8 respectively).
Table 4.
Mean symptom scores for ELAPE and SAPE patients. The higher the score, the worse the symptom
| Symptom | ELAPE | SAPE | p-value |
| Fatigue | 25.1 (SD: 24.4) | 32.1 (SD: 23.3) | 0.61 |
| Nausea and vomiting | 1.9 (SD: 5.2) | 9.0 (SD: 14.0) | 0.32 |
| Pain | 10.5 (SD: 23.7) | 23.1 (SD: 21.2) | 0.22 |
| Dyspnoea | 14.0 (SD: 27.2) | 20.5 (SD: 24.6) | 0.60 |
| Insomnia | 13.2 (SD: 24.5) | 28.2 (SD: 31.6) | 0.51 |
| Appetite loss | 7.0 (SD: 23.1) | 12.8 (SD: 20.8) | 0.48 |
| Constipation | 8.8 (SD: 18.2) | 12.8 (SD: 20.8) | 0.77 |
| Diarrhoea | 5.9 (SD: 12.7) | 19.4 (SD: 16.4) | 0.23 |
| Financial difficulties | 13.0 (SD: 27.5) | 5.6 (SD: 18.4) | 0.66 |
| Urinary frequency | 30.7 (SD: 22.5) | 30.6 (SD: 19.0) | 0.99 |
| Blood and mucus in stool | 4.4 (SD: 9.1) | 6.9 (SD: 10.7) | 0.72 |
| Stool frequency | 21.3 (SD: 17.4) | 11.5 (SD: 13.7) | 0.43 |
| Urinary incontinence | 10.5 (SD: 21. 8) | 19.4 (SD: 25.3) | 0.57 |
| Dysuria | 3.5 (SD: 14.9) | 3.0 (SD: 9.6) | 0.88 |
| Abdominal pain | 12.2 (SD: 27.0) | 11.1 (SD: 15.7) | 0.80 |
| Buttock pain | 14.0 (SD: 24.9) | 11.1 (SD: 20.8) | 0.94 |
| Bloating | 12.3 (SD: 24.7) | 8.3 (SD: 19.8) | 0.87 |
| Dry mouth | 19.3 (SD: 29.3) | 13.9 (SD: 25.3) | 0.78 |
| Hair loss | 5.3 (SD: 22.3) | 0 (SD: 0) | 0.76 |
| Taste loss | 3.5 (SD: 10.2) | 8.3 (SD: 19.8) | 0.79 |
| Sore skin | 14.8 (SD: 22.8) | 20.5 (SD: 24.6) | 0.73 |
| Embarrassment of stoma | 31.6 (SD: 36.6) | 17.9 (SD: 31.0) | 0.56 |
| Stoma care problems | 3.5 (SD: 10.2) | 5.1 (SD: 12.0) | 0.85 |
| Impotence | 89.7 (SD: 24.1) | 78.8 (SD: 32.6) | 0.55 |
| Dyspareunia | 33.3 (SD: 47.1) | 0 (SD: 0) | 1.00 |
ELAPE = extralevator abdominoperineal excision; SAPE = standard abdominoperineal excision; SD = standard deviation
Laparoscopic vs open ELAPE
There was no statistically significant difference in mean global health status scores between patients undergoing laparoscopic or open ELAPE (70.4 vs 74.1 respectively, p=0.36). Similarly, there were no significant differences in functional scale or symptom scores between the two types of ELAPE procedure.
Discussion
This is the largest reported study to date and over a follow-up period greater than six months comparing QoL for patients after ELAPE with that after SAPE as well as for patients after laparoscopic versus open ELAPE. Over the six-year study period, an increasing proportion of patients underwent ELAPE rather than SAPE at our tertiary centre. From 2012 ELAPE was deemed to be the gold standard owing to our trust perceiving ELAPE as oncologically superior. After this time point, all consultants performing abdominoperineal resections were trained to carry out this technique.
Initially, ELAPE was performed by only three trained surgeons. However, by the end of our study, all consultants were performing ELAPE. Consequently, the tumour stage did not affect the decision to perform ELAPE or SAPE. Rates of tumour recurrence were not found to be significantly lower following ELAPE in our study. The rise in the number of ELAPE procedures performed reflects surgeons’ preferences for the procedure, supported by the lower rates of local recurrence and improved survival reported by previous studies.3,6,8
Our study did not demonstrate any oncological superiority for either of the two procedures in terms of disease free survival or long-term survival. Furthermore, there was no significant difference in overall long-term QoL between patients who had ELAPE and those who had SAPE, which is consistent with previous findings on short-term outcomes.9,15
Standardised pathological grading of resected specimens is now recognised as essential for patient care and as a marker of surgical technique. An additional three-stage classification system was proposed by Nagtegaal et al in 2002 to grade specimens at the level of the levators.16 The lack of standardisation among hospitals prompted further discussion in 2015 by Campa-Thompson et al.17 Although the three-stage classification system became a standard part of routine pathological reporting in our unit, this was not the case during the earlier years of the study, which prevented further analysis of our data. It is therefore possible that some surgeons may have inadvertently performed an extralevator resection, which may have affected the oncological results for these two cohorts.
There has been much debate about the potential superiority of ELAPE over SAPE in the treatment of rectal cancer. Asplund et al looked at two consecutive, unselected groups of 79 patients and found no significant difference in CRM positivity, tumour perforation or local recurrence.10 This is contrary to a meta-analysis published in 2015, which demonstrated lower rates of intraoperative perforation and local recurrence following ELAPE.18 On the other hand, many of the trials included in the meta-analysis were retrospective with small sample sizes (similar to our study).
Perineal wound complications are among the most common complications of abdominoperineal resection, occurring in 20–58% of cases.19 While no significant differences were observed between the two patient groups in our study, our findings are generally similar to previous results.15
With regard to QoL, the mean global health status score for the ELAPE cohort of 79.2 is lower than reported previously.15 This could be explained by the longer follow-up period in our study. Other factors such as initial health status prior to surgery and postoperative aftercare could also help to explain this difference. There was no significant difference between functional scale scores for patients who underwent ELAPE and those who had SAPE, nor was there a significant difference between scores for psychological symptoms, urinary and bowel symptoms or abdominal pain after the two procedures. This may reassure surgeons who choose not to perform ELAPE owing to reservations about the operation being too radical for patients who are frail or who have multiple co-morbidities.15
In addition, there was no significant difference between the oncological outcomes or longer-term QoL after laparoscopic and open ELAPE. Although not statistically significant, a greater total number of laparoscopic resections were followed by recurrent disease. It would, however, not be appropriate to conclude that laparoscopic surgery has a higher risk of recurrence than open surgery as a higher number of laparoscopic procedures were performed (33 vs 15) and our overall sample size was small. Nevertheless, while the advantages of laparoscopic colorectal surgery are well established4 (particularly in the short term), no ill effects were observed on long-term QoL in our study.
Impotence was by far the most common problem in men after both ELAPE and SAPE. The mean impotence scores were 89.7 and 78.8 respectively. These findings are similar to another study, reporting scores of 75 and 100 respectively.15 Impotence is a significant adverse side effect; it can be secondary to direct trauma to the nerves and vessels in the pelvic cavity during the resection. The surgeons at our trust perform the perineal component of ELAPE via an extended prone perineal approach with removal of the coccyx, as described by Holm et al.2 We believe this provides better visualisation of the operative field.8 As a result, the pudendal nerve and pelvic plexus can be better visualised and preserved. Alternatively, with either procedure, impotence can be due to acute oedema in the perineum that has led to longstanding nerve damage.15 Further research is required to develop surgical techniques that minimise this risk.
Meanwhile, it is important that patients are well informed of the high likelihood of suffering erectile dysfunction with either operation. Shared decision making is a major aspect of tailoring care to individual cases.20 Surgeons must discuss with their patients the impact that both ELAPE and SAPE can have on QoL as well as the impact of these procedures on recurrence rates. Once a patient has been fully informed of the risks and benefits of both operations, he or she should be allowed to make a decision that is most appropriate for that individual. Differences in co-morbidities, age and the ability to cope are all factors that need to be taken into consideration.
Study limitations
The retrospective component of the study design could have introduced recall bias for some of the questionnaire responses. Furthermore, this study was performed across two hospitals in one trust in Northern England and the findings cannot therefore be extrapolated nationally. When reporting results of such patients from a single centre, using a single QoL assessment, the follow-up periods will be variable.
Despite this being the largest study to date, no significant difference in QoL was observed between the ELAPE and SAPE groups because of the large variability in QoL scores. It is possible that our study was not sufficiently powered to meet our objective. The data generated in this study could be used to compare QoL in patients undergoing these two procedures as part of a future adequately powered multicentre study.
Future research
A prospective multicentre study involving a large sample of patients is needed to compare ELAPE versus SAPE and laparoscopic ELAPE versus open ELAPE, and to determine differences in longer-term oncological and patient outcomes. In order to ensure robust findings, QoL could be measured both pre and postoperatively to gauge the impact of the procedure on each individual case more accurately. Development of techniques that preserve genitourinary function and of treatments that prevent the recurrence of distant metastases would also improve the QoL for patients with lower rectal cancer.
Conclusions
There has been an increasing preference for ELAPE over SAPE at our unit. This is likely to be due to our surgeons’ preferences, informed by the lower local recurrence rates reported by previous studies. Conversely, in our study, ELAPE did not reduce the rate of CRM positivity or improve the overall oncological outcome. There was no significant difference in QoL between the cohorts (measured at least six months following the procedure) despite the general belief that ELAPE is a more radical operation with greater morbidity. However, both procedures result in a very high incidence of impotence in men. It is important that the impact of QoL and evidence-based recurrence rates are discussed with patients so that they are fully informed and able to make a decision that is appropriate for them.
Acknowledgements
The authors would like to thank Rochelle Bates for her help with case note retrieval.
The material in this paper was presented at the 9th European Colorectal Congress held in St Gallen, Switzerland, December 2015, as well as at the Society of American Gastrointestinal and Endoscopic Surgeons Annual Meeting held in Boston, US, March 2016.
References
- 1.MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993; : 457–460. [DOI] [PubMed] [Google Scholar]
- 2.Holm T, Ljung A, Häggmark T et al. Extended abdominoperineal resection with gluteus maximus flap reconstruction of the pelvic floor for rectal cancer. Br J Surg 2007; : 232–238. [DOI] [PubMed] [Google Scholar]
- 3.Wibe A, Syse A, Andersen E et al. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: anterior vs abdominoperineal resection. Dis Colon Rectum 2004; : 48–58. [DOI] [PubMed] [Google Scholar]
- 4.Chi P, Chen ZF, Lin HM et al. Laparoscopic extralevator abdominoperineal resection for rectal carcinoma with transabdominal levator transection. Ann Surg Oncol 2013; : 1,560–1,566. [DOI] [PubMed] [Google Scholar]
- 5.West NP, Anderin C, Smith KJ et al. Multicentre experience with extralevator abdominoperineal excision for low rectal cancer. Br J Surg 2010; : 588–599. [DOI] [PubMed] [Google Scholar]
- 6.Huang A, Zhao H, Ling T et al. Oncological superiority of extralevator abdominoperineal resection over conventional abdominoperineal resection: a meta-analysis. Int J Colorectal Dis 2014; : 321–327. [DOI] [PubMed] [Google Scholar]
- 7.Martijnse IS, Dudink RL, West NP et al. Focus on extralevator perineal dissection in supine position for low rectal cancer has led to better quality of surgery and oncologic outcome. Ann Surg Oncol 2012; : 786–793. [DOI] [PubMed] [Google Scholar]
- 8.West NP, Finan PJ, Anderin C et al. Evidence of the oncologic superiority of cylindrical abdominoperineal excision for low rectal cancer. J Clin Oncol 2008; : 3,517–3,522. [DOI] [PubMed] [Google Scholar]
- 9.Welsch T, Mategakis V, Contin P et al. Results of extralevator abdominoperineal resection for low rectal cancer including quality of life and long-term wound complications. Int J Colorectal Dis 2013; : 503–510. [DOI] [PubMed] [Google Scholar]
- 10.Asplund D, Haglind E, Angenete E. Outcome of extralevator abdominoperineal excision compared with standard surgery: results from a single centre. Colorectal Dis 2012; : 1,191–1,196. [DOI] [PubMed] [Google Scholar]
- 11.Aaronson NK, Ahmedzai S, Bergman B et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993; : 365–376. [DOI] [PubMed] [Google Scholar]
- 12.European Organisation for Research and Treatment of Cancer. The EORTC QLQ-C30 Scoring Manual. 3rd edn Brussels: EORTC; 2001. [Google Scholar]
- 13.Heald RJ, Moran BJ, Ryall RD et al. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg 1998; : –899. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence Colorectal cancer: diagnosis and management. http://www.nice.org.uk/guidance/cg131/ (cited January 2017).
- 15.Vaughan-Shaw PG, Cheung T, Knight JS et al. A prospective case-control study of extralevator abdominoperineal excision (ELAPE) of the rectum versus conventional laparoscopic and open abdominoperineal excision: comparative analysis of short-term outcomes and quality of life. Tech Coloproctol 2012; : 355–362. [DOI] [PubMed] [Google Scholar]
- 16.Nagtegaal ID, Marijnen CA, Kranenbarg EK et al. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol 2002; : 350–357. [DOI] [PubMed] [Google Scholar]
- 17.Campa-Thompson M, Weir R, Calcetera N et al. Pathologic processing of the total mesorectal excision. Clin Colon Rectal Surg 2015; : 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Xu H, Shang Z et al. Outcome of extralevator abdominoperineal excision over conventional abdominoperineal excision for low rectal tumor: a meta-analysis. Int J Clin Exp Med 2015; : 14,855–14,862. [PMC free article] [PubMed] [Google Scholar]
- 19.Butt HZ, Salem MK, Vijaynagar B et al. Perineal reconstruction after extra-levator abdominoperineal excision (eLAPE): a systematic review. Int J Colorectal Dis 2013; : 1,459–1,468. [DOI] [PubMed] [Google Scholar]
- 20.Page AE. Safety in surgery: the role of shared decision-making. Patient Saf Surg 2015; : 24. [DOI] [PMC free article] [PubMed] [Google Scholar]


