Abstract
Genetic constructs with promoters fused to reporter genes for simultaneous monitoring of cellular events have been the focus of attention in recent years. Adenoviral vectors, which have distinctive characteristics, have been used to monitor the differentiation of stem cells in vitro. In the present study, a modified adenoviral vector was constructed, containing a mouse, rat, and human general albumin promoter sequence fused to a ZsGreen reporter gene, and evaluated its efficiency in different cell types. Two hepatocyte cell lines (Hepa1-6 and HepG2), rat primary hepatocytes, rat bone marrow mesenchymal stem cells (BM-MSCs) and rat BM-MSCs-derived hepatocyte-like cells were transduced with this vector, and the transfection efficiency and functional capabilities of the promoter were evaluated by fluorescent microscopy. The results demonstrated efficient expression of ZsGreen in Hepa1-6 cells, HepG2 cells, rat primary hepatocytes, and rat BM-MSCs-derived hepatocyte-like cells, but not in rat BM-MSCs. In conclusion, the current study demonstrates a simple, high-efficiency, general tool for real-time monitoring of the differentiation status of hepatocytes from stem cells in mice, rats, and humans. This tool may be useful for evaluating different protocols to generate functional hepatocytes from stem cells in multiple species.
Keywords: albumin promoter, adenoviral vector, hepatocyte differentiation, stems cells, gene reporter
Introduction
Shortages of donor livers and hepatocytes are the major limitations of liver transplantation. Thus, generation of hepatocyte-like cells may provide an alternative source of cells for use in therapeutic applications. A number of recent studies have demonstrated that hepatic stem cells from (1) bone marrow (BM-MSCs), (2) umbilical cord blood, (3) hepatic progenitor cells, (4) hematopoietic cells, and (5) adipose tissue can be differentiated into hepatocyte-like cells, which may overcome the limited supply of donor hepatocytes and provide a new cell-based therapeutic option for life-threatening liver diseases, regenerative medicine, toxicity testing for pharmacological drug screenings, and other medical-related applications. A simple, high-efficiency, liver-specific tool as a sensor for real-time monitoring of stem cell differentiation into functional hepatocytes is urgently required. In recent years, genetic constructs with tissue-specific promoters fused to reporter genes, such as green fluorescent protein (GFP), for use in simultaneous monitoring of various cellular events, have gained increased attention. Albumin (ALB) is a protein that is synthesized primarily in the liver, and is specifically expressed in adult animal hepatocytes. The ALB promoter contains at least six potential cis-acting elements (6), and these can bind specifically to hepatocyte nuclear factor 1 (HNF1), CCAAT/enhancing binding protein (C/EBP), D site ALBp binding protein (DBP), leucine arylamidase (LAP), and nuclear factors-Y and −1 (NF-Y and NF-1), thereby stimulating transcription in the liver (7). Since the ALB promoter has highest activity in the liver, it has been already used as a target for cell-specific gene delivery (8). Furthermore, the ALB promoter is highly homologous among different species, including rat, mouse, and human (9,10). Adenoviral transduction provides a mechanism for expressing proteins in >95% of hepatocytes in as little as 24 h following infection, and it can be used in whole animals for liver-specific gene expression (11). One of the newest reporter genes, Zoanthus green fluorescent protein (ZsGreen), encodes a bright green fluorescent protein that has been modified for high solubility, bright emission, and rapid chromophore maturation. In the present study, we constructed a recombinant adenovirus vector that is co-expressed in rat, mouse, and human, containing a general ALB promoter fragment and ZsGreen as a simple, high-efficiency, general sensor for real-time monitoring of the hepatic differentiation status of stem cells in mice, rats, and humans.
Materials and methods
Human hepatoma HepG2 cells, mouse liver hepatoma Hepa1-6 cells, human umbilical vein endothelial cells (HUVEC) and human macrophage U937 cells were cultured in DMEM (Cyagen, Guangzhou, China) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Primary rat hepatocytes were isolated from male Sprague-Dawley rats (body weight, 180–220 g) by a two-step collagenase perfusion method, and their viability was confirmed to be >95% by the trypan blue exclusion method (12). Hepatocytes were cultured in serum-free medium as previously described (13). Commercial rat BM-MSCs (Cyagen) from passage 6–8 were used in this study, and these cells were cultured in Sprague-Dawley rat MSC growth medium (Cyagen). All cells were grown in the same culture medium at 37°C and 5% CO2 in a humidified incubator. The culture medium was replaced at least every 2–3 days. Cells in the exponential phase of growth were counted, and after 24 h, cells at 50–70% confluence were transfected with the recombinant plasmids or adenovirus.
Hepatocyte differentiation of rat BM-MSCs in vitro
To induce hepatocyte differentiation, a two-step induction protocol was applied. Prior to the induction protocol, the hepatocytes were serum-deprived for two days in Iscove's modified Dulbecco's Medium (IMDM, HyClone, Beijing, China) supplemented with 20 ng/ml epidermal growth factor (EGF, Sigma-Aldrich, St Louis, MO, USA) and 10 ng/ml basic fibroblast growth factor (bFGF, Sigma-Aldrich). The induction protocol was as follows: i) Differentiation medium consisting of IMDM medium supplemented with 20 ng/ml hepatocyte growth factor, 10 ng/ml basic fibroblast growth factor, and 0.61 g/l nicotinamide (all from Sigma-Aldrich) for 7 days; ii) maturation medium, which consisted of IMDM supplemented with 20 ng/ml oncostatin M (Sigma-Aldrich), 1 mmol/l dexamethasone (Sigma-Aldrich), and 50 mg/ml insulin-transferrin-selenium premix (Gybcol) for 2 weeks.
Construction of the pDRIVE-SV40-Albp-LacZ recombinant plasmids
The ALB promoter sequences from human, rat, and mouse were obtained from a Gene Bank BLAST search. The predicted ALB promoter in the Promoter Database contains 1,000 bp (−700/+299). Based on the payload size of a gene delivery vector (11,14) and the potential cis-acting element binding sites, conserved clusters of transcription factor binding sites were identified at −247/+36 (284 bp, Fragment 1), −173/-23 (151 bp, Fragment 2), and −173/+36 (210 bp, Fragment 3). All amplified promoter fragments were cloned into the digested expression vector to generate pDRIVE-SV40-Albp-LacZ recombinant plasmids. Each promoter fragment drives the expression of the LacZ reporter gene, which allows for assessment of the promoter's activity in transient transfection experiments. The LacZ gene encodes β-galactosidase, an enzyme that catalyzes the hydrolysis of 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal), producing a blue precipitate that can be easily visualized under a microscope. Plasmids were transformed into Escherichia coli (E. coli) by electroporation, and then amplified, and purified by using the E. coli Fast-Media Zeo kit (InvivoGen, San Diego, CA, USA). LacZ expression was assessed by counting blue stained cells in each visual field and determining the proportion of stained cells among all cells. ALB promoter transcriptional levels were assessed by manual counting, which was aided by using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Plasmid transfection
In an attempt to evaluate the transcriptional efficiency of the three ALB promoter fragments, Hepa1-6 cells were transfected with the three different pDRIVE-SV40-Albp-LacZ constructs using LipoFiter™ Liposomal Transfection Reagent (Hanbio, Shanghai, China), according to the manufacturer's instructions. At 48 h post-transfection, the cells were incubated with 1 mg/ml X-gal in culture medium overnight at 37°C, and the blue staining was evaluated. The blue precipitate produced by LacZ expression can be examined using a Leica Microsystems DM40000B upright microscope. Blue staining was evaluated by counting blue stained cells in ten 400X visuafieldsl and determining the proportion of stained cells among all cells.
Construction of Albp-ZsGreen adenoviral vectors
The final ALB promoter fragment sequence was chosen based on the results of the plasmid transfection experiment. The ALB promoter and ZsGreen fragments were inserted into the pHBAd-U6-CMV (Hanbio) vector to generate the recombinant plasmid pHBAd-Albp-ZsGreen. Recombinant adenoviruses were produced by transfecting 293T cells (Hanbio) with the adenoviral expression plasmid pHBAd-Albp-ZsGreen and the framework plasmid pHBAd-BHGlox (Hanbio).
Adenovirus transduction
Hepa1-6 cells, HepG2 cells, rat MSCs, and rat BM-MSC-derived hepatocyte-like cells (at a density of 2×106 cells) and HUVEC, U937 cells and rat primary hepatocytes (at a density of 1×106 cells) were cultured in 6-well tissue culture plates. The cells were transduced with the same titer (in 10 µl) of recombinant ALB promoter adenovirus and CMV promoter adenovirus with GFP (positive control). During transduction, 1 ml of culture medium was added to each well. Following 2 h of transduction, 2 ml of fresh culture medium was added to the wells, and the cells were cultured for an additional 36 h. Following this, a fluorescence microscope (Leica, Germany) was used to detect ZsGreen fluorescence at 495 nm in the transduced cells.
Immunofluorescence staining
Hepa1-6 cells, HepG2 cells, rat primary hepatocytes, rat BM-MSCs, and rat BM-MSC-derived hepatocyte-like cells were cultured on glass coverslips for 24 h. After the cells were fixed, they were incubated with a primary antibody against ALB (1:100, cat no. ab8940; Abcam, Cambridge, MA, USA) and a FITC-conjugated secondary anti-sheep IgG (cat no. ab6743; Abcam), according to the manufacturer's instructions. After nuclear staining with DAPI (Santa Cruz Biotechnology, Dallas, TX, USA) slides were observed with a fluorescence microscope (Leica, Germany).
Results
Construction of the pDRIVE-SV40-Albp-LacZ recombinant plasmid
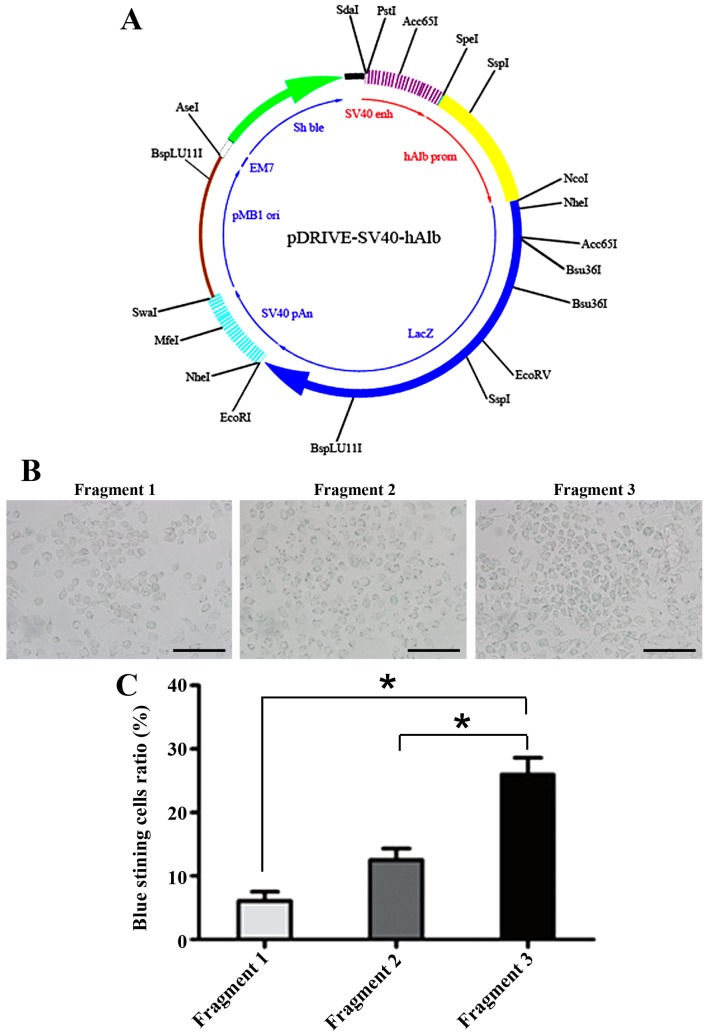
The ALB promoter fragment sequences are shown in Table I as Fragments 1, 2 and 3. All ALB promoter fragments were successfully amplified, and the recombinant pDRIVE-SV40-Albp-LacZ plasmids were successfully constructed (Fig. 1A) and verified by restriction digestion and sequencing (data not shown).
Table I.
Nucleotide sequences of the albumin (ALB) promoter used in the experimental constructs.
| Promoter fragment | Sequence | bp | Site |
|---|---|---|---|
| 1 | TTAAACTCTTATGTAAAATTTGATAAGATGTTTTACACAACTTTA ATACATTGACAAGGTCTTGTGGAGAAAACAGTTCCAGATGGTAAA TATACACAAGGGATTTAGTCAAACAATTTTTTGGCAAGAATATTA TGAATTTTGTAATCGGTTGGCAGCCAATGAAATACAAAGATGAGT CTAGTTAACACGTATATTAATCTACAATTATTGGTTAAAGAATAG TGCTAATTTCCCTCCGTTTGTCCTAGCTTTTCTCTTCTGTCAACC CCACACGCCTTTGG | 284 | −247/+36 |
| 2 | AGTTCCAGATGGTAAATATACACAAGGGATTTAGTCAAACAATTT TTTGGCAAGAATATTATGAATTTTGTAATCGGTTGGCAGCCAATG AAATACAAAGATGAGTCTAGTTAATAATCTACAATTATTGGTTAAA GAAGTATATTAGTGC | 151 | −173/-23 |
| 3 | AGTTCCAGATGGTAAATATACACAAGGGATTTAGTCAAACAATTT TTTGGCAAGAATATTATGAATTTTGTAATCGGTTGGCAGCCAATG AAATACAAAGATGAGTCTAGTTAATAATCTACAATTATTGGTTAA AGAAGTATATTAGTGCTAATTTCCCTCCGTTTGTCCTAGCTTTTC TCTTCTGTCAACCCCACACGCCTTTGGCAC | 210 | −173/+36 |
Figure 1.
Identification of the ALB promoter sequence with highest transcriptional activity. (A) Map of the pDRIVE-SV40-Albp-LacZ plasmid. The LacZ gene, encoding β-galactosidase, is marked in blue. The region in yellow is the insertion site for the human albumin (ALB) promoter fragment 1, 2, and 3. (B) X-gal staining of Hepa1-6 cells transfected with pDRIVE-SV40-Albp-LacZ. (C) The transcriptional levels of the ALB promoter were assessed by manually counting blue stained cells, aided by the use of Image-pro Plus version. Scale bar, 100 µm.
Comparison of the transcription levels of the ALB promoters in Hepa1-6 cells
Hepa1-6 cells transfected with the three recombinant pDRIVE-SV40-Albp-LacZ plasmids exhibited β-gal activity, as evidenced by the X-gal staining (blue in Fig. 1B). Fragment 3 exhibited the highest LacZ gene expression among the three plasmid constructs, and the number of cells transfected with the Fragment 3 construct with LacZ expression was much higher than the number of cells transfected with the other constructs (Fig. 1C). Therefore, we used Fragment 3 as the core ALB promoter in all subsequent experiments.
Construction of the recombinant Albp-ZsGreen reporter adenovirus
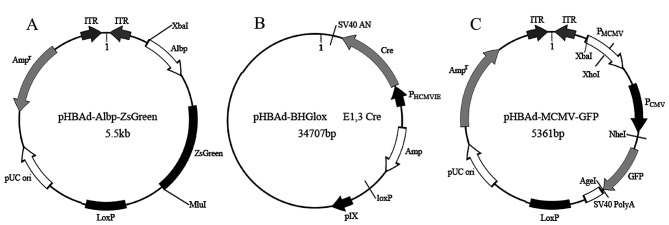
The recombinant pHBAd-Albp-ZsGreen plasmids containing Fragment 3 were successfully constructed, as shown in Fig. 2A. The authenticity of the Albp-ZsGreen fragment was confirmed by sequencing (data not shown). The Albp-ZsGreen reporter adenovirus vectors were successfully packaged and amplified in 293T cells by transfecting with the framework plasmid pHBAd-BHGlox (Fig. 2B). The Albp-ZsGreen-tagged positive rate as determined by TCID50 was 2×1010 PFU/ml. The CMV promoter-containing adenovirus with GFP derived from the expression plasmid pHBAd-MCMV-GFP was used as positive control.
Figure 2.
Maps of the plasmids for construction of the Albp-ZsGreen adenovirus vector. (A) The adenoviral expression plasmid pHBAd-Albp-ZsGreen with ALB promoter fragment 3. (B) The framework plasmid pHBAd-BHGlox for 293T cells, producing reporter adenovirus vectors by transfecting with adenoviral expression plasmid. (C) The adenoviral expression plasmid pHBAd-MCMV-GFP as a positive control.
Detection of recombinant Albp-ZsGreen reporter adenovirus in transduced cells
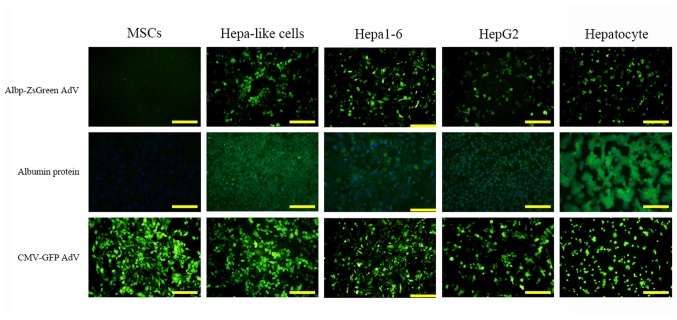
The Albp-ZsGreen reporter adenovirus or a CMV promoter-ZsGreen adenovirus (as a positive control) were used to infect and transduce Hepa1-6 cells, HepG2 cells, rat primary hepatocytes, rat BM-MSCs, and rat BM-MSCs-derived hepatocyte-like cells. As shown in Fig. 3, ALB gene expression (ZsGreen green fluorescence) was observed in Hepa1-6 cells, HepG2 cells, rat primary hepatocytes, and rat BM-MSCs-derived hepatocyte-like cells, but not in rat BM-MSCs, which was consistent with the results of the ALB immunofluorescence. In addition, an overall higher intensity of ZsGreen fluorescence in all cells was noted for the CMV promoter positive control group. HUVEC and U937 cells were tested as negative control. They were transduced with the same titer (in 10 µl) of recombinant ALB promoter adenovirus with ZsGreen, and no green fluorescence was detected (data not shown).
Figure 3.
Detection of the recombinant Albp-ZsGreen reporter adenovirus in transduced cells. ALB promoter expression following transfection with the recombinant adenovirus, was detected as green fluorescence (ZsGreen) in mouse Hepa1-6 cells, human HepG2 cells, rat primary hepatocytes, and rat BM-MSCs-derived hepatocyte-like cells, but not in rat MSCs (upper row). This was consistent with the albumin protein immunofluorescence staining results (lower row, the cell nucleus were stained blue, and albumin protein in green). The second row are albumin protein immunofluorescence staining results. The cell nucleus were stained blue (DAPI), and albumin protein in green An overall higher intensity of CMV promoter-driven green fluorescence was noted in all cells in the positive control group (third row). Hepa-like cell, hepatocyte-like cell differentiated from rat MSCs; AdV, adenoviral vector; Albp, ALB promoter; CMV, cytomegalovirus. Scale bar, 100 µm.
Therefore, the recombinant adenoviral vector confirmed that Fragment 3 is the core region of the ALB promoter, and that it is appropriate for the detection of hepatocyte-like cells in rat, mouse and human.
Discussion
The aim of the present study was to construct a highly active, hepatocyte-like cell-specific detection tool to identify human, rat, and mouse stem cell-derived hepatocytes. An important criterion for the successful application of tissue-specific detecting elements is that the transcriptional elements should restrict the expression of the reporter gene to hepatocytes. Transcriptional targeting to the liver can be achieved using liver-specific promoters. Some promoters that are expressed mainly in the liver have been used for cell-specific gene delivery (15,16). ALB is an abundant protein in adult serum. It is predominantly synthesized in the liver, and is regulated at the transcriptional level. The ALB promoter shows high homology among a number of species, including rat, mouse, and human. Since promoters do not have a set number of basepairs, the ALB gene core promoter and its main regulatory elements were identified by comparing putative promoter elements in the transcription initiation site, proximal to the 5′-flanking region of the human ALB gene. Within the proximal ALB promoter region, conserved clusters of transcription factor binding sites were identified at −247/+36 (284 bp, Fragment 1), −173/-23 (151 bp, Fragment 2), and −173/+36 (210 bp, Fragment 3).
Plasmids are circular or linear DNA molecules that are templates for chromosomal propagation in bacteria. Evolutionarily shaped plasmids are inherently chimeric, with individual functional units represented by distinct segments in the plasmid genome. The patchwork of plasmid genetic modules is a convenient template, and can be used as a model for the application of artificial plasmid vehicles for gene delivery into mammalian cells (14). We used a plasmid system, which is easy and fast, to probe three different fragments of the human ALB promoter region, fused to a LacZ gene for transient expression. We used separate plasmid vectors to deliver the three human ALB promoter elements into Hepa1-6 cells. In all transfected cells, X-gal staining indicated that the proteins were co-localized intracellularly. Global alignment of the blue staining in cytoplasm of the transfected cells revealed that Fragment 3 exhibited the highest activities of the three fragments. What discriminates the conserved elements from the nonfunctional, non-conserved sequences is unclear, as all three fragments contain a similar repertoire of transcription factor binding sites for liver-expressed transcription factors.
The adenoviral vector is a highly evolved, natural delivery agent for genetic material, and it is commonly used for its remarkable transduction efficiency. Based on Fragment 3, an Albp-ZsGreen adenoviral vector was constructed, and its biological function was confirmed in mouse, rat, and human hepatocytes. The Albp-ZsGreen adenoviral vector worked in Hepa1-6 cells, HepG2 cells, rat primary hepatocytes, and rat BM-MSCs-derived hepatocyte-like cells, which was in good agreement with the ALB protein expression results. These data demonstrated the authenticity of the core region in this fragment of the ALB promoter and showed that it is appropriate for the detection of hepatocyte-like cells in rats, mice and humans.
In conclusion, the present study has identified a cross-species core region of the ALB promoter that can be used as a simple, high-efficiency and general tool for real-time monitoring of the differentiation status of hepatocytes from stem cells in mice, rats, and humans, which is useful for evaluating different protocols to generate functional hepatocytes from stem cells in different species.
Acknowledgements
The present study was funded by the National Natural Scientific Foundations of China (grant no. 81200315).
References
- 1.Kuo TK, Hung SP, Chuang CH, Chen CT, Shih YR, Fang SC, Yang VW, Lee OK. Stem cell therapy for liver disease: Parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111–2121, 2121.e1-e3. doi: 10.1053/j.gastro.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campard D, Lysy PA, Najimi M, Sokal EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833–848. doi: 10.1053/j.gastro.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Li W, Liu B, Wang P, Li W, Zhang H. Efficient generation of functional hepatocyte-like cells from human fetal hepatic progenitor cells in vitro. J Cell Physiol. 2012;227:2051–2058. doi: 10.1002/jcp.22934. [DOI] [PubMed] [Google Scholar]
- 4.Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- 5.Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Quinn G, Okochi H, Ochiya T. Adipose tissue-derived mesenchymal stem cells as a source of human hepatocytes. Hepatology. 2007;46:219–228. doi: 10.1002/hep.21704. [DOI] [PubMed] [Google Scholar]
- 6.Herbomel P, Rollier A, Tronche F, Ott MO, Yaniv M, Weiss MC. The rat albumin promoter is composed of six distinct positive elements within 130 nucleotides. Mol Cell Biol. 1989;9:4750–4758. doi: 10.1128/MCB.9.11.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tronche F, Rollier A, Bach I, Weiss MC, Yaniv M. The rat albumin promoter: Cooperation with upstream elements is required when binding of APF/HNF1 to the proximal element is partially impaired by mutation or bacterial methylation. Mol Cell Biol. 1989;9:4759–4766. doi: 10.1128/MCB.9.11.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loomes KM, Russo P, Ryan M, Nelson A, Underkoffler L, Glover C, Fu H, Gridley T, Kaestner KH, Oakey RJ. Bile duct proliferation in liver-specific Jag1 conditional knockout mice: Effects of gene dosage. Hepatology. 2007;45:323–330. doi: 10.1002/hep.21460. [DOI] [PubMed] [Google Scholar]
- 9.Cereghini S, Raymondjean M, Carranca AG, Herbomel P, Yaniv M. Factors involved in control of tissue-specific expression of albumin gene. Cell. 1987;50:627–638. doi: 10.1016/0092-8674(87)90036-5. [DOI] [PubMed] [Google Scholar]
- 10.Schorpp M, Kugler W, Wagner U, Ryffel GU. Hepatocyte-specific promoter element HP1 of the Xenopus albumin gene interacts with transcriptional factors of mammalian hepatocytes. J Mol Biol. 1988;202:307–320. doi: 10.1016/0022-2836(88)90460-3. [DOI] [PubMed] [Google Scholar]
- 11.Suchanek AL, Salati LM. Construction and evaluation of an adenoviral vector for the liver-specific expression of the serine/arginine-rich splicing factor, SRSF3. Plasmid. 2015;82:1–9. doi: 10.1016/j.plasmid.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Q, Bao J, Zhou YJ, Wang YJ, Du ZG, Shi YJ, Li L, Bu H. Optimizing perfusion-decellularization methods of porcine livers for clinical-scale whole-organ bioengineering. BioMed Res Int. 2015;2015:785474. doi: 10.1155/2015/785474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bao J, Fisher JE, Lillegard JB, Wang W, Amiot B, Yu Y, Dietz AB, Nahmias Y, Nyberg SL. Serum-free medium and mesenchymal stromal cells enhance functionality and stabilize integrity of rat hepatocyte spheroids. Cell Transplant. 2013;22:299–308. doi: 10.3727/096368912X656054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolmachov OE. Building mosaics of therapeutic plasmid gene vectors. Curr Gene Ther. 2011;11:466–478. doi: 10.2174/156652311798192798. [DOI] [PubMed] [Google Scholar]
- 15.Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, Lübbert H, Bujard H. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice; Proc Natl Acad Sci USA; 1996; pp. 10933–10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuriyama S, Yoshikawa M, Ishizaka S, Tsujii T, Ikenaka K, Kagawa T, Morita N, Mikoshiba K. A potential approach for gene therapy targeting hepatoma using a liver-specific promoter on a retroviral vector. Cell Struct Funct. 1991;16:503–510. doi: 10.1247/csf.16.503. [DOI] [PubMed] [Google Scholar]