Abstract
There is a crosstalk between mood disorders and oxidative stress. Chlorpheniramine (CPA), a first generation antihistamine, is hypothesized to have an anxiolytic role at high doses; however, its antidepressant and antioxidant roles have not previously been investigated. The aim of the current study was to evaluate the antidepressant and anxiolytic effects of CPA treatment in association with nitric oxide (NO) and super oxide dismutase (SOD) activity in a mouse model of anxiety. BALB/c mice were divided into unstressed (naïve), control, and CPA- (0.5 mg/kg) and escitalopram- (ESC; 10 mg/kg) treated groups for 3 weeks. Subsequently, they were immobilized for 6 h and subjected to behavioural paradigms as follows: The open field test, the elevated plus maze (EPM) and the forced swim test to investigate motor function, anxiety and depression, respectively. The mice were sacrificed and serum was obtained to detect NO and SOD activity. Compared with the control group, the CPA-treated group demonstrated an antidepressant effect similar to that of the ESC-treated group. In addition, CPA prevented stress-induced NO without affecting SOD activity. CPA did not improve anxiety-like behaviour in the EPM, nor did it improve stress-induced locomotion and rearing, as demonstrated by the OFT. Thus, to the best of our knowledge, this is the first study to evaluate the antidepressant role of CPA in association with NO metabolism. However, further studies are required to elucidate the underlying mechanism.
Keywords: chlorpheniramine, antidepressant, nitric oxide
Introduction
Anxiety and depression are widespread neurological or psychological illnesses with high prevalence rates worldwide. According to epidemiological studies, approximately one-third of people will suffer from anxiety disorders (1). It is well accepted that neurotransmitter deficiency is associated with mood disorders, specifically serotonin and other catecholamines. Therefore, antidepressants, such as the selective serotonin reuptake inhibitor (SSRI), escitalopram (ESC), represent a fundamental pharmacotherapeutic option.
Histamine is a neurotransmitter in the brain with numerous physiological functions; it has also been implicated in anxiety-like behaviour in animal models (2,3). Previous evidence indicates a potential role for the influence of histaminergic neurons in anxiety-associated behaviour via H1, H2 and H3 receptor activation. H3 receptor activation is hypothesized to be involved in diminishing the release of serotonin, dopamine and norepinephrine, which are all implicated in anxiety and mood disorders (3,4). In addition, H3 receptor knockout in mice demonstrated reduced anxiety (5).
The precise role of antihistamines in anxiety and depression remains controversial. First generation antihistamines have been widely used to alleviate anxiety and panic attacks (6). The anxiolytic and antidepressant effects of chlorpheniramine (CPA), a first generation antihistamine, are proposed to be associated with its serotonergic functions (7). Furthermore, the anxiolytic and antidepressant effect of CPA was evident in animal models (8,9). However, these results have been challenged by previous findings (10) in which CPA was demonstrated to exert anxiogenic effects in mice.
In addition to the histaminergic system, growing evidence from animal and human trials implicates oxidative stress in anxiety and stress disorders in the central nervous system and in peripheral tissues (11,12). Oxidative stress is defined as the overproduction of free radicals, such as reactive oxygen species and reactive nitrogen species accompanied by the inability of endogenous defensive antioxidant enzymes to detoxify these free radicals (11). Nitrates, a nitric oxide (NO) metabolite present in different tissues and the chief reactive nitrogen species constituent, is a marker of oxidative stress and is implicated in associated disorders (13); NO increases in acute anxiety (14,15). Furthermore, various animal studies have reported higher NOx in the hippocampus and brain cortex of a stressed animal (16). Furthermore, SSRIs were found to exert an antioxidant role peripherally (17). Super oxide dismutase (SOD) is an abundant antioxidant enzyme responsible for super oxide (O2−) species detoxification to yield hydrogen peroxide (H2O2). SOD activation occurs as a response to an oxidative stress status (18).
Clinically, anxiolytics or antidepressants are used chronically, rather than as a single dose. Although CPA anxiolytic and antidepressant effects were investigated using a single dose (8,18,19), their effects, however, were not evaluated following repeated doses. Additionally, the role of CPA in oxidative stress modulation has yet to be addressed. Therefore, the aim of the present study was to evaluate the protective anxiolytic and antidepressant effects of three weeks of CPA administration in comparison to ESC, and to correlate CPA behavioural effects on serum NOx levels and SOD activity in mice anxiety.
Materials and methods
Study animals and design
Forty BALB/c mice (Animal House Facility of the Applied Science University, Amman, Jordan) were used in the present study. The mice were aged 6–8 weeks and weighed ~25 g. The BALB/c line was selected due to its suitability in anxiety studies (20). Mice were maintained in separate cages, at a temperature of ~25°C, with 50–60% humidity and continuous air ventilation. The research was conducted according to the international ethical standards for the Care and Use of Laboratory Animals and the study was approved by the American University of Madaba research committee (Madaba, Jordan). The mice were divided into four groups (n=10 per group) as follows: Group 1, naïve (unstressed and untreated); group 2, control (treated with distilled water for 3 weeks subsequently stressed); group 3, CPA (treated for 3 weeks and subsequently stressed); group 4, ESC (treated for three weeks and subsequently stressed); and group 5 received a combination of 0.5 mg/kg CPA and 10 mg/kg ESC (treated for three weeks and subsequently stressed). A treatment period of 3 weeks was selected in order to investigate the antidepressant potential. Following completion of the 3-week treatment, groups 2, 3 and 4 were restrained in Falcon tubes for 6 h between 9:00 and 15:00, then all mice were subjected to behavioural tests. The mice were sacrificed, blood samples (~1.5 ml) were collected and the serum was obtained by centrifugation at 2,000 × g for 10 min at room temperature.
Treatment strategies
The CPA-treated group received a dose of 0.5 mg/kg intraperitoneally for 3 weeks. The ESC-treated group received a dose of 10 mg/kg orally for 3 weeks. CPA and ESC were generously donated by the Arab Pharmaceutical Manufacturing Co., Ltd. (Amman, Jordan) and the JOSWE Medical (Amman, Jordan), respectively. The control group received an equal volume of distilled water intraperitoneally for 3 weeks. The majority of similar studies employed acute dosing regimens (1 h prior to the behavioural tests); however, chronic dosing for 3 weeks with ESC has previously been performed (21). In addition, the effect of chronic dosing (at least 2 weeks) was evaluated in the present study to establish the possible antidepressant effect that may resemble clinical settings.
Acute immobility stress
In order to induce anxiety in mice, an acute immobility stress test was performed according to the method of Machawal and Kumar (22) with slight modifications. The current findings indicate that acute immobility stress increases serum levels of nerve growth factor (data not published), which is associated with anxiety (23).
In the present study, the mice were immobilised individually for 6 h in a 50-ml Falcon tube while proper ventilation was maintained. In the naïve group, the mice were maintained in an animal cage with soft bedding under the same experimental conditions. After performing the immobility stress test, the mice were subjected to behavioural tests.
Behavioural tests
Forced swim test (FST)
The FST is the most commonly used behavioural model for screening antidepressant-like activity in rodents (24). Mice were individually forced to swim for 5 min in an open glass chamber (25×15×25 cm3) containing fresh water to a height of 15 cm and maintained at 26±1°C. Floating time (FT) was defined as the time in which mice stop moving completely while in the water.
Elevated plus maze (EPM)
The EPM test, a model for screening anxiolytics, was performed as described previously (25) with certain modifications. The apparatus was elevated 25 cm above the floor. The maze is composed of two closed arms (30×5×10 cm) and two open arms (30×5cm). Mice were placed at the centre, facing the closed arm, and allowed to move freely for 10 min. The frequency of open arm exists (OAE) and the open arm time (OAT) spent were recorded by an experienced technician.
Open field test (OFT)
An OFT was performed to assess the locomotion activity and sedation of the mice (26). Briefly, the mice were placed in a central square and allowed to move freely for 5 min. The field was located in a test room and lit by indirect lighting. The procedure was performed in an empty room to minimise noise and distractions. The open field maze was cleaned between each mouse, using 70% ethyl alcohol. The locomotion activity (represented by the number of lines crossed) and the sedation (represented by the rearing frequency) were recorded by an experienced technician.
Biochemical tests
Subsequent to performing the behavioural tests, the mice were sacrificed, blood (~1.5 ml) was collected and serum was obtained using standard protocols. The accumulation of nitrate, an indicator of the production of NO, was determined using a colorimetric assay with a Griess reagent (27). Serum nitrate was assayed using a Nitric Oxide Assay kit (cat. no. ab65328; Abcam, Cambridge, MA, USA) according to the manufacturer's instructions. The nitrate concentration was obtained according to the standard curve generated after measuring absorbance at a wavelength of 540 nm using a Multiskan™ FC Microplate Photometer (Thermo Fisher Scientific, Waltham, MA, USA). All samples and standards were processed in duplicate.
SOD activity was assayed using an SOD Assay kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany; cat. no. 19160) as previously described (28). The kit utilises Dojindo's highly water-soluble tetrazolium salt, WST-1 [2-(4-Iodophenyl)- 3-(4-nitrophenyl)-5-(2,4-disulfophenyl)- 2H-tetrazolium, monosodium salt], which produces a water-soluble formazan dye upon reduction with a superoxide anion. The rate of the reduction with O2 is linearly associated with the xanthine oxidase activity, and is inhibited by SOD. The SOD activity, as an inhibition activity, is quantified by measuring the decrease in the colour development at a wavelength of 440 nm.
Statistical analysis
All data obtained from the behavioural tests, and the NO and SOD activity were analyzed with a one-way ANOVA and a subsequent Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference and quantitative data are presented as the mean ± standard error of the mean.
Results
FST
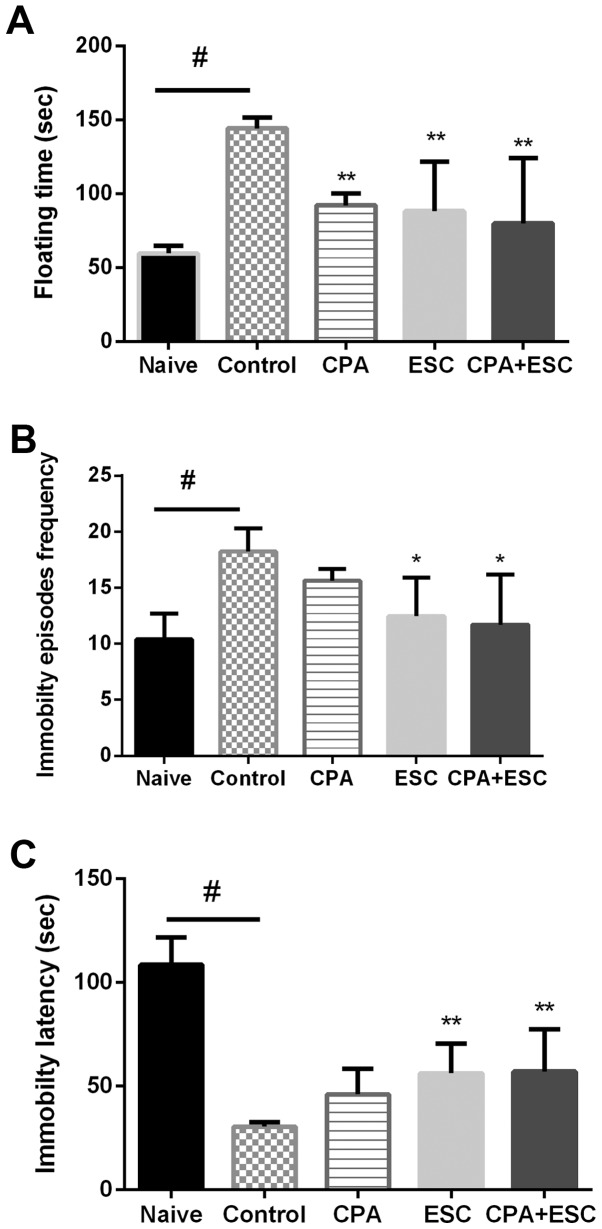
The results of the FST are presented in Fig. 1. The results of the FT revealed that the CPA- and the ESC-treated groups exhibited a similar and significant decrease when compared with the control group. The result of combination therapy did not vary when compared with that of each therapy alone (P>0.05).
Figure 1.
(A) Effect of 3 weeks of treatment with CPA (0.5 mg/kg), ESC (10 mg/kg) and a combination of the two on FT following immobilization stress. Values are expressed as the mean ± SEM (ANOVA followed by Tukey's test). F(4, 45)=10.83; **P<0.05 and #P<0.00001. (B) Effect of treatment with CPA (0.5 mg/kg), ESC (10 mg/kg) and a combination of the two for 3 weeks on the immobility episodes frequency. Each bar represents the mean ± SEM (ANOVA followed by Tukey's test). F(4, 39)=5.037; #P=0.002 and *P<0.05. (C) Effect of treatment with CPA (0.5 mg/kg), ESC (10 mg/kg) and a combination of the two for 3 weeks on immobility latency. Each bar represents the mean ± SEM (ANOVA followed by Tukey's test). F(4, 43)=41.83; #P=0.0001 and **P<0.001. CPA, chlorpheniramine; ESC, escitalopram; FT, floating time; SEM, standard error of the mean.
In addition, the control group demonstrated increased FT when compared with the naïve group (P<0.00001) and the CPA group demonstrated higher FT than the naïve group (Fig. 1A; P<0.05). Regarding the immobility episodes frequency (Fig. 1B), the control group demonstrated a higher frequency of immobility episodes when compared with the naïve group (P<0.05). The ESC-treated group demonstrated a significant decrease in FT when compared with the control group (P<0.05), the CPA-treated group exhibited lower, but non-significant immobility episodes when compared with the control, with no difference between the CPA- and ESC-treated groups (P>0.05). The result of combination therapy did not vary when compared with that of each therapy alone (P>0.05).
The results of the immobility latency (Fig. 1C) revealed significantly lower immobility latency in the control group when compared with the naïve group (P<0.05). The ESC-treated group and the combination therapy group significantly improved the immobility latency (P<0.05). The CPA-treated group demonstrated higher, but non-significant immobility latency when compared with the control group. The result of combination therapy did not vary when compared with that of each therapy alone (P>0.05).
EPM
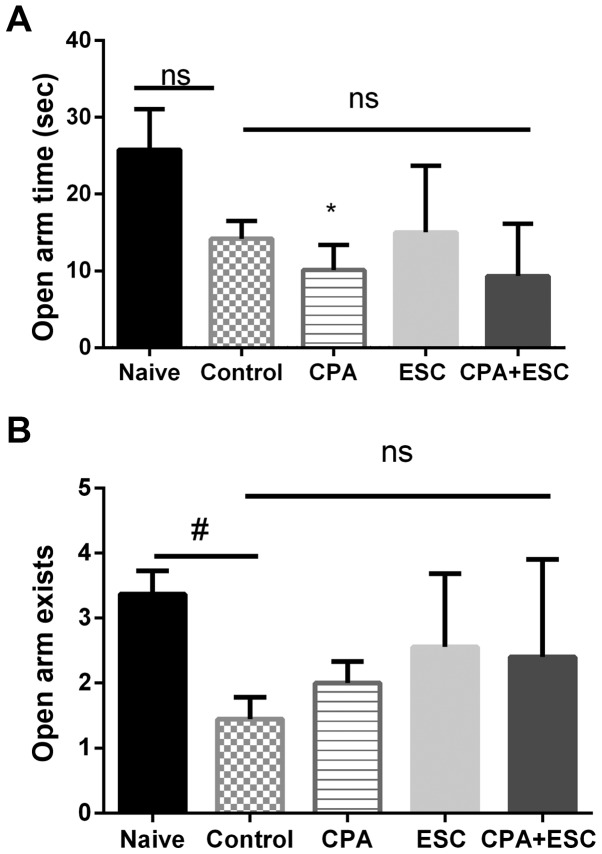
The OAT and OAE were significantly higher in the naïve group when compared with the control group (P<0.05). The administration of CPA (0.5 mg/kg), ESC (10 mg/kg) and a combination of the two for 3 weeks did not alter the OAT and OAE measurements when compared with the control group (P>0.05; Fig. 2A and B).
Figure 2.
(A) Effect of 3 weeks of treatment with CPA (0.5 mg/kg), ESC (10 mg/kg) and a combination of the two on OAT following immobilization stress. Values are expressed as the mean ± SEM (ANOVA followed by Tukey's test). F(4, 38)=3.648; *P<0.05. (B) Effect of treatment with CPA (0.5 mg/kg), ESC (10 mg/kg) and a combination of the two for 3 weeks on OAE. ANOVA followed by Tukey's test. F(4, 39)=3.725; #P<0.05. CPA, chlorpheniramine; ESC, escitalopram; FT, floating time; OAE, open arm exists; SEM, standard error of the mean; ns, non-significant.
OFT
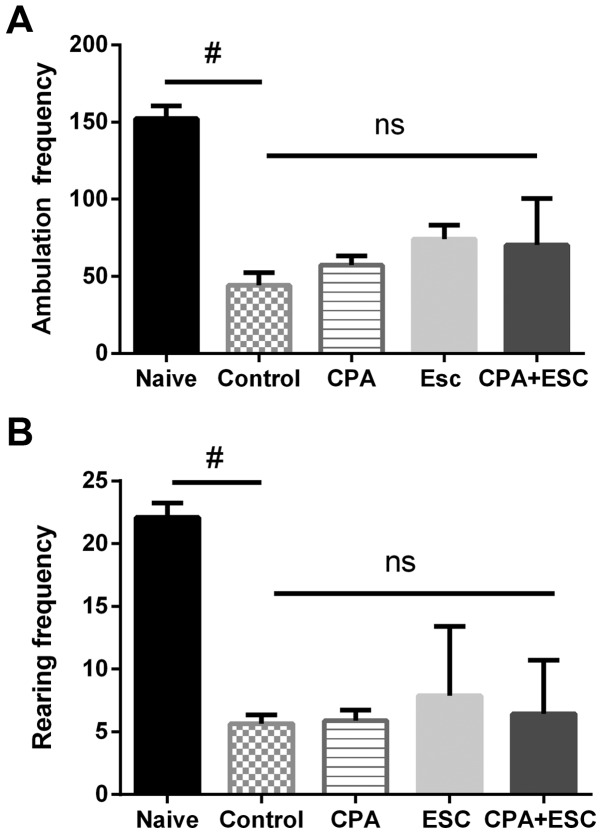
The results demonstrated a significant decrease in the locomotion activity in the control group when compared with the naïve group (P<0.0001). The administration of CPA (0.5 mg/kg), ESC (10 mg/kg) and a combination of the two for 3 weeks did not increase the ambulation frequency (P>0.05). Similar results were observed for the rearing frequency. Acute restraint significantly decreased rearing in the control group when compared with the naïve group (P<0.0001). The administration of CPA (0.5 mg/kg), ESC (10 mg/kg) and a combination of the two for 3 weeks did not increase the rearing frequency (P=0.98; Fig. 3A and B).
Figure 3.
(A) Effect of treatment with CPA (0.5 mg/kg), ESC (10 mg/kg) and a combination of the two for 3 weeks on the ambulation frequency. Values are expressed as the mean ± SEM (ANOVA followed by Tukey's test). F(4,38)=33.21; #P=0.0001. No difference in ambulation frequency was observed between the treated groups and the control despite a trend of increase. (B) Effect of treatment with CPA (0.5 mg/kg), ESC (10 mg/kg) and a combination of the two for 3 weeks on ambulation frequency. Values are expressed as the mean ± SEM (ANOVA followed by Tukey's test). F(4, 41)=33.5; #P=0.0001. CPA, chlorpheniramine; ESC, escitalopram; SEM, standard error of the mean; ns, non-significant.
Biochemical tests
Serum NO
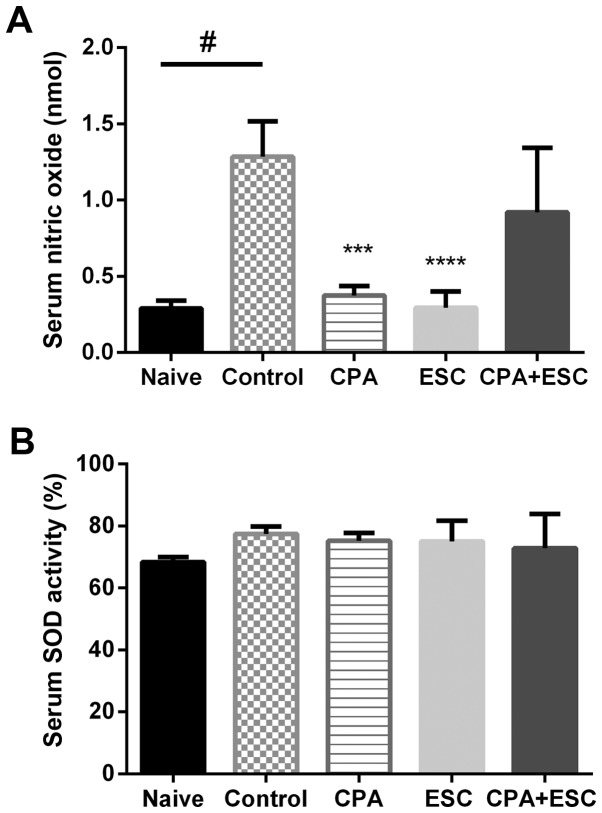
The immobility stress resulted in a significant increase in serum NOx in the control group when compared with the naïve group (P=0.0002). The CPA- and the ESC-treated groups demonstrated lower NOx when compared with the control group (P<0.05). However, the combination therapy did not result in any reduction (P>0.05; Fig. 4A).
Figure 4.
(A) Effect of treatments with CPA (0.5 mg/kg), ESC (10 mg/kg) and a combination of the two for 3 weeks on serum NO following immobilization stress. Values are expressed as the mean ± SEM (ANOVA followed by Tukey's test). F(4, 35)=11.98; #P<0.0001, ***P<0.001 and ****P<0.00001. (B) Effect of treatments with CPA (0.5 mg/kg), ESC (10 mg/kg) and a combination of the two for 3 weeks on serum SOD activity after immobilization stress. Values are expressed as the mean ± SEM (ANOVA followed by Tukey's test). F(4, 35)=1.662; P=0.183. CPA, chlorpheniramine; ESC, escitalopram; SEM, standard error of the mean.
SOD activity
SOD serum activity did not vary among the study groups (P>0.05; Fig. 4B).
Discussion
The current study revealed an antidepressant, but not anxiolytic role of CPA following 3 weeks of administration in mice. Additionally, CPA prevented stress-induced NOx increase, although it did not alter SOD activity with respect to the control.
In the current study, CPA treatment demonstrated antidepressant action similar to that of ESC treatment. A possible explanation could be attributed to its possible serotonergic activity, as CPA shares structural properties with SSRIs (29). Another explanation is that CPA exerts an antidepressant role by enhancing neurotransmitters additional to serotonin, such as norepinephrine (30). A previous st udy (8) demonstrated a CPA antidepressant effect via an FST in rodents following repeated acute doses of 7.5 and 15 mg/kg that were administered in the 24 h prior to the FST. In the current study, a lower dose of CPA (0.5 mg/kg) was used for 3 weeks, which demonstrated antidepressant properties. A reduced dose of CPA was selected in order to minimise the cholinergic and sedative side effects (31). Notably, the combination treatment of CPA with ESC was not synergistic. Typically, synergism is achieved by combining drugs that act on different receptors. Although further studies are required to explain this finding, one hypothesis is that CPA may be competing for the same serotonin transporter as ESC, based on its postulated serotonergic activity.
An unexpected finding was that CPA failed to exert anxiolytic effects in the EPM and the OFT. These findings are consistent with Serafim et al (10) who demonstrated the anxiogenic effect of CPA in mice at various doses. It was proposed that the H1 receptor antagonists supress acetylcholine release from the ventral striatum; furthermore, acetylcholine is associated with anxiety-like behaviours in rodents (10).
Conversely, certain studies reported the anxiolytic role of CPA in animals (25,32). Indeed, it has been suggested that CPA anxiolysis may be attributed to modulation of the serotonergic and cholinergic systems (7). In fact, CPA inhibits the serotonin (5-hydroxytryptamine) transporter (6) and, therefore, inhibits serotonin reuptake.
Our findings may be due to methodological differences; for example, prior to performing the anxiety behavioural tests in the present study, the mice were restrained. Previous findings indicate that acute restraint induces anxiety (22). Therefore, it is hypothesized that the low dose of CPA employed in the present study was insufficient to alleviate anxiety-like behaviours. This explanation is supported by the fact that previous studies did not use immobility stress. Furthermore, other studies employed higher CPA doses (8). Therefore, it may be inferred that a low dose of CPA is only useful in mild anxiety.
Acute anxiety provokes an increase in NOx in various tissues, including the hippocampus, in blood and in saliva (13,14). To the best of our knowledge, this is the first study demonstrating the antioxidant effects of CPA. A novel finding in the current study was the ability of CPA treatment to normalise the stress-induced increase in serum NOx. Similarly, ESC treatment reduced serum NOx. Although the exact mechanism is yet to be established, this may be due to similarities in the mechanisms between CPA and ESC.
Although SOD activity was increased following acute stress induction, neither CPA nor ESC diminished its activity. The exact cause of the rise in SOD activity due to anxiety is yet to be clarified. It may be a compensatory mechanism to overcome oxidative stress. Recently, SOD has been gaining attention in mood disorders. For example, SOD mRNA levels were upregulated following acute stress exposure (31) while in another study, mild chronic stressful events decreased SOD activity in the hippocampus and cortex of mice (33). This may indicate a potential role for the antioxidant enzyme in acute stress. The authors suggest that investigating the transcripts of antioxidant enzymes could be more beneficial than analyzing enzymatic activity. It was reported that long term use of antidepressants upregulated the mRNA expression levels of SOD and other antioxidant enzymes (34).
The present study presents novel ideas, which may lay the foundation for future investigations; however, there were certain limitations. Only a single concentration of CPA was evaluated for its antidepressant and antioxidant effects. Future studies should administer different doses that could determine potential dose-dependent effects and potential synergism between CPA and ESC. Furthermore, future studies may focus on the potential role of CPA or ESC in modulating the levels or the activity of the inducible NO synthase [the enzyme responsible for NOx synthesis under stressful conditions (13)], which may demonstrate the cross talk between mood disorders and oxidative stress in the serum, as well as in the brain cortex, allowing definitive conclusions to be derived.
In conclusion, this is the first study, to the best of our knowledge, describing the antidepressant and the potential antioxidant role of CPA in a mouse model of anxiety. This preliminary finding provides novel hypotheses for future studies regarding depression. Thus, further investigations are required to clarify the underlying mechanisms and the possible implementation in clinical practice.
Acknowledgements
The authors would like to thank Mr. Salem Al-Shawabkeh (Department of Pharmacy, Applied Science University, Amman, Jordan), Dr Amjad Abu Rumaileh (Associate Professor of Pharmacology, Al-Isra University; Amman, Jordan) and Professor S. G. Moscati (Department of Pharmacy, American University of Madaba) for their dedication and efforts. The authors would like to thank Mr. Rafael Angelo Custode (Department of Logistics, American University of Madaba), Dr Moscati and Mr. Nour Hamarneh (Department of Pharmacy, American University of Madaba) for their help with the current study. The project was funded by the American University of Madaba.
References
- 1.Hawgood J, De Leo D. Anxiety disorders and suicidal behaviour: An update. Curr Opin Psychiatry. 2008;21:51–64. doi: 10.1097/YCO.0b013e3282f2309d. [DOI] [PubMed] [Google Scholar]
- 2.Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/S0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 3.Kumar KV, Krishna DR, Palit G. Histaminergic H1 receptors mediate L-histidine-induced anxiety in elevated plus-maze test in mice. Behav Pharmacol. 2007;18:213–217. doi: 10.1097/FBP.0b013e328157f450. [DOI] [PubMed] [Google Scholar]
- 4.Zarrindast MR, Nasehi M, Piri M, Bina P. Anxiety-like behavior induced by histaminergic agents can be prevented by cannabinoidergic WIN55,212-2 injected into the dorsal hippocampus in mice. Pharmacol Biochem Behav. 2010;94:387–396. doi: 10.1016/j.pbb.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 5.Rizk A, Curley J, Robertson J, Raber J. Anxiety and cognition in histamine H3 receptor-/- mice. Eur J Neurosci. 2004;19:1992–1996. doi: 10.1111/j.1460-9568.2004.03251.x. [DOI] [PubMed] [Google Scholar]
- 6.Hellbom E, Humble M. Panic disorder treated with the antihistamine chlorpheniramine. Ann Allergy Asthma Immunol. 2003;90:361. doi: 10.1016/S1081-1206(10)61811-X. 362, author reply 361–362. [DOI] [PubMed] [Google Scholar]
- 7.Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;40:249–258. doi: 10.1016/S0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- 8.Hirano S, Miyata S, Onodera K, Kamei J. Effects of histamine H(1) receptor antagonists on depressive-like behavior in diabetic mice. Pharmacol Biochem Behav. 2006;83:214–220. doi: 10.1016/j.pbb.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Onodera K, Sakurai E, Niwa H. Effect of chlorpheniramine on muricide induced by thiamine deficiency: Pharmacokinetic and behavioral studies. Agents Actions. 1987;20:229–232. doi: 10.1007/BF02074677. [DOI] [PubMed] [Google Scholar]
- 10.Serafim KR, Kishi MS, Canto-de-Souza A, Mattioli R. H1 but not H2 histamine antagonist receptors mediate anxiety-related behaviors and emotional memory deficit in mice subjected to elevated plus-maze testing. Braz J Med Biol Res. 2013;46:440–446. doi: 10.1590/1414-431X20132770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gałecki P, Szemraj J, Bieńkiewicz M, Zboralski K, Gałecka E. Oxidative stress parameters after combined fluoxetine and acetylsalicylic acid therapy in depressive patients. Hum Psychopharmacol. 2009;24:277–286. doi: 10.1002/hup.1014. [DOI] [PubMed] [Google Scholar]
- 12.Wei YC, Zhou FL, He DL, Bai JR, Hui LY, Wang XY, Nan KJ. The level of oxidative stress and the expression of genes involved in DNA-damage signaling pathways in depressive patients with colorectal carcinoma. J Psychosom Res. 2009;66:259–266. doi: 10.1016/j.jpsychores.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Chen HJC, Spiers JG, Sernia C, Lavidis NA. Response of the nitrergic system to activation of the neuroendocrine stress axis. Front Neurosci. 2015;9:3. doi: 10.3389/fnins.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gammoh OS, Al-Smadi A, Al-Awaida W, Badr MM, Qinna NA. Increased salivary nitric oxide and G6PD activity in refugees with anxiety and stress. Stress Health. 2016;32:435–440. doi: 10.1002/smi.2666. [DOI] [PubMed] [Google Scholar]
- 15.Jin L, Qin L, Xia D, Liu X, Fan Z, Zhang C, Gu L, He J, Ambudkar IS, Deng D, Wang S. Active secretion and protective effect of salivary nitrate against stress in human volunteers and rats. Free Radic Biol Med. 2013;57:61–67. doi: 10.1016/j.freeradbiomed.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Fernández AP, Rodrigo J, Boscá L, Leza JC. Chronic stress induces the expression of inducible nitric oxide synthase in rat brain cortex. J Neurochem. 2000;74:785–791. doi: 10.1046/j.1471-4159.2000.740785.x. [DOI] [PubMed] [Google Scholar]
- 17.Matchkov VV, Kravtsova VV, Wiborg O, Aalkjaer C, Bouzinova EV. Chronic selective serotonin reuptake inhibition modulates endothelial dysfunction and oxidative state in rat chronic mild stress model of depression. AJP Regulatory Integrative and Comparative Physiology. 2015;15:R814–R823. doi: 10.1152/ajpregu.00337.2014. [DOI] [PubMed] [Google Scholar]
- 18.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann Bot. 2003;91:179–194. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimazoe T, Shibata S, Ueki S. A new forced swimming test for the evaluation of antidepressants in rats by recording vibration of a water tank. J Pharmacobiodyn. 1987;10:639–643. doi: 10.1248/bpb1978.10.639. [DOI] [PubMed] [Google Scholar]
- 20.Roy V, Belzung C, Delarue C, Chapillon P. Environmental enrichment in BALB/c mice: Effects in classical tests of anxiety and exposure to a predatory odor. Physiol Behav. 2001;74:313–320. doi: 10.1016/S0031-9384(01)00561-3. [DOI] [PubMed] [Google Scholar]
- 21.Matchkov VV, Kravtsova VV, Wiborg O, Aalkjaer C, Bouzinova EV. Chronic selective serotonin reuptake inhibition modulates endothelial dysfunction and oxidative state in rat chronic mild stress model of depression. Am J Physiol Regul Integr Comp Physiol. 2015;309:R814–R823. doi: 10.1152/ajpregu.00337.2014. [DOI] [PubMed] [Google Scholar]
- 22.Machawal L, Kumar A. Possible involvement of nitric oxide mechanism in the neuroprotective effect of rutin against immobilization stress induced anxiety like behaviour, oxidative damage in mice. Pharmacol Rep. 2014;66:15–21. doi: 10.1016/j.pharep.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Gioiosa L, Iannitelli A, Aloe L. Stress, anxiety and schizophrenia and neurotrophic factors: The pioneer studies with nerve growth factor. Riv Psichiatr. 2009;44:88–94. doi: 10.1708/420.4978. [DOI] [PubMed] [Google Scholar]
- 24.Porsolt RD, Bertin A, Jalfre M. ‘Behavioural despair’ in rats and mice: Strain differences and the effects of imipramine. Eur J Pharmacol. 1978;51:291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- 25.Privou C, Knoche A, Hasenöhrl RU, Huston JP. The H1- and H2-histamine blockers chlorpheniramine and ranitidine applied to the nucleus basalis magnocellularis region modulate anxiety and reinforcement related processes. Neuropharmacology. 1998;37:1019–1032. doi: 10.1016/S0028-3908(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 26.Dishman RK, Armstrong RB, Delp MD, Graham RE, Dunn AL. Open-field behavior is not related to treadmill performance in exercising rats. Physiol Behav. 1988;43:541–546. doi: 10.1016/0031-9384(88)90206-5. [DOI] [PubMed] [Google Scholar]
- 27.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- 28.Omori A, Yoshimura Y, Deyama Y, Suzuki K. Rosmarinic acid and arbutin suppress osteoclast differentiation by inhibiting superoxide and NFATc1 downregulation in RAW 264.7 cells. Biomed Rep. 2015;3:483–490. doi: 10.3892/br.2015.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Domino EF. History of modern psychopharmacology: A personal view with an emphasis on antidepressants. Psychosom Med. 1999;61:591–598. doi: 10.1097/00006842-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berl) 1999;147:162–167. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- 31.Kala M, Nivsarkar M. Role of cortisol and superoxide dismutase in psychological stress induced anovulation. Gen Comp Endocrinol. 2016;225:117–124. doi: 10.1016/j.ygcen.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 32.Miyata S, Hirano S, Ohsawa M, Kamei J. Chlorpheniramine exerts anxiolytic-like effects and activates prefrontal 5-HT systems in mice. Psychopharmacol. 2011;213:441–452. doi: 10.1007/s00213-009-1695-0. [DOI] [PubMed] [Google Scholar]
- 33.Biala G, Pekala K, Boguszewska-Czubara A, Michalak A, Kruk-Slomka M, Budzynska B. Behavioral and biochemical interaction between nicotine and chronic unpredictable mild stress in mice. Mol Neurobiol. 2017;54:904–921. doi: 10.1007/s12035-016-9701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt AJ, Heiser P, Hemmeter UM, Krieg JC, Vedder H. Effects of antidepressants on mRNA levels of antioxidant enzymes in human monocytic U-937 cells. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1567–1573. doi: 10.1016/j.pnpbp.2008.05.024. [DOI] [PubMed] [Google Scholar]