Abstract
Cancer remains a leading cause of mortality worldwide, therefore food products are being investigated for potential prevention or treatment strategies. The ingredient, barley grass extract (Hordeum vulgare L.; Bex) is used to prevent or ameliorate various types of disease. In cancer, Bex has been revealed to inhibit tumor growth. However, its effect on cancer cells is yet to be clearly defined. In the present study, the effect of Bex on cancer cell growth was investigated. Bex inhibited the viabilities of breast and prostate cancer cells according to the results of MTT assays. Accordingly, Bex caused apoptosis, which was confirmed by Annexin V staining and western blot analysis for poly (ADP-ribose) polymerase and caspases. Furthermore, Bex increased the intracellular levels of reactive oxygen species (ROS), and N-acetyl-L-cystein blocked Bex-induced apoptosis. Therefore, the study demonstrated that Bex causes apoptosis of breast and prostate cancer cells by increasing intracellular ROS levels.
Keywords: barley grass extract, cancer, reactive oxygen species
Introduction
Cancer is one of the leading causes of mortality worldwide (1,2). Despite numerous cancer studies and the development of various anti-cancer therapeutic agents, cancer remains dangerous. Anti-cancer therapeutic agents are chemically or biologically produced, and their effects are well defined (3–7). However, treatments continue to be associated with adverse effects and the majority of patients have an aversion to them (8).
Herbal products have long been used to prevent or treat diseases, including cancer (9–12). Furthermore, certain anti-cancer therapeutic agents that are chemically produced originate from herbal products and their chemical characteristics are modified (7,12–14). Typically, patients prefer to take herbal products (15–18); herbal products have historically been used as traditional medicines, such as traditional Chinese and Korean medicines, Kampo medicines and Ayurvedic medicine (13,14,19). Certain herbal products were demonstrated to treat cancer and/or reduce the side effects of cancer treatment (13,15–17,19–21). Therefore, herbal products are considered to be promising for cancer prevention and treatment.
Barley grass extract (Hordeum vulgare L.; Bex) has long been used as a food product. Its biological effects have also been addressed by various in vitro and in vivo studies, although evidence there is limited evidence of the efficacy of Bex against specific conditions (22). The effect of Bex on the immune system was revealed in in vitro and in vivo experimental sets (23–25). Accordingly, Bex inhibited atopic dermatitis in NC/Nga mice by altering the expression levels of cytokines (26). Similarly, Bex repressed lipopolysaccharide-induced inflammation (27). Furthermore, its effect in type 2 diabetes was revealed in a genetically engineered mouse model and patients (28,29). Therefore, the effects of Bex on particular diseases have been demonstrated at least in experimental systems. A previous study revealed that Bex caused apoptosis of leukemia and lymphoma cell lines (30); however, its effect in cancer remains unclear.
The present study examined the effect of Bex in different cancer cell lines, including breast cancer MDA-MB-231 cells and prostate cancer DU145 cells. Bex induced apoptotic cell death in MDA-MB-231 and DU145 cells. Furthermore, its effect resulted from an increased intracellular reactive oxygen species (ROS) level. Thus, the current study suggests that Bex may be useful for treating cancer, particularly breast and prostate cancer.
Materials and methods
Cell culture and herbal extract
MDA-MB-231 and DU-145 cells (American Type Culture Collection, Manassas, VA, USA) were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum and 1% penicillin-streptomycin (all Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Barley grass extract (Bex) was obtained from Chungbuk Agricultural Cooperation (Jecheon, South Korea). Bex was solubilized in water containing 0.01% dimethyl sulfoxide (DMSO). Therefore, all control groups in the experiment were treated with 0.01% DMSO.
Cell viability assay
Cell viability was examined using an EZ-CYTOX cell viability/cytotoxicity assay kit (cat. no. EZ-000, Daeil Lab Service, Seoul, South Korea) according to the manufacturer's instructions. Briefly, 100,000 cells per well were cultured in 96-well plates and treated with different doses (0, 0.01, 0.1, 1, 10, 100, 250 and 500 µg/ml) of Bex for 72 h. Cell viability at 24, 48 and 72 h was measured using a microplate reader at a wavelength of 450 nm. Experiments were performed in triplicate and repeated three times independently.
Apoptosis assay
Cells (3×106) were treated with different concentrations (0, 0.01, 0.1, 1, 10, 100, 250 and 500 µg/ml) of Bex for 24 h and stained with Annexin V-fluorescein isothiocyanate (FITC) and 7-aminoactinomycin D (7-AAD). Apoptotic cell death was determined using BD FACSCalibur flow cytometry with BD MultiSET software (BD Biosciences, San Jose, CA, USA). For western blot analysis, 1×106 cells were treated with 100 µg/ml Bex for 24 h and lysed with RIPA buffer. Protein (30 µg) was loaded onto SDS-PAGE and transferred to the membrane. After blocking with 5% milk, the membrane was incubated with an appropriate primary antibody for 1 h at room temperature. Anti-poly(ADP-ribose) polymerases (PARP) (cat. no. 9542), anti-cleaved caspase 8 (cat. no. 9496), anti-cleaved caspase 9 (cat. no. 7237), anti-cleaved caspase-3 (cat. no. 9664) and anti-β-tubulin (cat. no. 2146) antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Intracellular ROS detection assay
ROS levels were measured using 10 µM 2′,7′-dichlorofluorescin diacetate (H2DCF-DA; Molecular Probes; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cells (1×106) were treated with 100 µg/ml Bex for 5 min and treated with H2DCF-DA for a further 24 h. The flow cytometry experiments were conducted in triplicate and repeated three times independently. Sigma-Aldrich N-acetyl-L-cystein (NAC; Merck KGaA, Darmstadt, Germany) at 10 mM was used to inhibit ROS induction. Cells (1×106) were pretreated with 10 mM NAC for 1 h before being treated with 100 µg/ml Bex for 24 h.
Statistical analysis
All experiments were performed in triplicate and repeated three times independently. Statistical significance was evaluated using Student's t-test and analysis was conducted using SPSS version 24.0 software (IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Bex inhibits cancer cell viability
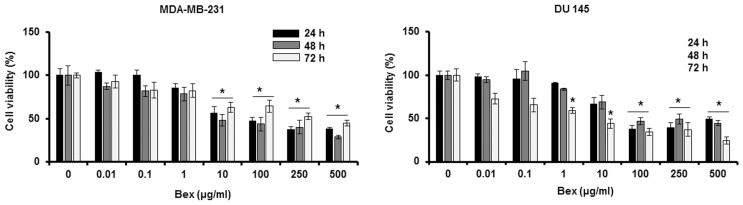
To examine the effect of Bex on cancer cell viability, MDA-MB-231 breast cancer cells and DU-145 prostate cancer cells were treated with different concentrations (0, 0.01, 0.1, 1, 10, 100, 250 and 500 µg/ml) of Bex for 24, 48 and 72 h. Bex reduced the viability of those cancer cells in a dose-dependent manner (Fig. 1). Thus, the MTT assay data indicates that Bex inhibits cancer cell viability.
Figure 1.
Bex reduces MDA-MB-231 and DU-145 cell viabilities. Cells were treated with different concentrations (0, 0.01, 0.1, 1, 10, 100, 250 and 500 µg/ml) of Bex for 72 h. Cell viabilities were measured at 24, 48 and 72 h. *P<0.05 vs. 0 µg/ml. Bex, barley grass extract.
Bex causes apoptosis of cancer cells
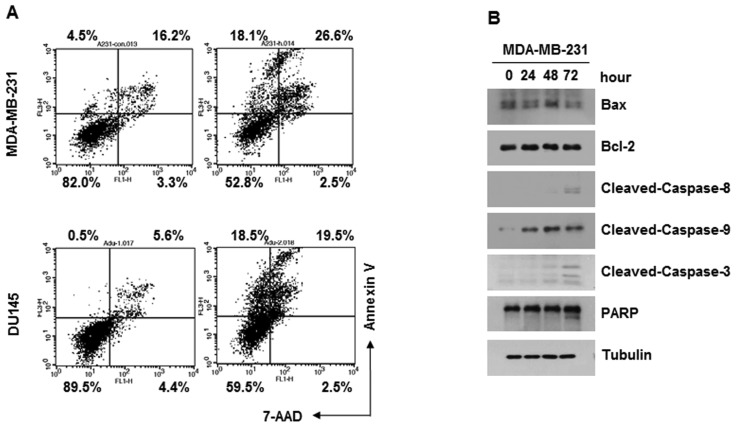
Annexin V assays were performed to examine whether Bex induces apoptosis of cancer cells. MDA-MB-231 or DU-145 cells were treated with 100 µg/ml Bex for 24 h, followed by Annexin V-FITC and 7-AAD. Flow cytometry data indicated that Bex induced apoptosis of the two types of cancer cell (Fig. 2A). Consistently, Bex induced PARP cleavage and caspase activation in the MDA-MB-231 cells (Fig. 2B; data for DU-145 not shown). Thus, the current data indicates that Bex causes apoptosis of cancer cells.
Figure 2.
Bex induces apoptotic cell death. (A) Cells were treated with 100 µg/ml Bex for 24 h and subjected to Annexin V-fluorescein isothiocyanate and 7-AAD staining. (B) MDA-MB-231 cells were treated with 100 µg/ml Bex for 24 h and subjected to western blot analysis. Bex, barley grass extract; 7-AAD, 7-aminoactinomycin D; Bax, BCL2 associated X, apoptosis regulator; Bcl-2, B-cell lymphoma 2; PARP, poly (ADP-ribose) polymerase.
Bex reduces the level of intracellular ROS
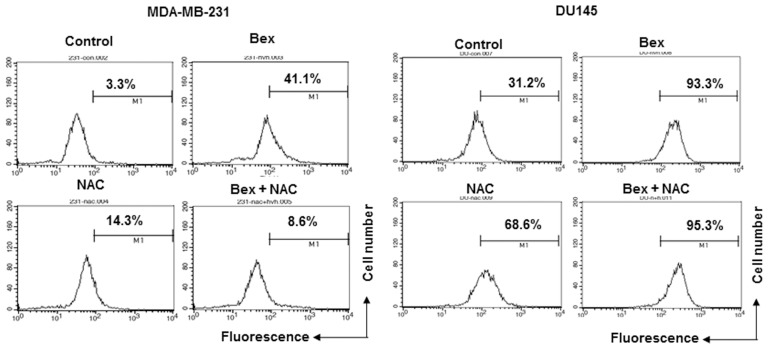
To examine whether Bex affects the production of intracellular ROS, MDA-MB-231 and DU-145 cells were treated with 100 µg/ml Bex for 1 h, and the intracellular ROS level was measured by detecting H2DCF-DA fluorescence using flow cytometry. Bex increased the intracellular ROS level in MDA-MB-231 breast and DU-145 prostate cancer cells (Fig. 3).
Figure 3.
Bex increases intracellular reactive oxygen species level. MDA-MB-231 and DU-145 cells were pretreated with 10 mM NAC for 1 h before being treated with 100 µg/ml Bex for 24 h. Bex, barley grass extract; NAC, N-acetyl-L-cystein.
Bex-mediated increase of intracellular ROS level is critical for apoptosis
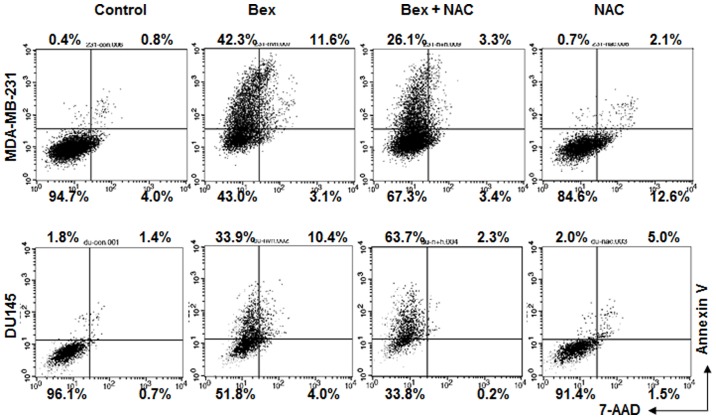
Whether Bex-induced apoptosis required an increase of intracellular ROS level was subsequently examined. Bex-induced apoptosis was blocked in cells treated with NAC (Fig. 4). The data indicate that Bex-induced ROS accumulation is important for apoptosis.
Figure 4.
Bex-induced apoptosis requires intracellular reactive oxygen species production. Cells were treated with Bex for 24 h with or without NAC, and then subjected to Annexin V-fluorescein isothiocyanate and 7-AAD staining. Bex, barley grass extract; NAC, N-acetyl-L-cystein; 7-AAD, 7-aminoactinomycin D.
Discussion
Bex has long been incorporated into diets for disease prevention. However, to the best of our knowledge, its effects in cancer are yet to be investigated. In the present study, Bex caused apoptosis of breast and prostate cancer cells by increasing the intracellular ROS level. The present data indicate that cancer could be, in part, treated using natural products in food. Bex is widely used in food. Therefore, the present study demonstrates that foods containing Bex may be useful for cancer treatment during therapeutic interventions.
A recent study demonstrated that Bex induced apoptosis of leukemia and lymphoma cell lines (30). While not shown in the present study, the data demonstrated no apoptotic effect of Bex in Jurkat T cells (data not shown). It is possible that the experimental conditions, such as the extraction method and concentration, may have influenced the controversial results. In the present study, Bex induced apoptotic cell death of highly metastatic MDA-MB-231 breast cancer cells and DU-145 prostate cancer cells. Thus, the anti-cancer effect of Bex is not limited to blood cancer. This is consistent with results obtained using Bex-treated B16 melanoma cells or HepG2 hepatoma cells (31,32). Bex is one of the ingredients in cereal and the anti-cancer effect of peptides from cereal has previously been demonstrated (33). Furthermore, meta-analyses indicated that cereal reduces cancer risk (34,35). Thus, the present study provides evidence that dietary components are beneficial for cancer prevention and treatment.
In conclusion, Bex induction of ROS was crucial for apoptotic cell death. While the chemical components to produce ROS and induce apoptotic cell death in those breast and prostate cancer cells require further investigation, this is the first study, to the best of our knowledge, that shows the role of Bex in cancer cell death.
Acknowledgements
The present study was supported by the Korean National University of Transportation 2016.
References
- 1.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 2.McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr. 2016;7:418–419. doi: 10.3945/an.116.012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv Drug Deliv Rev. 2017;1:25–38. doi: 10.1016/j.addr.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 4.Dawidczyk CM, Kim C, Park JH, Russell LM, Lee KH, Pomper MG, Searson PC. State-of-the-art in design rules for drug delivery platforms: Lessons learned from FDA-approved nanomedicines. J Control Release. 2014;187:133–144. doi: 10.1016/j.jconrel.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellou S, Pentheroudakis G, Murphy C, Fotsis T. Anti-angiogenesis in cancer therapy: Hercules and hydra. Cancer Lett. 2013;338:219–228. doi: 10.1016/j.canlet.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Ali I, Wani WA, Saleem K, Haque A. Platinum compounds: A hope for future cancer chemotherapy. Anticancer Agents Med Chem. 2013;13:296–306. doi: 10.2174/1871520611313020016. [DOI] [PubMed] [Google Scholar]
- 7.Fauzee NJ. Taxanes: Promising anti-cancer drugs. Asian Pac J Cancer Prev. 2011;12:837–851. [PubMed] [Google Scholar]
- 8.Vera-Badillo FE, Al-Mubarak M, Templeton AJ, Amir E. Benefit and harms of new anti-cancer drugs. Curr Oncol Rep. 2013;15:270–275. doi: 10.1007/s11912-013-0303-y. [DOI] [PubMed] [Google Scholar]
- 9.Ghorbani A. Clinical and experimental studies on polyherbal formulations for diabetes: Current status and future prospective. J Integr Med. 2014;12:336–345. doi: 10.1016/S2095-4964(14)60031-5. [DOI] [PubMed] [Google Scholar]
- 10.Jiang M, Yang J, Zhang C, Liu B, Chan K, Cao H, Lu A. Clinical studies with traditional Chinese medicine in the past decade and future research and development. Planta Med. 2010;76:2048–2064. doi: 10.1055/s-0030-1250456. [DOI] [PubMed] [Google Scholar]
- 11.Ilyas U, Katare DP, Aeri V, Naseef PP. A review on hepatoprotective and immunomodulatory herbal plants. Pharmacogn Rev. 2016;10:66–70. doi: 10.4103/0973-7847.176544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahmani M, Shirzad H, Shahinfard N, Sheivandi L, Rafieian-Kopaei M. Cancer phytotherapy: Recent views on the role of antioxidant and angiogenesis activities. J Evid Based Complementary Altern Med. 2016 doi: 10.1177/2156587215625157. 2156587215625157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu H, Zhao X, Liu X, Xu P, Zhang K, Lin X. Antitumor effects of traditional Chinese medicine targeting the cellular apoptotic pathway. Drug Des Devel Ther. 2015;9:2735–2744. doi: 10.2147/DDDT.S80902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ichikawa H, Nakamura Y, Kashiwada Y, Aggarwal BB. Anticancer drugs designed by mother nature: Ancient drugs but modern targets. Curr Pharm Des. 2007;13:3400–3416. doi: 10.2174/138161207782360492. [DOI] [PubMed] [Google Scholar]
- 15.Poonthananiwatkul B, Howard RL, Williamson EM, Lim RH. Cancer patients taking herbal medicines: A review of clinical purposes, associated factors, and perceptions of benefit or harm. J Ethnopharmacol. 2015;175:58–66. doi: 10.1016/j.jep.2015.08.052. [DOI] [PubMed] [Google Scholar]
- 16.Lee JW, Lee WB, Kim W, Min BI, Lee H, Cho SH. Traditional herbal medicine for cancer pain: A systematic review and meta-analysis. Complement Ther Med. 2015;23:265–274. doi: 10.1016/j.ctim.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 17.Leggett S, Koczwara B, Miller M. The impact of complementary and alternative medicines on cancer symptoms, treatment side effects, quality of life, and survival in women with breast cancer - a systematic review. Nutr Cancer. 2015;67:373–391. doi: 10.1080/01635581.2015.1004731. [DOI] [PubMed] [Google Scholar]
- 18.Bao Y, Kong X, Yang L, Liu R, Shi Z, Li W, Hua B, Hou W. Complementary and alternative medicine for cancer pain: An overview of systematic reviews. https://doi.org/10.1155/2014/170396. eCAM. 2014:170396. doi: 10.1155/2014/170396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CY, Bai XY, Wang CH. Traditional Chinese medicine: A treasured natural resource of anticancer drug research and development. Am J Chin Med. 2014;42:543–559. doi: 10.1142/S0192415X14500359. [DOI] [PubMed] [Google Scholar]
- 20.Ohnishi S, Takeda H. Herbal medicines for the treatment of cancer chemotherapy-induced side effects. Front Pharmacol. 2015;6:14. doi: 10.3389/fphar.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park B, Jun JH, Jung J, You S, Lee MS. Herbal medicines for cancer cachexia: Protocol for a systematic review. BMJ Open. 2014;4:e005016. doi: 10.1136/bmjopen-2014-005016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lahouar L, El-Bok S, Achour L. Therapeutic potential of young green barley leaves in prevention and treatment of chronic diseases: An overview. Am J Chin Med. 2015;43:1311–1329. doi: 10.1142/S0192415X15500743. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki Y, Tokunaga Y, Takagaki K, Tsusaki S, Tachibana H, Yamada K. Effect of dietary cabbage fermentation extract and young barley leaf powder on immune function of Sprague-Dawley rats. J Nutr Sci Vitaminol (Tokyo) 2001;47:253–257. doi: 10.3177/jnsv.47.253. [DOI] [PubMed] [Google Scholar]
- 24.Cremer L, Herold A, Avram D, Szegli G. Inhibitory capacity of some fractions isolated from a green barley extract upon TNF alpha production by the cells of the THP-1 human monocytes line. Roum Arch Microbiol Immunol. 1996;55:285–294. [PubMed] [Google Scholar]
- 25.Cremer L, Herold A, Avram D, Szegli G. A purified green barley extract with modulatory properties upon TNF alpha and ROS released by human specialised cells isolated from RA patients. Roum Arch Microbiol Immunol. 1998;57:231–242. [PubMed] [Google Scholar]
- 26.Iguchi T, Kawata A, Watanabe T, Mazumder TK, Tanabe S. Fermented barley extract suppresses the development of atopic dermatitis-like skin lesions in NC/Nga mice, probably by inhibiting inflammatory cytokines. Biosci Biotechnol Biochem. 2009;73:489–493. doi: 10.1271/bbb.80436. [DOI] [PubMed] [Google Scholar]
- 27.Choi KC, Hwang JM, Bang SJ, Son YO, Kim BT, Kim DH, Lee SA, Chae M, Kim DH, Lee JC. Methanol extract of the aerial parts of barley (Hordeum vulgare) suppresses lipopolysaccharide-induced inflammatory responses in vitro and in vivo. Pharm Biol. 2013;51:1066–1076. doi: 10.3109/13880209.2013.768274. [DOI] [PubMed] [Google Scholar]
- 28.Hong H, Jai Maeng W. Effects of malted barley extract and banaba extract on blood glucose levels in genetically diabetic mice. J Med Food. 2004;7:487–490. doi: 10.1089/jmf.2004.7.487. [DOI] [PubMed] [Google Scholar]
- 29.Yu YM, Chang WC, Chang CT, Hsieh CL, Tsai CE. Effects of young barley leaf extract and antioxidative vitamins on LDL oxidation and free radical scavenging activities in type 2 diabetes. Diabetes Metab. 2002;28:107–114. [PubMed] [Google Scholar]
- 30.Robles-Escajeda E, Lerma D, Nyakeriga AM, Ross JA, Kirken RA, Aguilera RJ, Varela-Ramirez A. Searching in mother nature for anti-cancer activity: Anti-proliferative and pro-apoptotic effect elicited by green barley on leukemia/lymphoma cells. PLoS One. 2013;8:e73508. doi: 10.1371/journal.pone.0073508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghavami L, Goliaei B, Taghizadeh B, Nikoofar A. Effects of barley β-glucan on radiation damage in the human hepatoma cell line HepG2. Mutat Res Genet Toxicol Environ Mutagen. 2014;775:1–6. doi: 10.1016/j.mrgentox.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Meng TX, Irino N, Kondo R. Melanin biosynthesis inhibitory activity of a compound isolated from young green barley (Hordeum vulgare L.) in B16 melanoma cells. J Nat Med. 2015;69:427–431. doi: 10.1007/s11418-015-0902-z. [DOI] [PubMed] [Google Scholar]
- 33.Ortiz-Martinez M, Winkler R, García-Lara S. Preventive and therapeutic potential of peptides from cereals against cancer. J Proteomics. 2014;111:165–183. doi: 10.1016/j.jprot.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 34.Lei Q, Zheng H, Bi J, Wang X, Jiang T, Gao X, Tian F, Xu M, Wu C, Zhang L, et al. Whole grain intake reduces pancreatic cancer risk: A meta-analysis of observational studies. Medicine (Baltimore) 2016;95:e2747. doi: 10.1097/MD.0000000000002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716. doi: 10.1136/bmj.i2716. [DOI] [PMC free article] [PubMed] [Google Scholar]