Abstract
Combined heart–liver transplantation is a rare, life-saving procedure that treats complex and often fatal diseases including familial amyloidosis polyneuropathy and late stage congenital heart disease status-post previous repair. There were 159 combined heart–liver transplantations performed between January 1, 1988 and October 3, 2014 in the United States. A multitude of potential techniques to be used for combined heart and liver transplant including: orthotopic heart transplant (OHT) and orthotopic liver transplant (OLT) on full cardiopulmonary bypass (CPB), OHT with CPB and OLT with venovenous bypass (VVB), OHT with CPB and OLT without VVB, enbloc technique and sequential transplantation. Outcomes of combined heart–liver transplant have been demonstrated to be comparable to outcomes of isolated heart and isolated liver transplant. The liver graft may provide some tolerance of other allografts © 2016 Elsevier Inc. All rights reserved.
1. Introduction
The first combined heart–liver transplantation (CHLT) was performed in a six year-old female with familial hypercholesterolemia and heart failure secondary to coronary artery disease and was described by Starzl et al. in 1984 [1]. This index case patient survived for eight years post-transplant; the next two died shortly after transplant [2]. The second CHLT was a 2 year-old female with end-stage cardiomyopathy and biliary hypoplasia who underwent CHLT followed by low cardiac output and acidosis requiring intra-aortic balloon pump and finally re-transplant of the heart within 24 h. She showed initial improvement, but died shortly after. The third CHLT was a 17 year-old female with familial hypercholesterolemia with significant history of abdominal and cardiac surgery including portacaval shunt, aortic valve replacement, coronary artery bypass grafting, mitral valve replacement, periprosthetic valve leak repair, and prosthetic valve replacement. Immediately after her CHLT was performed there was compression of the heart and the chest and abdomen were reopened and partial liver resection performed. She was never able to be weaned from cardiopulmonary bypass (CPB) [2].
There were 192 combined heart–liver transplantations (CHLT) performed in the United States between January 1, 1988 and December 31, 2015 [3]. The majority of these were performed at high-volume centers: Mayo Clinic (n = 33), Hospital of the University of Pennsylvania (n = 31), University of Pittsburgh Medical Center (n = 14), University of Chicago Medical Center (n = 13), Methodist Hospital (n = 13), Cedars-Sinai Medical Center (n = 9). The remaining 33 centers performed 7 or less CHLTs each [4].
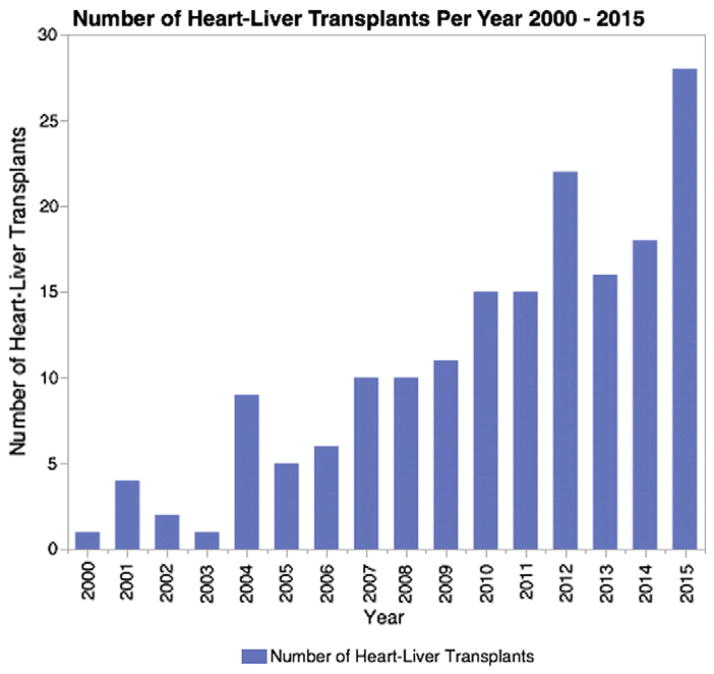
Graft survival after CHLT is similar to that of isolated heart and isolated liver transplantation, with 1-year survival greater than 80% and 10-year survival greater than 70% [5,6]. Here, we will review the past and current medical literature on the CHLT procedure with a focus on indications, procedure and outcome to assist clinicians in evaluating patients for CHLT, discussing potential complications and prognosis, and in procedural planning. Although still a rare procedure, it is increasing in frequency (Fig. 1) [3]. Therefore there is need to understand current indications and outcomes.
Fig. 1.
Frequency of combined heart–liver transplantations in the United States between January 1, 2000 and December 31, 2015 as reported to the OPTN [4].
The literature search was begun with a search of PubMed. Each of the citations for the papers originally pulled was then reviewed for additional articles for inclusion.
2. Indications
Current indications for CHLT include end-stage heart and liver disease of varying etiology, end-stage heart and liver disease of related etiology, and end-stage heart disease with liver transplantation performed to prevent damage to the cardiac allograft [7]. Familial amyloid polyneuropathy (FAP) and heart failure with associated cardiac cirrhosis are the most common indications for CHLT [5–8]. Additional indications reported in the medical literature are listed in Tables 1 and 2.
Table 1.
Indications for Combined Heart–Liver Transplantation (CHLT) As Reported in the Literature.
| Indication for Combined Heart–Liver Transplantation | |
|---|---|
| Familial amyloidosis [5–10,12,14,15,30,34,35,52] | |
| Familial hypercholesterolemia [1,2,17,19,21,35] | |
| Homozygous beta-thalassemia [19,35] | |
| Hemochromatosis [5,8,15,27,35,53] | |
| Alcoholic cardiomyopathy [35] | |
| Cryptogenic cirrhosis with underlying cardiomyopathy [35] | |
| Glycogen storage disease [8] | |
| Indications for Heart Portion | Indications for Liver Portion |
|
| |
| failure of single ventricle variations of congenital heart disease [22–25,40] | congestive hepatopathy or cardiac cirrhosis [5–8,15,22–24,29,31,36,37,40,54] |
| congenital heart disease [5,7,8,15,31] | hepatitis C [5,6,8,31,35,37,42] |
| idiopathic restrictive cardiomyopathy [5,7,8] | cryptogenic cirrhosis [5,6,8,33,35,38,44,55] |
| restrictive cardiomyopathy[15,37] | alcoholic cirrhosis [5,6,8,33,36,44,56] |
| arrhythmogenic right ventricular dysplasia [7] | chronic hepatitis — other [5] |
| coronary artery disease [8] | acute hepatic failure [5] |
| nonischemic cardiomyopathy [29,31] | primary biliary cirrhosis [5,6,8,15] |
| ischemic cardiomyopathy [31,33,36,37] | primary sclerosing cholangitis [5,6,8] |
| idiopathic cardiomyopathy [2,44] | biliary atresia/hypoplasia [2,5] |
| alcoholic cardiomyopathy [8,35] | metabolic disease – other [5] |
| viral dilated cardiomyopathy [8] | cirrhosis — other [5,25] |
| idiopathic dilated cardiomyopathy [5,8] | Carcinoid [5] |
| ischemic dilated cardiomyopathy [5] | cystic fibrosis [5] |
| alcoholic dilated cardiomyopathy [5] | Budd–Chiari syndrome [5,8] |
| dilated cardiomyopathy [33,36,37,42,44,56] | unknown [5] |
| apical variant hypertrophic cardiomyopathy [7] | alpha 1-antitrypsin [6,8,31] |
| hypertrophic cardiomyopathy [5,37,38] | autoimmune hepatitis [8] |
| valvular disease [5,8,35] | nodular regenerative hyperplasia [8] |
| primary pulmonary hypertension [5,15] | |
| glycogen storage disease [5] | |
| cystic fibrosis [5] | |
| sarcoidosis [5] | |
| unknown [5] | |
Table 2.
Combined Heart–Liver Transplantation (CHLT) Reviews in the Literature.
| Author (citation) | Te et al. [8] | Cannon et al. [5] |
|---|---|---|
| Year | 2008 | 2014 |
| # of Cases Included | 47 | 97 |
| Age | Mean 46, Range 22–65 | Mean 43.7 SD 16 Range 1–67 |
| Gender | 31 (67%) Male, 16 (33%) Women | 68 (70.1%) Male |
| BMI | NR | Mean 24.3 SD 5.1 |
| Number w/ Associated Kidney Tx | 6 | NR |
| MELD at Transplant | NR | Mean 13.8 SD 5.4 |
| Patients with MELD exception | NR | 17 (26.2%) |
| Follow-up | Mean 1362 days/3.7 year, Range 0–12.6 year | NR |
| Liver Graft Failure | NR | 12 of 97 |
| Cardiac Graft Failure | NR | 1 of 97 |
| Patient Survival, 1-year | 84.80% | 84.40% |
| Patient Survival, 3-year | 79.50% | 78.00% |
| Patient Survival, 5-year | 75.60% | 72.40% |
| Heart Graft Survival, 1-year | 84.80% | 83.00% |
| Heart Graft Survival, 5-year | 75.60% | 73.20% |
| Heart Graft Survival, 10-year | NR | 71.50% |
| Liver Graft Survival, 1-year | 82.40% | 83.40% |
| Liver Graft Survival, 3-year | 77.30% | NR |
| Liver Graft Survival, 5-year | 73.50% | 72.80% |
| Liver Graft Survival, 10-year | NR | 71.00% |
In a series of 27 CHLTs, 21 (78%) were performed for FAP [7]. In an analysis of the United Network for Organ Sharing (UNOS) database between 1988 and 2005 FAP was the most common indication (30%) [8]. In FAP, there is increase production of mutant transthyretin from the liver resulting in abnormal accumulation in the peripheral nervous system and in end-organs, such as the heart, soft tissues, urinary bladder and gastrointestinal tract [7,9,10]. In cases of significant heart involvement with TTR deposition, CHLT is considered in order to prevent accumulation in the cardiac allograft [5]. Some patients with FAP also have severe renal insufficiency and in that case combined heart–liver–kidney transplant may be performed [8,11]. Explanted liver from patients afflicted with FAP patients can be used for domino transplantation in select recipients [7,12–15].
Ninety-seven (97) cases in the UNOS database between 1987 and 2010 were reviewed and it was noted that FAP was the most common indication for heart transplantation (n = 26, 26.8%), followed by congenital heart disease (n = 17, 17.5%), and idiopathic dilated cardiomyopathy (n = 14, 14.4%). The most common indication for liver transplantation in this analysis was amyloidosis (n = 27, 27.8%), followed by cardiac cirrhosis (n = 17, 17.5%) [5]. In a pooled analysis of 36 cases the most common indications for CHLT were FAP (n = 11, 31%) or heart failure with associated cardiac cirrhosis (n = 6, 17%) [6].
The first reported CHLT was performed for familial hypercholesterolemia [1,2]. Familial hypercholesterolemia is a dominant inherited disease of low-density lipoprotein (LDL) caused by mutations of LDL receptors located in the hepatocyte, leading to severe cardiovascular disease in the second or third decade of life [5,16–18]. Typically CHLT is performed patients who have a homozygote genetic mutation, but can be performed for heterozygotes after failure of medical management [16,18]. Other treatments that are currently in use include lipid lowering medications; including statins, nicotinic acid and bezafibrates; plasma apheresis; partial ileal bypass; portacaval shunting; and isolated liver transplantation [16,18,19]. CHLT leads to a reduction in lipid values to normal levels [1,19,20] and resolution of tuberous xanthomas [6]. However, some patients may still require lipid lowering medications after CHLT to control lipid levels [16,17,21] or to encourage regression of xanthomas [17].
CHLT has also been performed in patients after surgical treatment for congenital heart disease. CHLT was performed for a 42-year-old patient who was born with situs ambiguous and had undergone five previous cardiac surgeries including Fontan palliation [22]. The authors also detail their institutional experience with CHLT, which includes 4 patients with failed Fontan physiology and 10 patients with biventricular heart failure. Both groups had good heart and liver function at 1 year (p = NS). There were two deaths in the first year in the biventricular group and none in the failed Fontan group [23]. In a series of three pediatric patients undergoing CHLT for failed single ventricle palliation, all three patients were alive at 2, 3 and greater than 5 years respectively [24]. CHLT has also been used to treat a patient with polysplenia and dextrocardia with situs ambiguous [25].
Accumulation of iron in the heart and liver is also an indication for CHLT [26]. Homozygous beta-thalassemia patients usually require blood transfusions leading to accumulation of iron in tissues, including the heart and liver, which is fatal without iron-chelating therapy. Additionally, CHLT may be performed for hereditary hemochromatosis and is also an indication for CHLT. These patients have abnormal absorption of iron in their intestines and develop tissue iron deposition leading to cirrhosis and cardiomyopathy [6,27].
3. Waitlist outcomes
In a single-center experience, eight patients were listed for CHLT from January 1997 to February 2004. Three patients survived to transplantation (42%), while four died awaiting transplantation and one remained on the waitlist at the time of study conclusion. For those patients who died on the waitlist, time on the list ranged from 63 to 1140 days [28].
Nationally, there were 110 patients listed for CHLT during this same time period and 33 of them were transplanted (30%), 30 patients died (27%) and 11 were still listed (10%). The remaining 34 received single organ transplants, sequential transplants or were awaiting single organ transplants after recovery of one of their organs. The authors asserted that current policies for organ allocation placed patients with MELD scores in the range of 20–29 who were cardiac status 2 at a disadvantage. They proposed exception MELD points for patients with both cardiac and liver failure [28].
4. Pre-transplant work-up
Few authors detail the pre-transplant evaluation of patients requiring CHLT. The typical approach seems to be independent evaluation for each organ. There are no published guidelines regarding evaluation for CHLT [29,30]. To optimize organ allocation to an orphan population of transplant candidates is an important area for future development.
5. Order of operation, cardiopulmonary bypass and venovenous bypass
There are a variety of approaches to the surgical procedure for CHLT. Originally, Starzl et al. [1] and Shaw et al. [2] performed dissection of both the abdomen and chest prior to the arrival of the donor organs. Both heart transplantation (OHT) and liver transplantation (OLT) were performed on cardiopulmonary bypass (CPB). Additionally, OLT was performed with portal bypass. The patients were heparinized throughout the entire period of CPB. Currently, most authors perform the heart transplant first with the patient on CPB. CPB is then discontinued and with reversal of anticoagulation for the liver transplant.
The more commonly described technique is performing the heart transplant on CPB, discontinuing bypass, leaving the chest open, and performing the liver transplant with selective use of venovenous bypass (VVB). Patients with FAP whose livers were used for domino transplant were put on VVB and the caval interposition technique was used. For other patients, the piggyback technique was used and VVB was avoided. The mediastinum was left open throughout the liver transplant. The abdomen was closed over drains and with biliary tube in place in the donor cystic duct stump, when possible. The chest was then closed over two chest tubes [15]. The majority of authors used this technique or a slightly modified version [Table 4] [7,9,10,12,16,21,29,31–35].
Table 4.
Combined Heart–Liver Transplantation (CHLT) Single-Center Outcomes.
| Citation | Year | Successful CHLT Completed | Perioperative Transfusion | Post-op Dialysis | Infection | DVT/PE | Return to OR for Bleeding | Duration of Mechanical Ventilation | LOS ICU | LOS Hospital | In-Hospital Mortality | Survival | Follow-up Time | Readmission | Rejection |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barbara et al. [7] | 2015 | 27 CHLT, 4 also with kidney | 7 patients with pre-op transfusions of blood products, RBC mean 3.2 ± 5.4 U, FFP mean 1.8 ± 5.0, Platelets mean 0.7 +/0 1.6, Cryoprecipitate mean 0.6 + −2.1 | 5/27 (18.5%) | NR | NR | NR | Mean 1.8 ± 2 | Mean 8.5 ± 7.1 | Mean 24.6 ± 27.8 | 0 (0%) | 30-day Survival: 25/26 (96.2%) | NR | NR | NR |
| Atluri et al. [31] | 2014 | 26/26 (100%) | 23 of 26 patients required transfusion of PRBC, mean 4 ± 4 U in the postoperative period | NR | 3.8 (1%) | NR | 3.8 (1%) | Mean 3 ± 2 | Mean 10 ± 5 days | Mean 25 ± 11 days | NR | 30-day mortality 3.8%, 1-Year Patient Survival: 87% ± 7%, 5-Year Patient Survival: 83% +/1 3% | NR | NR | “Long term” Rejection 3/26 (11.5%) |
| Nagpal et al. [29] | 2013 | 5/5 (100%) | NR | 1/5 (20%) | 2/5 (40%) | 1/5 DVT (20%) | 1/5 (20%) | Median 2 days (1 to 8) | Median 3 (R 3–11 days) | 26 days (12–80 days) | 0 (0%) | 30-day Survival 100%, Survival 100% at mean 38 ± 20 months | 38 ± 20 months | 1/5 (at 15 months post-op) | 0 (0%) |
| Nelson et al. [9] | 2012 | 6/7 (86%) and 1 Lung Followed By Liver | NR | 3/7 (42.9%) | 2 CMV, 2 sepsis, 1 Aspergillus | NR | NR | NR | NR | NR | 0 (0%) | 30-day Survival: 100%, 1-Year Survival: 71%, 4.5 Years Patient Survival: 71%, 4.5 Year Graft Survival: 71% | Mean 55 months | NR | 4/7 (57%) patients had 15 episodes of rejection |
| Eyraud et al. [33] | 2011 | 3/3 (100%) | Mean: 12 U PRBC, 0 U FFP | NR | NR | NR | NR | NR | NR | NR | 0 (0%) | 30-day Survival: 100%, 1-year Survival: 100% | Mean 26 months (Range 12–38) | NR | NR |
| Rauchfuss et al. [36] | 2011 | 4/4 (100%) | NR | 4/4 (100%) | NR | NR | 2/4 (50%) | 1, 3, 35 | NR | NR | 1/4 (25%) | 11 months, 25 months, 61 months | 11 months, 25 months, 61 months | NR | NR |
| Raichlin et al. [15] | 2009 | 13/15 (86.6%) | NR | 2/13 (15.3%) | 5/13 (39%) | 1/13 PE (7.7%) | 2/4 (50%) | NR | 13.8 ± 12.1 days | 26.5 ± 20.9 | NR | 1-Year Patient Survival 100%, 5-Year Patient Survival 75%, 10-Year Patient Survival 60% | Mean follow-up 61.6 ± 53.6 months | NR | Cardiac rejection: 8 patients, Liver rejection: 1 patient |
| Pilato et al. [10] | 2007 | 5/5 (100%) | NR | NR | NR | NR | NR | NR | 1, 4, 5 days | NR | 1/5 (20%) | 30-day Survival 100%, 1-Year Survival 80% | NR | NR | NR |
| Nardo et al. [12] | 2004 | 4/4 (100%) | Intraoperative PRBC: 5500, 6300, 4990, 1800, Intraoperative FFP: 5550, 7350, 2800, 4200 | 1/4 (25%) | NR | NR | NR | NR | 6 days, 6 days, 13 days, 60 days | 22 days, 24 days, 90 days | 1/4 (25%) | Pt. 1 alive at 38 months, Pt. 2 died at 2 days, Pt. 3 dead at 20 months, Pt. 4 alive at 1 months | NR | NR | 2/4 (50%) |
Although it is no longer the standard of practice to complete the entire procedure on CPB, experts report that while there are risks to CPB [39] during liver transplantation these are outweighed by the improved hemodynamic stability and decreased metabolic disturbances with hepatic reperfusion, protecting the cardiac allograft from hyperkalemia, fluid overload and acidosis, thereby lessening stress on the newly implanted cardiac graft [36–38]. The combined procedure on CPB also reduces liver cold ischemia time by eliminating the time period required for reversal of anticoagulation [36,38]. In a case series of 4 CHLTs, the associated bleeding risk with OHT and OLT on CPB is discussed [37]. The transfusion requirements in their study were similar to prior to those using alternative strategies, with the exception that patients required more platelets [37]. Continuing CPB with partial flow through the liver transplant portion has also been described in order to provide hemodynamic support to the cardiac allograft and to protect it from injury during reperfusion of the liver [30].
Some centers transplant the heart and liver en-bloc or use this technique selectively. When the en-bloc technique is performed the liver and heart remain connected by the inferior vena cava and the liver is cooled while the heart transplant is performed. The heart and liver are re-perfused simultaneously [34,40]. Authors using the en-bloc technique argue that the procedure minimizes hepatic cold ischemia time, CPB supports the transplantation of the heart as well as the liver, hemodynamic impact of liver reperfusion is minimized and optimal oxygenation is provided to help the cardiac and hepatic allografts recover from ischemia–reperfusion injury [40]. Arguments against the en-bloc approach center on the detrimental impact of simultaneous reperfusion [36].
Successful step-wise, or staged, CHLT has also been performed with a heart and liver from different donors [9,41]. A 12 year-old boy with terminal ischemic heart disease secondary to homozygous familial type IIa hypercholesterolemia underwent heart transplant, followed by the liver transplant 21 days later [41]. A patient underwent sequential transplant when during planned CHLT it was noted that the liver was unacceptable secondary to significant steatosis. He received a follow-up liver transplant from a different donor 175 days after his heart transplant [9].
Several unique situations have been reported in the literature including the use of an implantable centrifugal blood-pump (extracorporeal membrane oxygenation [ECMO]) as a bridge to transplantation [42], CHLT in a patient with pulmonary hypertension [33], early cardiac graft failure in a CHLT patient requiring ECMO support during the liver transplantation [43], and CHLT with a right split liver graft [32].
The operative techniques used, duration of operation, cold ischemia times for the heart and liver and duration of the anhepatic period reported are summarized in Table 3.
Table 3.
Combined Heart–Liver Transplantation (CHLT) Single Center Reports in the Literature, Operative Factors, 2004–2015.
| Citation | Citation | Year | Technique | Duration of Operation | Donor Heart Ischemic Time | Donor Liver Ischemic Time | CPB Duration | Anhepatic Period |
|---|---|---|---|---|---|---|---|---|
| Barbara et al. | [7] | 2015 | Heart–Liver 25/27 (93%), Liver–Heart 2/27 (7%), 12 with VVB for OLT and caval interposition, 11 without VVB with piggyback technique, 2 with CPB for OHT and OLT, CPB for all OHT | M 760 ± 125, Med 754; R 545–1124 | M 141 ± 57, Med 142, R 58–257 | M 390 ± 104, Med 413, R 151–536 | M 134 ± 40, M 122, R 151–536 | M 62 ± 11, Med 61, R 45–82 |
| Atluri et al. | [31] | 2014 | OLT after CPB discontinuation, OLT with VVB | NR | 175 ± 50 | NR | 175 | NR |
| Nagpal et al. | [29] | 2013 | OHT with CPB, CPB weaned, protamine administered, OLT with selective VVB with caval interposition or piggyback | NR | Med 141 m, R 125–177 | NR | NR | NR |
| Ravaioli et al. | [32] | 2012 | Simultaneous Heart and Split Liver (Right), OLT after CPB discontinuation, piggyback technique, no VVB | NR | NR | CIT 6 h | 45 m | NR |
| Nelson et al. | [9] | 2012 | OLT after CPB discontinuation, OLT with VVB in five patients, sequential heart then liver in 1 patient with liver 175 d after heart transplant | NR | CIT M 230 ± 65 m, R 120–324 m | CIT M 620 m ± 110 m, R 450–720 m | NR | NR |
| Chan et al. | [14] | 2012 | OLT after CPB discontinuation, OLT without VVB with side-to-side anastomosis | NR | <4 h | NR | NR | NR |
| Marriott et al. | [30] | 2011 | OHT with CPB, CPB with partial flow during OLT (10%), No VVB, modified piggyback technique | NR | 174 m | 346 m | 271 m | 55 m |
| Patel et al. | [21] | 2011 | OHT with CPB, OLT after CPB discontinuation, OLT with VVB | NR | NR | NR | NR | NR |
| Eyraud et al. | [33] | 2011 | OHT on CPB, OLT while patient on ECMO and double VVB | NR | 135 m | 11 h 52 m | NR | NR |
| Rauchfuss et al. | [36] | 2011 | OHT and OLT on CPB, no VVB | 293 ± 204 m | Cold 190 ± 72 m, Warm 98 ± 96 m | Cold 349 ± 101 m, Warm 36.25 ± 3.5 m | 534 ± 247 m | NR |
| Eyraud et al. | [43] | 2011 | Simultaneous Tx, OLT after CPB discontinuation, OLT with VVB | NR | 2 h 46 m | 12 h 47 m | NR | NR |
| Hennessey et al. | [37] | 2010 | OHT and OLT on CPB, no VVB | NR | M 138.75, R 83–201 | M 222.5, R 135–300 | M 188.5, R 121–248 m | NR |
| Raichlin et al. | [15] | 2009 | OLT after CPB discontinuation, OHT with selective VVB | NR | NR | NR | NR | NR |
| Barreiros et al. | [34] | 2009 | 3 OHT then OLT without VVB, 1 en-block | NR | NR | NR | NR | NR |
| Diaz et al. | [35] | 2007 | OLT after CPB discontinuation, OLT with VVB, piggyback technique | NR | 260 min | 473 m cold, 32 m warm | 177 min | NR |
| Bernier et al. | [38] | 2007 | OHT and OLT on CPB, no VVB | NR | 185 min | 288 min | 197 min | Cross-clamp time 73 min |
| Pilato et al. | [10] | 2007 | OHT with CPB, OLT 2 piggyback without VVB and 3 piggyback with VVB | NR | M 153 m, R 111–180 m | M 8.9 h, R 8.10–9.54 | M 151, R 105–235 | NR |
| Dell'Amore et al. | [42] | 2006 | Simultaneous Tx, no specification about the operation technique | NR | NR | NR | NR | NR |
| Alkofer et al. | [16] | 2005 | OHT on CPB, OLT after CPB discontinuation, OLT with VVB | NR | NR | NR | NR | NR |
| Haynes et al. | [27] | 2004 | Simultaneous Tx, no specification about the operation technique | NR | NR | NR | NR | NR |
| Nardo et al. | [12] | 2004 | OHT on CPB, OLT after CPB discontinuation and reversal, OLT with VVB in 2/4 (50%) of patients | hour.min: 14.20, 17.10, 16, 13.15 | hour.min: 2.22, 2.42, 2.30, 2.58 | hour.min: 9.10, 9.39, 8.4, 9.54 | min: 148, 112, 157, 105 | NR |
6. Outcomes
There are two large database studies of CHLT using the United Network for Organ Sharing (UNOS) database. In the first study based on the UNOS database, Te et al. described the United States experience from October of 1987 to December of 2005, at which time there were 41 cases of CHLT and 6 cases of combined heart–liver–kidney transplant (CHLKT). Amyloidosis was the most common indication for both heart and liver grafts. The most common immunosuppressive regimens were corticosteroids and either tacrolimus or cyclosporine, however 28.2% of patients were maintained on a single immunosuppressive agent. In this cohort there were 7 episodes of liver rejection observed in 6 patients (13%), that were treated with corticosteroids or corticoste-roids and tacrolimus, with all episodes except 1 occurring within the first two years after transplantation. There were 5 episodes of heart rejection in 5 (10.9%) patients. Four patients (8.7%) developed malignancy. There was one case of CMV and no cases of EBV. Patient 1- and 5-year survival was 84.8% and 75.6%. Heart graft survival at 1- and 5-years was 84.8% and 75.6%. Liver graft survival at 1- and 5-years was 82.4% and 73.5%. They concluded that CHLT is a viable option for candidates requiring combined organ transplantation and that outcomes were comparable to those for single-organ recipients [8].
Cannon et al. completed a review of the United States experience with CHLT using the UNOS data on 97 cases reported between October of 1987 and December of 2010. In 9 of these patients a simultaneous kidney transplant was performed. In 10 of these patients a simultaneous lung transplant was performed. Amyloidosis was the most common indication for both heart and liver transplantation (Heart, n = 26, 26.8%, Liver, n = 27, 27.8%). Liver graft survival at 1-, 5- and 10-years was similar between CHLT patients and isolated liver patients (83.4%, 72.8%, 71.0% versus 79.4%, 71%, 65.1%, p = 0.894). Cardiac allograft survival at 1-, 5- and 10-years was similar between CHLT and isolated cardiac transplantation (83.5%, 73.2%, 71.5%, versus 82.6%, 71.9%, 63.2%, p = 0.341). They concluded that CHLT is a safe procedure with graft survival rates similar to liver-alone and cardiac-alone transplantation. In patients with greater than 1 year survival, the incidence of acute liver rejection was lower in the CHLT group than in those undergoing liver transplant alone (5.2% versus 12.2%, p = 0.060). The incidence of acute cardiac rejection was also lower in the group undergoing CHLT than in the cardiac transplant alone group (8.9% versus 23.9%, p = 0.002). A multivariable analysis that controlled for recipient age, recipient gender and donor age, found no significant difference in risk for patient death or graft failure in the CHLT group versus isolated liver transplant (HR 1.30, 95% CI 0.93–1.84, p = 0.127 and HR 1.09, 95% CI 0.79–1.52, p = 0.589). There was also no significant difference between CHLT and isolated heart transplant in risk for patient death or graft failure (HR 0.90, 95% CI 0.64–1.26, p = 0.538 and HR 0.86, CI 0.62–1.21, p = 0.386) [5].
There are also a multitude of single center studies described in the literature (Table 4).
7. Graft tolerance
In determining order of transplantation for multi-organ transplantation, clinicians need to consider each graft's tolerance to cold ischemia [13,44]. In CHLT, the heart is typically transplanted first. This provides the benefit of a functioning heart adequately perfusing the liver graft once it is transplanted [13]. Additionally, the heart is less tolerant of ischemia.
In a large series, 25 of 27 (93%) patients had heart transplantation completed prior to liver transplantation. In the remaining 2 (7%) patients, liver transplantation was completed prior to heart transplantation in an effort to protect the cardiac graft from high titer donor-specific antibodies [7]. No data are currently available on the clinical outcomes of these different orders of transplantation.
In a case where liver transplantation proceeded heart transplantation to protect the cardiac allograft from donor specific antibodies. It has been demonstrated that a positive cross-match with a donor can become negative after liver transplantation and the authors hypothesized that if they transplanted the liver first and followed it with the heart they could protect the heart from antibody mediated rejection. This effect has also been described in combined liver–kidney transplantation [45]. Induction of tolerance of the heart allograft in the setting of concomitant liver allograft has been demonstrated in animal models [46].
In the case of a 41 year-old female with apical variant hypertrophic cardiomyopathy, congestive hepatopathy, and high titers of donor-specific anti-human leukocyte antigen (HLA) antibodies. The patient underwent preoperative plasma exchange and reverse order of transplantation to reduce exposure of the cardiac graft to donor specific antibodies [7].
In a study of 22 consecutive liver allograft recipients who tested positive for immunoglobulin (IgG) lymphocytotoxicity demonstrated that 14 of 22 (64%) of sensitized patients had a negative cross-match on post-operative day 1 and remained negative, while 8 of 22 (36%) had cross-match results that remained positive. Most of those who had a negative cross-match post-operatively had low titers prior to transplant (< or =1/16), while most of those with positive cross-match postoperatively had high-titers (>1/32–1024).18 The authors conclude that patients with titers >1/32 are more likely to have persistently positive cross-matches and an accompanying syndrome including increased total hemolytic complement activity, circulating immune complexes, and refractory thrombocytopenia [47].
It has been suggested that the liver allograft protects the kidney allograft from hyperacute rejection in combined liver–kidney transplantation. One mechanism proposed for this is reduction of donor specific antibodies [48]. It has been demonstrated that there is a reduction in donor specific antibodies (DSA) after transplantation of the liver and that in patients where DSA persist, while there is evidence of complement activation in the graft, there is not significant clinical impact in the first year [49]. Experts have postulated that there may be a differential reduction of class I versus class II donor specific antibodies and concomitant transplantation may not be sufficient to protect the kidney [48]. The exact mechanism of reduction of donor specific antibodies is unknown. Experts suggest several hypothesis: phagocytosis of antibodies by Kupffer cells, HLA antigen secretion, dilution of antibody concentrations caused by bleeding or a large vascular bed [7]. Reduction in donor specific HLA antibodies has been demonstrated after isolated liver and combined kidney–liver transplantations [7].
In a large single-center series, a lower than anticipated rate of rejection, which they state may be caused by hepatic allograft conferring a protective effect on the cardiac allograft [31]. In a single center study the rate of acute rejection was found to be lower in patients who have received a combined heart and liver transplantation than in those who have received an isolated liver transplant or isolated heart transplant and report that the incidence of acute liver rejection in patients with liver graft survival greater than 1 year was lower in the CHLT group than in those undergoing liver transplant alone (5.2% versus 12.2%, p = 0.060) [5]. Previous work found that CHLT recipients were maintained on much lower doses of immunosuppression than their isolated heart transplant patients, which suggests that the liver does provide some an immunoprotective effect [24]. A proposed mechanism for the protective effect of the liver is the shedding of soluble human leukocyte antigens [50]. Some authors propose as a result of this protective effect patients who have undergone CHLT may tolerate lower levels of immunosuppressive medications [11].
Rana et al. examined the protective effects of different allografts. The authors analyzed a total of 133,416 patients and concluded that in an allograft transplanted with a heart, liver or kidney rejection rates are significantly lower than for organs transplanted alone. The same did not apply for organs transplanted with small bowel or pancreas [51].
8. Conclusion
Extensive and unified efforts are needed to coordinate this complex care between two organ transplant programs to optimize patient outcomes with only a few select programs offering this type of combined transplantation. It has been demonstrated that there is a wide range of potentially successful techniques for completing this procedure. Majority of authors complete the heart transplantation on CPB with the patient heparinized and then discontinue heparinization prior performing the liver transplantation. Therefore, we would recommend this approach. Immunosuppressive regimens are similar to those used for isolated liver and heart transplants, but a large body of work regarding graft tolerance in combined liver transplantation suggests that these patients may not require high levels of immunosuppression. This is an important area for future exploration.
Acknowledgments
Eliza W. Beal would like to acknowledge her T32 funding support NIHT32AI 106704-01A1.
Footnotes
Authorship: Eliza W. Beal: Involved in the conception, drafting, design and revision of the manuscript. Sylvester Black: Involved in the conception, design and revision of the manuscript. Don Hayes, Khalid Mumtaz, and Bryan A. Whitson: Contributed equally to the review and revision of the manuscript.
Conflict of interest statement
All authors declare that they have no commercial, personal, political, intellectual or religious interests in relation to the submitted work. No outside funding was received.
References
- 1.Starzl TE, Bilheimer DW, Bahnson HT, et al. Heart-liver transplantation in a patient with familial hypercholesterolaemia. Lancet. 1984;1:1382–3. doi: 10.1016/s0140-6736(84)91876-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw BW, Bahnson HT, Hardesty RL, Griffith BP, Starzl TE. Combined transplantation of the heart and liver. Ann Surg. 1985;202:667–72. doi: 10.1097/00000658-198512000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.OPTN. Multiple Organ Transplants in the U.S. by Recipient ABO. U.S. Multiple Organ Transplants Performed January 1, 1988 – December 31, 2015. [Accessed March 30, 2016];Liver-Heart. 2016 https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/, Published 2016.
- 4.OPTN. Multi-Organ Transplants by Center. U.S. Multi-Organ Transplants Performed January 1, 1988 – December 31, 2015. [Accessed March 30, 2016];Liver-Heart. 2016 https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/, Published 2016.
- 5.Cannon RM, Hughes MG, Jones CM, Eng M, Marvin MR. A review of the United States experience with combined heart-liver transplantation. Transpl Int. 2012;25:1223–8. doi: 10.1111/j.1432-2277.2012.01551.x. [DOI] [PubMed] [Google Scholar]
- 6.Barshes NR, Udell IW, Joyce DL, Southard RE, O’Mahony CA, Goss JA. A pooled analysis of posttransplant survival following combined heart-liver transplantation. Transplantation. 2007;83:95–8. doi: 10.1097/01.tp.0000243731.29657.87. [DOI] [PubMed] [Google Scholar]
- 7.Barbara DW, Rehfeldt KH, Heimbach JK, Rosen CB, Daly RC, Findlay JY. The peri-operative management of patients undergoing combined heart-liver transplantation. Transplantation. 2015;99:139–44. doi: 10.1097/TP.0000000000000231. [DOI] [PubMed] [Google Scholar]
- 8.Te HS, Anderson AS, Millis JM, Jeevanandam V, Jensen DM. Current state of combined heart-liver transplantation in the United States. J Heart Lung Transplant. 2008;27:753–9. doi: 10.1016/j.healun.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Nelson LM, Penninga L, Sander K, et al. Long-term outcome in patients treated with combined heart and liver transplantation for familial amyloidotic cardiomyopathy. Clin Transplant. 2013;27:203–9. doi: 10.1111/ctr.12053. [DOI] [PubMed] [Google Scholar]
- 10.Pilato E, Dell’Amore A, Botta L, Arpesella G. Combined heart and liver transplantation for familial amyloidotic neuropathy. Eur J Cardiothorac Surg. 2007;32:180–2. doi: 10.1016/j.ejcts.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Raichlin E, Um JY, Duncan KF, et al. Combined heart and liver transplantation against positive cross-match for patient with hypoplastic left heart syndrome. Transplantation. 2014;98:e100–2. doi: 10.1097/TP.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 12.Nardo B, Beltempo P, Bertelli R, et al. Combined heart and liver transplantation in four adults with familial amyloidosis: experience of a single center. Transplant Proc. 2004;36:645–7. doi: 10.1016/j.transproceed.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 13.Faenza S, Arpesella G, Bernardi E, et al. Combined liver transplants: main characteristics from the standpoint of anesthesia and support in intensive care. Transplant Proc. 2006;38:1114–7. doi: 10.1016/j.transproceed.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Chan SC, Cheng LC, Ho KL, et al. Improvising hepatic venous outflow and inferior vena cava reconstruction for combined heart and liver and sequential liver transplantations. Asian J Surg. 2013;36:89–92. doi: 10.1016/j.asjsur.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Raichlin E, Daly RC, Rosen CB, et al. Combined heart and liver transplantation: a single-center experience. Transplantation. 2009;88:219–25. doi: 10.1097/TP.0b013e3181ac60db. [DOI] [PubMed] [Google Scholar]
- 16.Alkofer BJ, Chiche L, Khayat A, et al. Liver transplant combined with heart transplant in severe heterozygous hypercholesterolemia: report of the first case and review of the literature. Transplant Proc. 2005;37:2250–2. doi: 10.1016/j.transproceed.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 17.Ibrahim M, El-Hamamsy I, Barbir M, Yacoub MH. Translational lessons from a case of combined heart and liver transplantation for familial hypercholesterolemia 20 years post-operatively. J Cardiovasc Transl Res. 2012;5:351–8. doi: 10.1007/s12265-011-9311-1. [DOI] [PubMed] [Google Scholar]
- 18.Ahualli L, Stewart-Harris A, Bastianelli G, et al. Combined cardiohepatic transplantation due to severe heterozygous familiar hypercholesteremia type II: first case in Argentina–a case report. Transplant Proc. 2007;39:2449–53. doi: 10.1016/j.transproceed.2007.07.068. [DOI] [PubMed] [Google Scholar]
- 19.Offstad J, Schrumpf E, Geiran O, Søreide O, Simonsen S. Plasma exchange and heart-liver transplantation in a patient with homozygous familial hypercholesterolemia. Clin Transplant. 2001;15:432–6. doi: 10.1034/j.1399-0012.2001.150612.x. [DOI] [PubMed] [Google Scholar]
- 20.Barbir M, Khaghani A, Kehely A, et al. Normal levels of lipoproteins including lipoprotein(a) after liver-heart transplantation in a patient with homozygous familial hypercholesterolaemia. Q J Med. 1992;85:807–12. [PubMed] [Google Scholar]
- 21.Patel SR, D’alessandro D, Shin JJ. Combined heart and liver transplantation in an adult with familial heterozygous hypercholesterolemia and severe ischemic cardiomyopathy. J Heart Lung Transplant. 2012;31:229. doi: 10.1016/j.healun.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Vallabhajosyula P, Komlo C, Wallen TJ, Olthoff K, Pochettino A. Combined heart-liver transplant in a situs-ambiguous patient with failed Fontan physiology. J Thorac Cardiovasc Surg. 2013;145:e39–41. doi: 10.1016/j.jtcvs.2012.12.066. [DOI] [PubMed] [Google Scholar]
- 23.Vallabhajosyula PKC, Molina M, Roche L, Kim Y, Goldberg L, Pochettino A. Combined Heart-Liver Transplantation for Failed Single Ventricle/Fontan Physiology. J Heart Lung Transplant. 2012;31:S112. [Google Scholar]
- 24.Hollander SA, Reinhartz O, Maeda K, Hurwitz M, Rosenthal ND, Bernstein D. Intermediate-term outcomes after combined heart-liver transplantation in children with a univentricular heart. J Heart Lung Transplant. 2013;32:368–70. doi: 10.1016/j.healun.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Horai T, Bhama JK, Fontes PA, Toyoda Y. Combined heart and liver transplantation in a patient with situs ambiguous. Ann Thorac Surg. 2011;91:600–1. doi: 10.1016/j.athoracsur.2010.07.078. [DOI] [PubMed] [Google Scholar]
- 26.Olivieri NF, Liu PP, Sher GD, et al. Brief report: combined liver and heart transplantation for end-stage iron-induced organ failure in an adult with homozygous beta-thalassemia. N Engl J Med. 1994;330:1125–7. doi: 10.1056/NEJM199404213301605. [DOI] [PubMed] [Google Scholar]
- 27.Haynes H, Farroni J. Successful combined heart-liver transplantation in a patient with hemochromatosis. Prog Transplant. 2004;14:39–40. doi: 10.1177/152692480401400106. [DOI] [PubMed] [Google Scholar]
- 28.Porrett PM, Desai SS, Timmins KJ, Twomey CR, Sonnad SS, Olthoff KM. Combined orthotopic heart and liver transplantation: the need for exception status listing. Liver Transpl. 2004;10:1539–44. doi: 10.1002/lt.20279. [DOI] [PubMed] [Google Scholar]
- 29.Nagpal AD, Chamogeorgakis T, Shafii AE, et al. Combined heart and liver transplantation: the Cleveland Clinic experience. Ann Thorac Surg. 2013;95:179–82. doi: 10.1016/j.athoracsur.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 30.Marriott AJ, Hwang NC, Lai FO, et al. Combined heart-liver transplantation with extended cardiopulmonary bypass. Singapore Med J. 2011;52:e48–51. [PubMed] [Google Scholar]
- 31.Atluri P, Gaffey A, Howard J, et al. Combined heart and liver transplantation can be safely performed with excellent short- and long-term results. Ann Thorac Surg. 2014;98:858–62. doi: 10.1016/j.athoracsur.2014.04.100. [DOI] [PubMed] [Google Scholar]
- 32.Ravaioli M, Serenari M, Cescon M, et al. Combined kidney-liver, heart-liver, and kidney-pancreas transplantations from a single deceased donor. Case Rep Transplant. 2012;2012:849619. doi: 10.1155/2012/849619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eyraud D, Ben Menna M, Vaillant JC, et al. Perioperative management of combined heart-liver transplantation in patients with cirrhosis, renal insufficiency, or pulmonary hypertension. Clin Transplant. 2011;25:228–34. doi: 10.1111/j.1399-0012.2010.01240.x. [DOI] [PubMed] [Google Scholar]
- 34.Barreiros AP, Post F, Hoppe-Lotichius M, et al. Liver transplantation and combined liver-heart transplantation in patients with familial amyloid polyneuropathy: a single-center experience. Liver Transpl. 2010;16:314–23. doi: 10.1002/lt.21996. [DOI] [PubMed] [Google Scholar]
- 35.Diaz GC, Renz JF, Nishanian E, Kinkhabwala M, Emond JC, Wagener G. Anesthetic management of combined heart-liver transplantation. J Cardiothorac Vasc Anesth. 2007;21:253–6. doi: 10.1053/j.jvca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 36.Detry O, Honoré P, Meurisse M, et al. Advantages of inferior vena caval flow preservation in combined transplantation of the liver and heart. Transpl Int. 1997;10:150–1. doi: 10.1007/s001470050030. [DOI] [PubMed] [Google Scholar]
- 37.Bernier PL, Grenon M, Ergina P, et al. Combined simultaneous heart and liver transplantation with complete cardiopulmonary bypass support. Ann Thorac Surg. 2007;83:1544–5. doi: 10.1016/j.athoracsur.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Rauchfuss F, Breuer M, Dittmar Y, et al. Implantation of the liver during reperfusion of the heart in combined heart-liver transplantation: own experience and review of the literature. Transplant Proc. 2011;43:2707–13. doi: 10.1016/j.transproceed.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Hennessey T, Backman SB, Cecere R, et al. Combined heart and liver transplantation on cardiopulmonary bypass: report of four cases. Can J Anaesth. 2010;57:355–60. doi: 10.1007/s12630-010-9263-y. [DOI] [PubMed] [Google Scholar]
- 40.Hill AL, Maeda K, Bonham CA, Concepcion W. Pediatric combined heart-liver transplantation performed en bloc: a single-center experience. Pediatr Transplant. 2012;16:392–7. doi: 10.1111/j.1399-3046.2012.01695.x. [DOI] [PubMed] [Google Scholar]
- 41.Figuera D, Ardaiz J, Martín-Júdez V, et al. Combined transplantation of heart and liver from two different donors in a patient with familial type IIa hypercholesterolemia. J Heart Transplant. 1986;5:327–9. [PubMed] [Google Scholar]
- 42.Dell’Amore A, Botta L, Gallieri S, Arpesella G. Extracorporeal membrane oxygenator assistance as “bridge” to combined heart and liver transplantation. Transplant Proc. 2006;38:3004–5. doi: 10.1016/j.transproceed.2006.08.129. [DOI] [PubMed] [Google Scholar]
- 43.Eyraud D, Vaillant JC, Ionescu C, et al. Early primary cardiac graft failure and combined heart-liver transplantation: need for an uncommon double bypass. Br J Anaesth. 2011;107:280–1. doi: 10.1093/bja/aer217. [DOI] [PubMed] [Google Scholar]
- 44.Befeler AS, Schiano TD, Lissoos TW, et al. Successful combined liver-heart transplantation in adults: report of three patients and review of the literature. Transplantation. 1999;68:1423–7. doi: 10.1097/00007890-199911150-00034. [DOI] [PubMed] [Google Scholar]
- 45.Daly RC, Topilsky Y, Joyce L, et al. Combined heart and liver transplantation: protection of the cardiac graft from antibody rejection by initial liver implantation. Transplantation. 2013;95:e2–4. doi: 10.1097/TP.0b013e318277226d. [DOI] [PubMed] [Google Scholar]
- 46.Calne RY, Sells RA, Pena JR, et al. Induction of immunological tolerance by porcine liver allografts. Nature. 1969;223:472–6. doi: 10.1038/223472a0. [DOI] [PubMed] [Google Scholar]
- 47.Mañez R, Kelly RH, Kobayashi M, et al. Immunoglobulin G lymphocytotoxic antibodies in clinical liver transplantation: studies toward further defining their significance. Hepatology. 1995;21:1345–52. doi: 10.1002/hep.1840210519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dar W, Agarwal A, Watkins C, et al. Donor-directed MHC class I antibody is preferentially cleared from sensitized recipients of combined liver/kidney transplants. Am J Transplant. 2011;11:841–7. doi: 10.1111/j.1600-6143.2011.03467.x. [DOI] [PubMed] [Google Scholar]
- 49.Taner T, Gandhi MJ, Sanderson SO, et al. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. 2012;12:1504–10. doi: 10.1111/j.1600-6143.2012.03995.x. [DOI] [PubMed] [Google Scholar]
- 50.McMillan RW, Gelder FB, Zibari GB, Aultman DF, Adamashvili I, McDonald JC. Soluble fraction of class I human histocompatibility leukocyte antigens in the serum of liver transplant recipients. Clin Transplant. 1997;11:98–103. [PubMed] [Google Scholar]
- 51.Rana A, Robles S, Russo MJ, et al. The combined organ effect: protection against rejection? Ann Surg. 2008;248:871–9. doi: 10.1097/SLA.0b013e31817fc2b8. [DOI] [PubMed] [Google Scholar]
- 52.Barrio IM, Mtnez de Guereñu MA, Real MI, Del Campo I, Pérez-Cerdá F, Moreno E. Anesthetic management of a combined heart and liver transplantation in an amyloidotic patient: a case report. Transplant Proc. 2007;39:2458–9. doi: 10.1016/j.transproceed.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 53.Surakomol S, Olson LJ, Rastogi A, et al. Combined orthotopic heart and liver transplantation for genetic hemochromatosis. J Heart Lung Transplant. 1997;16:573–5. [PubMed] [Google Scholar]
- 54.Ramamurthi A, Pandian NG, Kiernan MS, Lee H, DeNofrio D. Eroded valves, recurrent pleural effusions, and ultimately heart-liver transplant. Echocardiography. 2012;29:1139–41. doi: 10.1111/j.1540-8175.2012.01811.x. [DOI] [PubMed] [Google Scholar]
- 55.Sulewski ME, Wolf JH, Hasz R, et al. Combined heart-liver transplantation; implications for liver-alone wait list mortality. Transplantation. 2014;98:e45–7. doi: 10.1097/TP.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tazbir JS, Cronin DC. Indications, evaluations, and postoperative care of the combined liver-heart transplant recipient. AACN Clin Issues. 1999;10:240–52. doi: 10.1097/00044067-199905000-00010. [DOI] [PubMed] [Google Scholar]