Abstract
BACKGROUND
The role of routine lymphadenectomy for perihilar cholangiocarcinoma is still controversial and no study has defined the minimum number of lymph nodes examined (TNLE). We sought to assess the prognostic performance of American Joint Committee on Cancer/Union Internationale Contre le Cancer (7th edition) N stage, lymph node ratio, and log odds (LODDS; logarithm of the ratio between metastatic and nonmetastatic nodes) in patients with perihilar cholangiocarcinoma and identify the optimal TNLE to accurately stage patients.
METHODS
A multi-institutional database was queried to identify 437 patients who underwent hepatectomy for perihilar cholangiocarcinoma between 1995 and 2014. The prognostic abilities of the lymph node staging systems were assessed using the Harrell’s c-index. A Bayesian model was developed to identify the minimum TNLE.
RESULTS
One hundred and fifty-eight (36.2%) patients had lymph node metastasis. Median TNLE was 3 (interquartile range, 1 to 7). The LODDS had a slightly better prognostic performance than lymph node ratio and American Joint Committee on Cancer, in particular among patients with <4 TNLE (c-index = 0.568). For 2 TNLE, the Bayesian model showed a poor discriminatory ability to distinguish patients with favorable and poor prognosis. When TNLE was >2, the hazard ratio for N1 patients was statistically significant and the hazard ratio for N1 patients increased from 1.51 with 4 TNLE to 2.10 with 10 TNLE. Although the 5-year overall survival of N1 patients was only slightly affected by TNLE, the 5-year overall survival of N0 patients increased significantly with TNLE.
CONCLUSIONS
Perihilar cholangiocarcinoma patients undergoing radical resection should ideally have at least 4 lymph nodes harvested to be accurately staged. In addition, although LODDS performed better at determining prognosis among patients with <4 TNLE, both lymph node ratio and LODDS outperformed compared with American Joint Committee on Cancer N stage among patients with ≥4 TNLE.
Cholangiocarcinoma can arise from carcinomatous degeneration of the biliary ductal epithelium and, possibly, direct transdifferentiation of hepatocytes within the substance of the liver.1 Cholangiocarcinoma accounts for roughly 3% of all gastrointestinal tumors, 15% of hepatobiliary malignancies, and about 10% of liver tumors.2 Cholangiocarcinoma can be classified according to its anatomic location as intrahepatic, perihilar (PHCC), and distal tumors.3 Perihilar cholangiocarcinoma accounts for 60% to 70% of all cholangiocarcinoma4,5 and has an incidence of 1 to 2 per 100,000 individuals in the United States.6 Perihilar cholangiocarcinoma can be an aggressive malignancy with <50% of patients resectable at the time of diagnosis.7,8 In addition, even among patients who are resected, the 5-year overall survival (OS) after curative intent surgery ranges from only 20% to 42%.9 Long-term prognosis has been strongly correlated with lymph node (LN) status with 5-year survival <20% to 25% among patients who have metastatic disease in the LNs.10–12 When compared with other prognostic factors, such as margin status, grade of tumor differentiation, and carbohydrate antigen 19-9 serum level, the presence of metastatic LNs has been demonstrated to be one of the strongest predictors of poor prognosis.5,13–16
Traditionally, LN status has been categorized by the American Joint Committee on Cancer (AJCC) cancer staging system (7th edition) as absent (N0) vs present (N1/N2), as well as regional (N1) vs nonregional/periarotic/pericaval (N2).17 A few studies have evaluated the prognostic impact of lymph node ratio (LNR), defined as the ratio of number of metastatic LNs relative to the total number of LN examined (TNLE),18–20 as well as log odds of metastatic lymph nodes (LODDS), defined as the natural logarithm of the ratio between metastatic and nonmetastatic LNs.21,22 Although TNLE has been recognized as an important variable for many diseases, including colon and pancreas,23 the expected or “appropriate” TNLE to be harvested at the time of surgery for PHCC remains poorly defined. Data from several high-volume institutions have found a wide variation in the TNLE with PHCC surgery. Specifically, the TNLE ranged from 3 nodes in a study from Memorial Sloan Kettering Cancer Center24 to 11 nodes in one large study from Japan.20
The TNLE has been proposed as an important quality metric for other types of surgery.23,25,26 In addition, TNLE can impact the likelihood of identifying metastatic LNs and have implications for accurately staging patients. Previous studies have failed to examine the impact of TNLE on the prognostic performance of AJCC (7th edition) N stage, LNR, and LODDS. In addition, the ideal number of LNs to examine to accurately stage patients with PHCC has yet to be defined. Therefore, the objective of the current study was to define the minimum TNLE needed to optimize the prognostic performance of the various LN staging systems for patients with PHCC. In addition, we sought to examine the prognostic impact not only of the number of nodes (ie TNLE), but also the location of disease in the relative different LN stations.
METHODS
Patient selection
Patients who underwent hepatectomy for PHCC between January 1, 1995 and December 31, 2014 in 1 of 12 major hepatobiliary centers in the United States and Europe were identified (Johns Hopkins University, Baltimore, MD; Emory University, Atlanta, GA; Stanford University, Stanford, CA; University of Wisconsin, Milwaukee, WI; The Ohio State University, Columbus, OH; Washington University, St Louis, MO; Vanderbilt University, Nashville, TN; New York University, New York, NY; University of Louisville, Louisville, KY; Wake Forest University, Winston-Salem, NC; University of Verona, Verona, Italy, and University of Rotterdam, Rotterdam, The Netherlands). The IRBs of the participating institutions approved the study. Only patients with histological-ly confirmed PHCC who underwent a curative-intent tumor resection were included in the analysis. Details on the clinicopathologic patient characteristics collected for this study are reported in the Appendix (available online). In particular, the extent of lymphadenectomy, the number of LNs harvested, as well as number of nodes with metastatic disease, were recorded. Based on this information, data on LN status was categorized for comparative purposes into several different LN staging/scoring systems: 7th edition AJCC/Union Internationale Contre le Cancer N categories, LNR, and LODDS.27 The LODDS was defined as log of the ratio between the number of metastatic LNs relative to the number of nodes without metastasis.21 In a subset of patients, information on nodal stations was available and was classified according to the classification of the Japanese Society of Hepato-Biliary-Pancreatic Surgery.28 In brief, group 1 nodes were classified as hepatoduodenal ligament nodes (stations 12); group 2 nodes were along the left gastric artery (station 7), along the common hepatic artery (station 8), and on the posterior aspect of the head of the pancreas (station 13); and group 3 were nodes around the celiac artery (station 9), along the superior mesenteric artery (station 14), and periaortic area (station 16).
Statistical analyses
Continuous variables were summarized as median with interquartile range (IQR) and categorical variables were reported as whole numbers and percentages. The primary end point was OS, defined as the time interval between the date of surgery and the date of death. Time was censored at the date of last follow-up for all patients who were found to be alive. Survival curves were estimated using the Kaplan-Meier method and differences between curves were tested using the log-rank test. Statistically significant variables (p < 0.05) in the univariable analysis were analyzed using a multivariable Cox proportional hazard model. Using a backward elimination method (likelihood ratio test), variables were selected for inclusion in the final Cox model. The coefficients from the Cox models were subsequently reported as hazard ratio (HR) and corresponding 95% CI. Additionally, 3 separate models were built to explore the role of different variables used to stage nodal status: in model 1, AJCC N staging system vs model 2 LNR vs model 3 LODDS. The LNR and LODDS were analyzed according to established cutoff values based on previous data from studies that had examined LODDS for other types of cancers.29–31 A Bayesian model was developed to analyze the hypothesis that the number of harvested nodes influenced the survival of patients with (N1) and without (N0) metastatic nodes (Appendix; available online). The results of the Bayesian model were presented as overall survival proportion, as well as HR with associated 95% credible interval (CrI). A p value <0.05 was considered to be statistically significant. For statistical analyses, OpenBugs (2011), and R CRAN software (version 3.2.2, 2015) with the packages “survival,” “Hmisc,” and “R2OpenBUGS” were used.
RESULTS
Patient- and disease-specific characteristics
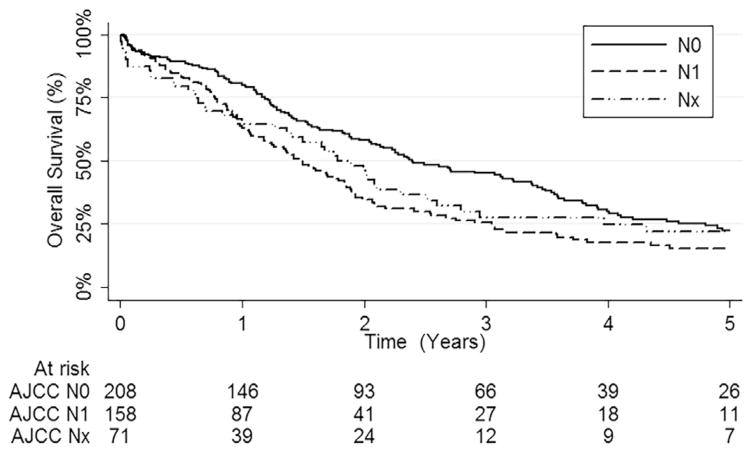
A total of 437 patients who underwent surgery for PHCC and met inclusion criteria were identified from the multi-institutional international database (Table 1; Appendix; available online). Of note, 158 (36.2%) patients had LN metastasis. Median number of LNs harvested was 3 (IQR 1 to 7 LNs). Only 1 to 3 LNs were harvested in 162 (44.3%) patients, 4 to 10 LNs in 130 (35.5%), and >10 LNs in 74 (20.2%) patients. Median LNR and LODDS were 0 (IQR, 0 to 0.25) and −1.47 (IQR −2.20 to −0.87), respectively. Of note, the 1-, 3-, and 5-year OS rates of N0 patients were 80.2% (95% CI, 74.8–86.0), 44.8% (95% CI, 37.9–53.0), and 22.4% (95% CI, 16.5–30.4), respectively, vs 62.9% (95% CI, 55.5–71.2), 25.4% (95% CI, 18.8–34.4), and 15.4% (95% CI, 9.9–23.9) for N1 patients (p < 0.001, Fig. 1). The N1 disease translated into a roughly 50% increased risk of death (HR = 1.55; 95% CI, 1.21–1.98; p < 0.001). In addition, N1 patients with 1 to 3 metastatic LNs had a 5-year OS rate of 18.6% (95% CI, 12.1–28.6) vs 11.1% (95% CI, 3.2–39.1) for N1 patients with >3 metastatic LNs (p = 0.022).
Table 1.
Baseline Clinical and Pathologic Characteristics of Patients Who Underwent Liver Resection for Perihilar Cholangiocarcinoma (N = 437)
| Characteristic | Data |
|---|---|
| Age, y, median (IQR) | 66.5 (58.2–73.0) |
| Age, n (%) | |
| Younger than 65 y | 197 (45.1) |
| 65 y and older | 240 (54.9) |
| Sex, female, n (%) | 165 (37.8) |
| BMI, kg/m2, median (IQR) | 24.7 (22.2–28.2) |
| ASA 1/ASA 2, n (%) | 120 (42.6) |
| NA | 155 |
| Jaundice, present, n (%) | 284 (78.5) |
| NA | 75 |
| Ascites, n (%) | 9 (2.6) |
| NA | 84 |
| Carbohydrate antigen 19-9, U/mL, median (IQR) | 102.2 (38.0–349.2) |
| Preoperative biliary drainage/stent, performed, n (%) | 272 (75.8) |
| NA | 78 |
| Type of resection, n (%) | |
| Bile duct resection + right hepatectomy | 165 (37.7) |
| Bile duct resection + left hepatectomy | 158 (36.2) |
| Other | 114 (26.1) |
| Portal vein resection, performed, n (%) | 49 (11.2) |
| Hepatic artery resection, performed, n (%) | 15 (3.4) |
| Portal vein embolization, n (%) | 31 (7.1) |
| Estimated blood loss, mL, median (IQR) | 600 (350–1150) |
| RBC transfusion, performed, n (%) | 91 (22.9) |
| NA | 40 |
| Size, mm, median (IQR) | 25 (16–40) |
| Margin status, n (%) | |
| R0 | 296 (67.7) |
| R1 | 141 (32.3) |
| Grade, n (%) | |
| G1–G2 | 339 (77.6) |
| G3–G4 | 98 (22.4) |
| Lymph nodes examined, n, median (IQR) | 3 (1–7) |
| Metastatic nodes, median (IQR) | 0 (0–1) |
| Bismuth classification, n (%) | |
| I | 67 (15.3) |
| II | 70 (16.0) |
| IIIa/IIIb | 216 (49.5) |
| IV | 84 (19.2) |
| AJCC T stage, n (%) | |
| T1 | 49 (11.2) |
| T2a–T2b | 271 (62.0) |
| T3–T4 | 117 (26.8) |
| AJCC N stage, n (%) | |
| N0 | 208 (47.6) |
| N1 | 158 (36.2) |
| Nx | 71 (16.2) |
| Lymph node ratio, n (%) | |
| 0% | 208 (56.8) |
| 1%–25% | 57 (15.6) |
| 26%–50% | 66 (18.0) |
| >50% | 35 (9.6) |
| NA | 71 |
| Log odds of positive lymph node, n (%) | |
| Less than <2 | 100 (27.3) |
| −1.99 to −0.9 | 174 (47.5) |
| −0.89 to 1.5 | 79 (21.6) |
| >1.5 | 13 (3.6) |
| NA | 71 |
| Lymphovascular invasion, present, n (%) | 209 (47.8) |
| Perineural invasion, present, n (%) | 356 (81.5) |
| Length of stay, d, median (IQR) | 10 (7–17) |
| Patients status, death, n (%) | 309 (70.6) |
| Neoadjuvant therapy, performed, n (%) | 16 (3.9) |
| Adjuvant therapy, performed, n (%) | 110 (25.2) |
AJCC, American Joint Committee on Cancer; ASA, American Society of Anesthesiologists; IQR, interquartile range; NA, not available.
Figure 1.
Kaplan-Meier curve depicting overall survival (OS) among 437 patients who underwent liver resection for perihilar cholangiocarcinoma stratified by American Joint Committee on Cancer (AJCC) N stages.
Performance of the lymph node ratio and log odds of metastatic lymph nodes
Initial performances of AJCC N staging, LNR, and LODDS were evaluated to examine the impact of TNLE on the discriminatory ability to predict prognosis. The 5-year OS rates, according to the previously proposed LNR categories were LNR 0: 22.4%, LNR 0.01 to 0.25: 20.7%, LNR 0.26 to 0.50: 13.7%, and LNR >0.50: 10.1%. The 5-year OS rates of the different LODDS categories were LODDS less than −2: 25.7%, LODDS −1.99 to −0.9: 19.2%, LODDS −0.89 to 1.5: 14.0%, and LODDS >1.5: 8.6%. When assessed using the established categorical cutoff values, LODDS (c-index = 0.586) had a slightly better prognostic performance than both the AJCC N staging (c-index = 0.561) and LNR (c-index = 0.575) staging systems. When stratified by TNLE (<4 vs ≥4 LN), LODDS performed better among patients who had <4 TNLE (c-index = 0.568) compared with AJCC N staging (c-index = 0.543) or LNR (c-index = 0.553). In contrast, among patients with ≥4 TNLE, LNR (c-index = 0.605) and LODDS (c-index = 0.613) performed similarly, yet better than AJCC N staging (c-index = 0.580).
Examining each scoring system as continuous variables was then performed to further assess the discriminatory ability of LNR and LODDS. The LODDS (c-index = 0.588) had more discriminatory power than either AJCC N staging (c-index = 0.543) or LNR (c-index = 0.577). When stratified by TNLE, LODDS (c-index = 0.572) as a continuous prognostic factor was better than both AJCC N staging (c-index = 0.543) and LNR (c-index = 0.553) among patients with low TNLE (<4 LN). However, among patients with ≥4 LNs examined, LODDS (c-index = 0.615) and LNR (c-index = 0.616) again had a similar prognostic performance, yet better than the AJCC N staging (AJCC, c-index = 0.543).
The prognostic ability of AJCC N stage, LNR, and LODDS were then tested in 3 different multivariable models to adjust for the possible confounding effect of other prognostic factors (Table 2). Both carbohydrate antigen 19-9 and AJCC T stage were confirmed as independent predictors of OS in all 3 models. In multivariable analysis, LODDS demonstrated the best ability to stratify patient prognosis according to nodal status (AJCC N c-index = 0.626 vs LNR c-index = 0.630 vs LODD c-index = 0.640). Interestingly, on multivariable analysis, patients who were classified as Nx and N1 had a similar risk of death.
Table 2.
Multivariable Survival Analysis for 366 Patients Who Underwent Lymphadenectomy
| Variable | Model 1
|
Model 2
|
Model 3
|
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Carbohydrate antigen 19-9* | 1.16 (1.08–1.24) | <0.001 | 1.15 (1.07–1.23) | <0.001 | 1.14 (1.07–1.22) | <0.001 |
|
| ||||||
| AJCC T stage | ||||||
|
| ||||||
| T1 | Ref | Ref | Ref | |||
|
| ||||||
| T2a–T2b | 1.48 (0.91–2.41) | 0.11 | 1.45 (0.89–2.36) | 0.13 | 1.46 (0.90–2.37) | 0.12 |
|
| ||||||
| T3–T4 | 1.92 (1.14–3.23) | 0.014 | 1.96 (1.16–3.29) | 0.011 | 1.97 (1.18–3.29) | 0.009 |
|
| ||||||
| AJCC N stage | ||||||
|
| ||||||
| N0 | Ref | |||||
|
| ||||||
| N1 | 1.36 (1.05–1.75) | 0.019 | ||||
|
| ||||||
| Lymph node ratio | ||||||
|
| ||||||
| 0% | Ref | |||||
|
| ||||||
| 1% to 25% | 1.12 (0.77–1.62) | 0.56 | ||||
|
| ||||||
| 25% to 50% | 1.39 (1.00–1.94) | 0.049 | ||||
|
| ||||||
| >50% | 1.76 (1.17–2.65) | 0.007 | ||||
|
| ||||||
| Log odds of positive lymph node | ||||||
|
| ||||||
| Less than −2 | Ref | |||||
|
| ||||||
| −1.99 to −0.9 | 1.34 (0.99–1.85) | 0.06 | ||||
|
| ||||||
| −0.89 to 1.5 | 1.67 (1.16–2.40) | 0.006 | ||||
|
| ||||||
| >1.5 | 2.80 (1.44–5.43) | 0.002 | ||||
|
| ||||||
| C-index | 0.626 | 0.630 | 0.640 | |||
Hazard ratio and CIs estimated for the natural logarithm of the carbohydrate antigen 19-9 serum level.
AJCC, American Joint Committee on Cancer; HR, hazard ratio; Ref, reference.
Bayesian model determining the minimum number of harvested node to stage patients
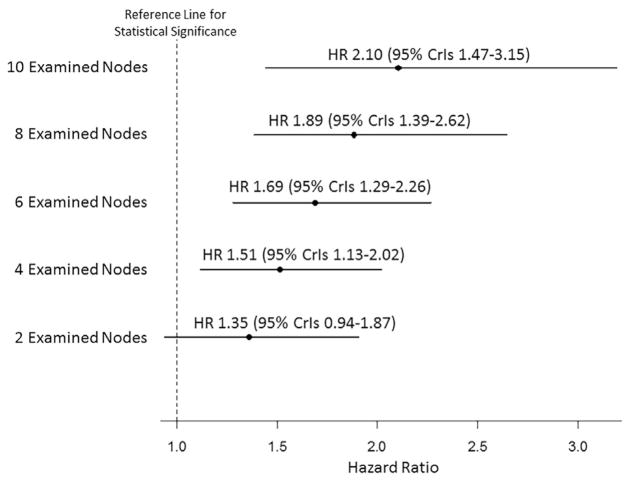
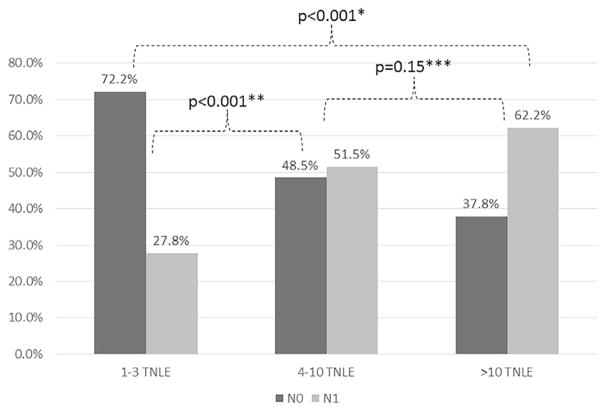
To further investigate the prognostic role of nodal status among patients undergoing liver resection for PHCC, a Bayesian Weibull model was developed. The model was built to compare the survival of patients without (N0) and with (N1) LNs metastasis discretized by the TNLE to identify the minimum TNLE to optimally stage patients with PHCC (Fig. 2 and Table 3). Among patients who had only 2 LNs examined, the HR (95% CrI) included the reference line for statistical significance, demonstrating a poor discriminatory ability to distinguish among patients with a favorable vs poor prognosis. For example, among patients who had only 2 LNs examined, 5-year OS rate for N0 patients was 23.9% (95% CrI, 17.9–30.7) vs 16.1% (95% CrI, 9.3–24.0) for N1 patients. In contrast, among patients with TNLE >2, the HR for N1 patients was above the reference line. In addition, the prognostic discriminatory of nodal status increased as the TNLE increased. For example, 5-year OS rates among N0 patients improved as the TNLE increased (TNLE 2: 23.9% vs TNLE 4: 26.9% vs TNLE 6: 29.9% vs TNLE 10: 36.2%). In contrast, 5-year OS rates among N1 patients were only slightly affected by the number of TNLE (TNLE 2: 16.1% vs TNLE 4: 15.0% vs TNLE 6: 14.0% vs TNLE 10: 12.2%). Among patients with N1 disease, the corresponding HR associated with OS was 1.51 (95% CI, 1.13–2.02) among patients who had 4 TNLE vs 2.10 (95% CI, 1.47–3.15) among patients who had 10 TNLE. The 5-year OS for N0 with <4 TNLE vs N0 with ≥4 TNLE was 19.7% (95% CI, 13.0–29.9) and 26.2% (95% CI, 16.7–41.1), respectively (p = 0.08). In addition, increasing the TNLE, the rate of metastatic LNs was statistically different, ranging from 27.8% when the TNLE was 1 to 3, to 51.5% when the TNLE was 4 to 10, and to 62.2% when the TNLN was >10 (both p < 0.001; Fig. 3). Interestingly, the proportion of metastatic LNs was not different when ≥4 or >10 nodes were harvested (p = 0.15).
Figure 2.
Results from the Bayesian Weibull proportional hazard model: hazard ratio (HR) between N1 and N0 patients stratified by the total number of lymph nodes examined.
Table 3.
Results from the Bayesian Weibull Proportional Hazard Model
| Node status | 5-Year overall survival, % | 95% CrI | Hazard ratio | 95% CrI |
|---|---|---|---|---|
| Node status actual survival* | 1.54 | 1.21–1.98 | ||
| N0 | 22.4 | 16.5–30.4 | ||
| N1 | 15.4 | 9.9–23.9 | ||
| Node status Bayesian model with 2 TNLE | 1.35 | 0.94–1.87 | ||
| N0 | 23.9 | 17.9–30.7 | ||
| N1 | 16.9 | 9.3–24.0 | ||
| Node status Bayesian model with 4 TNLE | 1.51 | 1.13–2.02 | ||
| N0 | 26.9 | 21.3–33.0 | ||
| N1 | 15.0 | 9.3–21.7 | ||
| Node status Bayesian model with 6 TNLE | 1.69 | 1.29–2.26 | ||
| N0 | 29.9 | 23.3–37.1 | ||
| N1 | 14.0 | 9.0–19.9 | ||
| Node status Bayesian model with 8 TNLE | 1.89 | 1.39–2.62 | ||
| N0 | 33.1 | 24.6–42.2 | ||
| N1 | 13.1 | 8.4–18.8 | ||
| Node status Bayesian model with 10 TNLE | 2.10 | 1.47–3.15 | ||
| N0 | 36.2 | 25.3–47.9 | ||
| N1 | 12.2 | 7.6–18.2 |
Three-year overall survival and 95% CI estimated with the Kaplan-Meier method, hazard ratio and 95% CI estimated with a univariate Cox model.
CrI, credible interval; TNLE, total number of lymph nodes examined.
Figure 3.
Frequency of patients with negative (N0) and metastatic (N1) nodes stratified by total number of lymph nodes examined (TNLE). p Value from chi-squared test comparing *1–3 TNLE vs >10 TNLE; **1–3 TNLE vs 4–10 TNLE; ***4–10 TNLE vs >10 TNLE.
Lymph node stations
A total of 107 patients had detailed data on LNs stations; the distribution and the incidence of metastatic LNs were examined relative to the various nodal stations (Table 4). In the overwhelming majority of patients (n = 100 [93.5%]), LNs from group 1 (stations 12) were removed. In this group of patients, median TNLE was 5 (IQR 3 to 8); 40 (40.0%) patients had LN metastasis. Of note, station 12a (63.6%) and 12p (52.3%) were the areas from which LNs were most commonly removed; the incidence of metastatic disease in these basins was relatively comparable (station 12h: 34.0%, 12a: 32.4%, 12p: 35.7%, and 12b: 27.1%). Group 2 stations 1, 7, 8, and 13 were examined in 63 (58.9%) patients; the median TNLE from these stations was 3 (IQR 2 to 7) and 26 (41.3%) patients had metastatic LNs. The incidence of metastatic LNs in stations 7, 8, and 13 (ranging between 31.3% and 50.0%) was comparable with stations 12 (ranging between 27.1% and 35.7%). Specifically, 6 (35.3%) patients had metastatic nodes in station 7, 15 (31.3%) in station 8, and 15 (50.0%) in station 13. Group 3 stations (9, 14, and 16) were examined in only 23 (21.5%) patients with a median TNLE of 3 (IQR 1 to 6); 5 (21.7%) patients had LN metastasis in this cohort. The incidence of metastatic disease ranged from 14.3% in station 9 to 20.0% in station 14.
Table 4.
Lymph Node Stations (n = 107)
| Node group | Lymphadenectomy performed, n (%) | TNLE, median (IQR) | Patients with metastatic nodes, n (%) |
|---|---|---|---|
| Group 1 | 100 (93.5) | 5 (3–8) | 40 (40.0) |
| 12h | 50 (46.7) | 2 (1–3) | 17 (34.0) |
| 12a | 68 (63.6) | 2 (1–4) | 22 (32.4) |
| 12p | 56 (52.3) | 2 (1–4) | 20 (35.7) |
| 12b | 48 (44.9) | 1 (1–2) | 13 (27.1) |
| Group 2 | 63 (58.9) | 3 (2–7) | 26 (41.3) |
| 1 | 2 (1.9) | 1 (1–2) | 0 |
| 7 | 17 (15.9) | 3 (2–4) | 6 (35.3) |
| 8 | 48 (44.9) | 1 (2–4) | 15 (31.3) |
| 13 | 30 (28.0) | 2 (1–3) | 15 (50.0) |
| Group 3 | 23 (21.5) | 3 (1–6) | 5 (21.7) |
| 9 | 14 (13.1) | 1.5 (1–5) | 2 (14.3) |
| 14 | 5 (4.7) | 3 (1–3) | 1 (20.0) |
| 16 | 12 (11.2) | 2.5 (1–5) | 2 (16.7) |
IQR, interquartile range; TNLE, total number of lymph nodes examined.
DISCUSSION
First described by Gerald Klatskin in 1965, PHCC is a challenging disease that often requires a complex treatment approach, including surgical resection, yet PHCC is generally associated with poor long-term outcomes.3 A wide range of factors predict prognosis after surgery for patients with PHCC, including positive surgical margins, advanced T stage, perineural and perivascular invasion, and poor tumor differentiation.3 In addition to these clinicopathologic variables, LN status is one of the most influential and well-established independent predictors of long-term survival.5,13,14,32–35 Despite this, the role of LN dissection during surgery for PHCC is controversial and the “ideal” TNLE remains poorly defined. Previous studies have demonstrated that an insufficient number of nodes harvested/examined at the time of surgery for other types of gastrointestinal tumors can lead to underestimation of tumor stage.36–41 For this reason, a minimum number of nodes to be examined has been established for diseases such as gastric, colon, and pancreatic cancer.36–42 In fact, a minimal TNLE has been proposed as a quality metric for colon cancer and adjuvant therapy can be delivered based on an inadequate TNLE.43 In contrast, the minimum number of LNs to be examined for PHCC has not been determined. The current study is important because it used a large cohort of patients with PHCC from 12 different major hepatobiliary centers. As such, we were able to define the practice patterns for lymphadenectomy and TNLE from a wide range of representative high-volume centers. Perhaps more importantly, we examined the prognostic discriminatory power of several different LN staging systems. Rather than AJCC N system, LODDS and LNR were noted to perform better in predicting long-term survival of patients with PHCC. In particular, LODDS performed best among those patients with fewer TNLE (<4 LN). These data emphasize that the need for logarithmic adjustment likely depends on the number of nodes examined—with a greater need to use LODDS among those patients who had fewer LNs examined (<4 nodes). In addition, using a Bayesian Weibull model to directly compare the survival of patients with and without metastatic LN disease, we noted that at least 4 nodes were required to stage accurately patients undergoing surgery for PHCC (Table 3).
The TNLE associated with surgery for PHCC varies considerably. In one study from Memorial Sloan Kettering Cancer Center of 144 patients,24 median number of LNs harvested during surgery was 3 (range 0 to 16 LNs), and in an Italian cohort of 53 patients, median TLNE was 7 (range 1 to 25).44 In contrast, several series from East-Asian centers have reported considerably higher TNLE. For example, in 2 different reports from Japan,13,20 median TNLE was 11 in a series of 320 patients and, amazingly, in a separate series of 110 patients undergoing surgery for PHCC, the median TNLE was 24.12,19 The current study consisted of 437 patients with PHCC with a median TNLE of 3 (IQR 1 to 7), which was similar to other published experiences from Western centers.18,45 Specifically, we noted that 117 (26.8%) patients had only 1 to 2 LNs examined, 64 (14.6%) had 3 to 4 LNs, and 185 (42.3%) patients had ≥4 LNs examined. Consistent with previous data,5,46,47 the incidence of LN metastasis was 36.2%. Perhaps not surprisingly, the incidence of finding LN metastasis increased as the number of TNLE increased (Fig. 3).
On multivariable analyses, several factors were associated with long-term prognosis, yet LN metastasis remained among the most powerful independent predictors of worse outcomes. In addition, a difference in survival was observed between patients with 1 to 3 metastatic LN vs >3.20 Unlike most previous studies, we investigated nodal status using not only the AJCC staging system, but also LNR and LODDS. Of note, LNR and LODDS both performed better than the AJCC N staging when assessed using both the established categorical cutoff values and as continuous values. When nodal status was investigated considering the number of LNs harvested, the prognostic power of LNR and LODDS varied. Specifically, LNR performed best among patients who had a larger TNLE; in contrast, among patients who had only a few TNLE, LODDS had the highest prognostic discriminatory power (Table 2). Previously, our group had suggested that LODDS might be the optimal manner to stratify the prognosis of patients with intrahepatic or gallbladder cancer.29–31 Although LNR is directly correlated with TNLE and number of metastatic LNs, LODDS represents the natural logarithmic transformation of LNs. For this reason, LODDS can better discriminate patients who might have similar LNR, but intuitively very different long-term prognoses (eg TNLE 1/number of metastatic LN 1 vs TNLE 6/number of metastatic LN 6). As such, although LNR might perform well in patients with high TNLE, the use of LODDS might be particularly relevant in surgical patients undergoing lymphadenectomy traditionally associated with a low TNLE yield.29–31
The ideal TNLE for patients undergoing surgery for PHCC has been a matter of some debate. Although investigators from Memorial Sloan Kettering Cancer Center have estimated the optimal TLNE to be 7, other investigators have suggested that the number of LNs to be examined for PHCC should be 5.20 The current study is unique in that we were the first to use more advance statistical modeling, using a Bayesian Weibull model in an attempt to define the optimal TNLE for PHCC. We noted that at least 4 nodes were required to accurately stage patients undergoing surgery for PHCC (Table 3). Interestingly, although 5-year OS of N1 patients was only slightly affected by the TNLE, the TNLE markedly impacted the OS of N0 patients. Specifically, when <4 LNs were harvested, there was a higher risk of a false negative (ie classification as N0 when really N1). These results from the Bayesian model were confirmed by the finding that the 5-year OS rate of N0 <4 TNLE patients (19.7%) tended to be worse compared with the 5-year OS rate of N0 ≥4 TNLE patients (26.2%; p = 0.08). The incidence of metastatic LNs was about doubled when we compared TNLE <4 to ≥4, and the incidence of metastatic LNs was similar when comparing TNLE 4 to 10 vs TNLE >10 (Fig. 3).
Another strength of the current study was the evaluation of LN metastasis according to the various nodal stations as classified by the Japanese Society of Hepato-Biliary-Pancreatic Surgery.28 Group 1 nodes (station 12) were the most frequently retrieved and examined, consistent with data reported by Guglielmi and colleagues44 in a series of 145 patients with ICC or PHCC. Of note, in about two-thirds of patients, the lymphadenectomy extended to the group 2 basin, nodes along the left gastric artery (station 7), the common hepatic artery (station 8), and posterior aspect of the head of the pancreas (station 13). Interestingly, among the 26 patients with metastatic nodes in group 2, eleven (42.3%) patients did not have any metastatic nodes in the examined group 1 nodes. Although the incidence of nodal metastasis was comparable in groups 1 and 2, the incidence of metastatic nodes in group 3 (nodes around the celiac artery, along the superior mesenteric artery, and periaortic) was lower, at about 20%. The incidence of nodal disease in basin 3 among the current cohort was comparable with that published in a different group of PHCC patients by Kitagawa and colleagues.13 We also found that every patient who had metastatic disease in the group 3 basin also had metastatic LNs found in either group 1 or 2 basins. As such, we believe that extirpation of both group 1 and group 2 LNs should be considered as standard lymphadenectomy for patients with PHCC.
Our study had several limitations that should be considered when interpreting the results. As with all retrospective studies, there might have been a selection bias with the diagnosis and treatment of patients with PHCC. Although the multicenter nature of the study is a considerable strength that conferred a larger sample size and more generalizability, it also led to some variability in surgical and treatment approaches. Finally, although LODDS cannot be empirically calculated at the bedside due to the need for the logarithmic computation, online calculators are becoming increasingly available to calculate LODDS.
CONCLUSIONS
The LODDS and LNR were both better predictors of survival after curative intent resection of PHCC than the AJCC nodal staging. In addition, although LODDS performed better at determining prognosis among patients with <4 LN, both LNR and LODDS outperformed AJCC N stage among patients with ≥4 LN examined. Our data strongly suggest that patients with PHCC undergoing radical resection should ideally have at least 4 LNs harvested. Lymph nodes from the hepatoduodenal ligament (stations 12), stations 7 (along left gastric artery), 8 (along the hepatic artery), and 13 (on the posterior aspect of the head of the pancreas) should be removed given the high risk of metastatic nodes in these stations and the possibility that metastatic nodes might skip stations 12.
Abbreviations and Acronyms
- AJCC
American Joint Committee on Cancer
- CrI
credible interval
- HR
hazard ratio
- IQR
interquartile range
- LN
lymph node
- LNR
lymph node ratio
- LODDS
log odds of metastatic lymph nodes
- OS
overall survival
- PHCC
perihilar cholangiocarcinoma
- TNLE
total number of lymph nodes examined
APPENDIX MATERIAL AND METHODS
Standard data on demographic, clinicopathologic, tumor, and therapy-related variables were collected. In particular, American Society of Anesthesiologists score, carbohydrate antigen 19-9 levels, presence of jaundice or ascites, and preoperative placement of biliary drainage or stent were recorded. Data on tumor-specific factors, such as tumor size, margin status, tumor type based on the Bismuth-Corlette classification, as well as tumor stage according to seventh edition of the American Joint Committee on Cancer (AJCC) staging system were obtained. Tumor grade was categorized as G1 to G2 vs G3 to G4 based on the grade of differentiation. Data on treatment-related variables, such as portal vein embolization, type of surgery, portal vein or hepatic artery resection, and radiation, or chemotherapy was recorded. In addition, information on receipt of lymphadenectomy was also collected. Data on short- and long-term outcomes, including intraoperative estimated blood loss, complications, as well as length of hospital stay, date of last follow-up, and vital status were also collected.
Bayesian model
A Bayesian framework was used to analyze the effects of the number of harvested and metastatic nodes on survival. Bayesians model are characterized by their ability of combining noninformative earlier distributions with clinical data to obtain posterior distributions from which probability statements can be made about all model parameters.
The survival distribution was assumed to be Weibull:
where ti is the failure time of the ith individual with covariate vector xi, β is a vector of unknown regression coefficients, and ρ the shape parameter of the Weibull distribution expressing the shape of the hazard function.
Defining μ = e−βi*xi gives the parameterization
For censored observations, the survival distribution is a truncated Weibull, with lower bound corresponding to the censoring time.
The regression β coefficients were assumed a priori to follow independent normal distributions with zero mean and “vague” precision 0.0001. The shape parameter ρ for the survival distribution was given a gamma earlier with one mean and “vague” precision 0.0001, which is slowly decreasing on the positive real line.
The R package R2OpenBUGS was used to implement the following code for the Bayesian model in OpenBugs according to the model presented by George G Woodworth.1
MODEL Weibull {
# Prior noninformative distribution of ρ, the shape
parameter of the Weibull distribution
r ~ dgamma(1,.0001)
# Earlier noninformative distribution of β, the vector of
unknown regression coefficients
for (i in 1:k) {
beta[i] ~ dnorm(0,.0001)
}
# Likelihood of the Weibull distribution
for (j in 1:n) {
mu[j] <- exp(inprod(x[j,],beta[]))
mu2[j] <- mu[j]
t.o[j] ~ dweib(r, mu2[j])I(t.c[j],)
}
# Survival rates for the analysis,
for (j in 1:m) {
mu.tab [j] <- exp(inprod(x.r[j,],beta[]))
Surv.r[j] <- exp(-mu.tab[j]*pow(t.r[j],r))
}
# Contrasts of interest in this analysis:
# N1/N0 RRs and HRs
for (j in 1:5) {
RRn1n0[j] <- (1- Surv.r[2*j])/(1- Surv.r[2*j-1])
HRn1n0[j] <- HRr[2*j]/HRr[2*j-1]
}
}
To assess convergence of samples to the posterior, parallel chains were simulated with different starting values and evaluated with the multivariate potential scale reduction factor of Brooks and Gelman as provided by the coda R package.
Patient characteristics
Median patient age was 66.5 years (interquartile range [IQR] 52.8 to 73.0 years) with a majority of patients being male (n = 272 [62.6%]). Although only a small subset of patients had ascites (n = 9 [2.6%]), the majority had jaundice (n = 284 [78.5%]). Preoperative biliary drainage was performed in 272 (75.8%) patients. The median carbohydrate antigen 19-9 serum level was 102.2 U/mL (IQR, 38.0 to 349.2 U/mL). Median PHCC tumor size was 25 mm (IQR 16 to 40 mm) and most patients had a Bismuth type IIIa/IIIb tumor (n = 216 [49.5%]); portal vein embolization was performed in 31 (7.1%) patients. Patients most often underwent either a bile duct resection plus right hepatectomy (n = 165 [37.7%]) or a bile duct resection plus left hepatectomy (n = 158 [36.2%]); a smaller subset of patients (n = 114 [26.1%]) underwent other procedures, including isolated bile duct resection or duodenal-pancreatectomy concomitant with liver resection. Major vascular resection was performed in a minority of patients (portal vein resection, n = 49 [11.2%]; hepatic artery resection, n = 15 [3.4%]). A complete R0 resection was achieved in 296 (67.7%) patients. Median intraoperative estimated blood loss was 600 mL (IQR 350 to 1,150 mL) and 91 (22.9%) patients received an intraoperative transfusion with at least 1 U of packed RBCs. On the final pathology report, the majority of patients had a G1 to G2 tumor (n = 339 [77.6%]) and a stage T2 tumor (n = 271 [62.0%]). Lymphovascular and perineural invasion were noted in 209 (47.8%) and 356 (81.5%) patients, respectively. Median length of say was 10 days (IQR 7 to 17 days). Half of all patients received some form of adjuvant therapy (n = 110 [25.2%]), and <5% (n = 16, 3.9%) received neoadjuvant therapy.
Long-Term Outcomes
The 1-, 3-, and 5-year OS rates of the entire cohort were 71.6% (95% CI, 66.9–75.7), 35.3% (95% CI, 30.3–40.3), and 19.8% (95% CI, 15.4–24.5), respectively; median survival was 22.7 months (IQR 20.1 to 25.1 months). Several patient- and tumor-specific factors were associated with worse overall survival rates, including carbohydrate antigen 19-9 serum level (HR = 1.15; 95% CI, 1.08–1.23; p < 0.001), margin status (R1, HR = 1.29; 95% CI, 1.10–1.64; p = 0.042), AJCC T stage (T2a–T2b; HR = 1.64; 95% CI, 1.07–2.49; p = 0.02; T3–T4, HR = 2.21; 95% CI, 1.39–3.54; p < 0.001), and AJCC N stage (N1, HR = 1.30; 95% CI, 1.01–1.69; p = 0.042).
- 1.Woodworth GG. Biostatistics: A Bayesian Introduction. Hoboken, NJ: Wiley-Interscience; 2004. p. xvi.p. 360. pages: illustrations; 25 cm. [Google Scholar]
Footnotes
Disclosure Information: Nothing to disclose.
Author Contributions
Study conception and design: Bagante, Tran, Spolverato, Ruzzenente, Buttner, Ethun, Koerkamp, Conci, Idrees, Isom, Fields, Krasnick, Weber, Salem, Martin, Scoggins, Shen, Mogal, Schmidt, Beal, Hatzaras, Vitiello, IJzermans, Maithel, Poultsides, Guglielmi, Pawlik
Acquisition of data: Bagante, Tran, Spolverato, Ruzzenente, Buttner, Ethun, Koerkamp, Conci, Idrees, Isom, Fields, Krasnick, Weber, Salem, Martin, Scoggins, Shen, Mogal, Schmidt, Beal, Hatzaras, Vitiello, IJzermans, Maithel, Poultsides, Guglielmi, Pawlik
Analysis and interpretation of data: Bagante, Pawlik
Drafting of manuscript: Bagante, Tran, Spolverato, Ruzzenente, Buttner, Ethun, Koerkamp, Conci, Idrees, Isom, Fields, Krasnick, Weber, Salem, Martin, Scoggins, Shen, Mogal, Schmidt, Beal, Hatzaras, Vitiello, IJzermans, Maithel, Poultsides, Guglielmi, Pawlik
Critical revision: Bagante, Tran, Spolverato, Ruzzenente, Buttner, Ethun, Koerkamp, Conci, Idrees, Isom, Fields, Krasnick, Weber, Salem, Martin, Scoggins, Shen, Mogal, Schmidt, Beal, Hatzaras, Vitiello, IJzermans, Maithel, Poultsides, Guglielmi, Pawlik
References
- 1.Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–3918. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergquist A, von Seth E. Epidemiology of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015;29:221–232. doi: 10.1016/j.bpg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Valero V, 3rd, Cosgrove D, Herman JM, et al. Management of perihilar cholangiocarcinoma in the era of multimodal therapy. Expert Rev Gastroenterol Hepatol. 2012;6:481–495. doi: 10.1586/egh.12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakeeb A, Pitt HA, Sohn TA, et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg. 1996;224:463–473. doi: 10.1097/00000658-199610000-00005. discussion 473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Oliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 7.Choi JY, Kim MJ, Lee JM, et al. Hilar cholangiocarcinoma: role of preoperative imaging with sonography, MDCT, MRI, and direct cholangiography. AJR Am J Roentgenol. 2008;191:1448–14457. doi: 10.2214/AJR.07.3992. [DOI] [PubMed] [Google Scholar]
- 8.Ruys AT, van Beem BE, Engelbrecht MR, et al. Radiological staging in patients with hilar cholangiocarcinoma: a systematic review and meta-analysis. Br J Radiol. 2012;85(1017):1255–1262. doi: 10.1259/bjr/88405305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suarez-Munoz MA, Fernandez-Aguilar JL, Sanchez-Perez B, et al. Risk factors and classifications of hilar cholangiocarcinoma. World J Gastrointest Oncol. 2013;5:132–138. doi: 10.4251/wjgo.v5.i7.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai EC, Lau WY. Aggressive surgical resection for hilar cholangiocarcinoma. ANZ J Surg. 2005;75:981–985. doi: 10.1111/j.1445-2197.2005.03595.x. [DOI] [PubMed] [Google Scholar]
- 11.Nagakawa T, Kayahara M, Ikeda S, et al. Biliary tract cancer treatment: results from the Biliary Tract Cancer Statistics Registry in Japan. J Hepatobiliary Pancreat Surg. 2002;9:569–575. doi: 10.1007/s005340200076. [DOI] [PubMed] [Google Scholar]
- 12.Nishio H, Nagino M, Nimura Y. Surgical management of hilar cholangiocarcinoma: the Nagoya experience. HPB (Oxford) 2005;7:259–262. doi: 10.1080/13651820500373010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitagawa Y, Nagino M, Kamiya J, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233:385–392. doi: 10.1097/00000658-200103000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SG, Song GW, Hwang S, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci. 2010;17:476–489. doi: 10.1007/s00534-009-0204-5. [DOI] [PubMed] [Google Scholar]
- 15.Song SC, Choi DW, Kow AW, et al. Surgical outcomes of 230 resected hilar cholangiocarcinoma in a single centre. ANZ J Surg. 2013;83:268–274. doi: 10.1111/j.1445-2197.2012.06195.x. [DOI] [PubMed] [Google Scholar]
- 16.Furusawa N, Kobayashi A, Yokoyama T, et al. Surgical treatment of 144 cases of hilar cholangiocarcinoma without liver-related mortality. World J Surg. 2014;38:1164–1176. doi: 10.1007/s00268-013-2394-x. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Byrd D, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7. New York: Springer; 2011. [Google Scholar]
- 18.Tamandl D, Kaczirek K, Gruenberger B, et al. Lymph node ratio after curative surgery for intrahepatic cholangiocarcinoma. Br J Surg. 2009;96:919–925. doi: 10.1002/bjs.6654. [DOI] [PubMed] [Google Scholar]
- 19.Lee YC, Yang PJ, Zhong Y, et al. Lymph node ratio-based staging system outperforms the Seventh AJCC System for Gastric Cancer: validation analysis with National Taiwan University Hospital Cancer Registry. Am J Clin Oncol. 2014 Aug 7; doi: 10.1097/COC.0000000000000110. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Aoba T, Ebata T, Yokoyama Y, et al. Assessment of nodal status for perihilar cholangiocarcinoma: location, number, or ratio of involved nodes. Ann Surg. 2013;257:718–725. doi: 10.1097/SLA.0b013e3182822277. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Hassett JM, Dayton MT, et al. The prognostic superiority of log odds of positive lymph nodes in stage III colon cancer. J Gastrointest Surg. 2008;12:1790–1796. doi: 10.1007/s11605-008-0651-3. [DOI] [PubMed] [Google Scholar]
- 22.Aurello P, Petrucciani N, Nigri GR, et al. Log odds of positive lymph nodes (LODDS): what are their role in the prognostic assessment of gastric adenocarcinoma? J Gastrointest Surg. 2014;18:1254–1260. doi: 10.1007/s11605-014-2539-8. [DOI] [PubMed] [Google Scholar]
- 23.Gleisner AL, Spolverato G, Ejaz A, et al. Time-related changes in the prognostic significance of the total number of examined lymph nodes in node-negative pancreatic head cancer. J Surg Oncol. 2014;110:858–863. doi: 10.1002/jso.23715. [DOI] [PubMed] [Google Scholar]
- 24.Ito K, Ito H, Allen PJ, et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg. 2010;251:675–681. doi: 10.1097/SLA.0b013e3181d3d2b2. [DOI] [PubMed] [Google Scholar]
- 25.Gleisner AL, Mogal H, Dodson R, et al. Nodal status, number of lymph nodes examined, and lymph node ratio: what defines prognosis after resection of colon adenocarcinoma? J Am Coll Surg. 2013;217:1090–1100. doi: 10.1016/j.jamcollsurg.2013.07.404. [DOI] [PubMed] [Google Scholar]
- 26.Nathan H, Shore AD, Anders RA, et al. Variation in lymph node assessment after colon cancer resection: patient, surgeon, pathologist, or hospital? J Gastrointest Surg. 2011;15:471–479. doi: 10.1007/s11605-010-1410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J, Hassett JM, Dayton MT, et al. Lymph node ratio: role in the staging of node-positive colon cancer. Ann Surg Oncol. 2008;15:1600–1608. doi: 10.1245/s10434-007-9716-x. [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki M, Ohtsuka M, Miyakawa S, et al. Classification of biliary tract cancers established by the Japanese Society of Hepato-Biliary-Pancreatic Surgery: 3(rd) English edition. J Hepatobiliary Pancreat Sci. 2015;22:181–196. doi: 10.1002/jhbp.211. [DOI] [PubMed] [Google Scholar]
- 29.Amini N, Spolverato G, Kim Y, et al. Lymph node status after resection for gallbladder adenocarcinoma: prognostic implications of different nodal staging/scoring systems. J Surg Oncol. 2015;111:299–305. doi: 10.1002/jso.23813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spolverato G, Ejaz A, Kim Y, et al. Prognostic performance of different lymph node staging systems after curative intent resection for gastric adenocarcinoma. Ann Surg. 2015;262:991–998. doi: 10.1097/SLA.0000000000001040. [DOI] [PubMed] [Google Scholar]
- 31.Kim Y, Spolverato G, Amini N, et al. Surgical management of intrahepatic cholangiocarcinoma: defining an optimal prognostic lymph node stratification schema. Ann Surg Oncol. 2015;22:2772–2778. doi: 10.1245/s10434-015-4419-1. [DOI] [PubMed] [Google Scholar]
- 32.Seyama Y, Kubota K, Sano K, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73–83. doi: 10.1097/01.SLA.0000074960.55004.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawasaki S, Imamura H, Kobayashi A, et al. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84–92. doi: 10.1097/01.SLA.0000074984.83031.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hemming AW, Reed AI, Fujita S, et al. Surgical management of hilar cholangiocarcinoma. Ann Surg. 2005;241:693–699. doi: 10.1097/01.sla.0000160701.38945.82. discussion 699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young AL, Prasad KR, Toogood GJ, et al. Surgical treatment of hilar cholangiocarcinoma in a new era: comparison among leading Eastern and Western centers, Leeds. J Hepatobiliary Pancreat Sci. 2010;17:497–504. doi: 10.1007/s00534-009-0203-6. [DOI] [PubMed] [Google Scholar]
- 36.Tamura S, Takeno A, Miki H. Lymph node dissection in curative gastrectomy for advanced gastric cancer. Int J Surg Oncol. 2011;2011:748745. doi: 10.1155/2011/748745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karpeh MS, Leon L, Klimstra D, et al. Lymph node staging in gastric cancer: is location more important than number? An analysis of 1,038 patients. Ann Surg. 2000;232:362–371. doi: 10.1097/00000658-200009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol. 2001;19:157–163. doi: 10.1200/JCO.2001.19.1.157. [DOI] [PubMed] [Google Scholar]
- 39.Caplin S, Cerottini JP, Bosman FT, et al. For patients with Dukes’ B (TNM Stage II) colorectal carcinoma, examination of six or fewer lymph nodes is related to poor prognosis. Cancer. 1998;83:666–672. [PubMed] [Google Scholar]
- 40.Mellon EA, Springett GM, Hoffe SE, et al. Adjuvant radiotherapy and lymph node dissection in pancreatic cancer treated with surgery and chemotherapy. Cancer. 2014;120:1171–1177. doi: 10.1002/cncr.28543. [DOI] [PubMed] [Google Scholar]
- 41.Ashfaq A, Pockaj BA, Gray RJ, et al. Nodal counts and lymph node ratio impact survival after distal pancreatectomy for pancreatic adenocarcinoma. J Gastrointest Surg. 2014;18:1929–1935. doi: 10.1007/s11605-014-2566-5. [DOI] [PubMed] [Google Scholar]
- 42.Tol JA, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS) Surgery. 2014;156:591–600. doi: 10.1007/978-1-4939-1726-6_59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsons HM, Begun JW, Kuntz KM, et al. Lymph node evaluation for colon cancer in an era of quality guidelines: who improves? J Oncol Pract. 2013;9:e164–e171. doi: 10.1200/JOP.2012.000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guglielmi A, Ruzzenente A, Campagnaro T, et al. Patterns and prognostic significance of lymph node dissection for surgical treatment of perihilar and intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2013;17:1917–1928. doi: 10.1007/s11605-013-2331-1. [DOI] [PubMed] [Google Scholar]
- 45.Guglielmi A, Ruzzenente A, Bertuzzo F, et al. Assessment of nodal status for perihilar cholangiocarcinoma location, number, or ratio of involved nodes. Hepatobiliary Surg Nutr. 2013;2:281–283. doi: 10.3978/j.issn.2304-3881.2013.08.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikeyama T, Nagino M, Oda K, et al. Surgical approach to bismuth Type I and II hilar cholangiocarcinomas: audit of 54 consecutive cases. Ann Surg. 2007;246:1052–1057. doi: 10.1097/SLA.0b013e318142d97e. [DOI] [PubMed] [Google Scholar]
- 47.Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]