Abstract
The goal of our study is to develop and characterize mucoadhesive films with entrapped lysozyme based on gelatin/sodium carboxymethyl cellulose as perspective antimicrobial preparation. Lysozyme in mucoadhesive films retains more than 95 % of its initial activity for 3 years of storage. Different physical-chemical and biochemical characteristics of entrapped enzyme were evaluated, such as film thickness, weight, time of dissolution in water, bioadhesive force, in vitro lysozyme release, pH- and thermoprofiles of hydrolytic activity, effect of γ-sterilization, etc. We have shown that gelatin/sodium carboxymethyl cellulose films have adhesive force on the level of 4380 Pa. Scanning electron microscopy images shows the relative uniformity of the gelatin surface with entrapped lysozyme. Mucoadhesive films with lysozyme have 100% bactericidal effect on the test strain, Staphylococcus aureus ATCC 25923 F - 49 and thus could be considered as a perspective antimicrobial preparation.
Keywords: Mucoadhesive films, entrapment, lysozyme, gelatin, sodium carboxymethyl cellulose, antimicrobial activity
1. Introduction
Lysozyme (muramidase, EC.3.2.1.17) catalyzes the hydrolysis of the cell walls of Gram-positive bacteria and results in a non-specific innate-immunity response against the invasion of bacterial pathogens (Jollès & Jollès, 1984). Additionally, some researchers reported that this enzyme is a unique enzybiotic that exerts not only antibacterial, but also antiviral, anti-inflammatory, anticancer, and immunomodulatory activities (Bachali et al., 2002; Bu et al., 2006; Ferrari, Callerio & Podio, 1959; Helal, Bader & Melzig, 2012; Karle, Hansen & Killmann, 1974; Kawamura et al., 1988; Morioka, Nishimura & Matsumura, 1970; Ogundele, 1998; Okada, 1977; Rymuszka A., 2000).
Reduction of lysozyme in the oral cavity contributes to the accelerated growth of conditionally pathogenic or pathogenic microorganisms (Iacono, MacKay, DiRienzo & Pollock, 1980). This and other factors are causes of the development of dental diseases (stomatitis, gingivitis and periodontitis), which affect ~70% of the human population. Thus, the reduction of lysozyme in the oral cavity constitutes a major health and social problem. (Petkar, 2014).
The oral cavity has been proposed as a potential topical delivery site for proteins in the treatment of dental diseases. Delivery at this site, however, is challenging due to the special structure of the mucin layer, salivation and muscle activity of the mouth (Sankar et al., 2011; Smart, 1993). Furthermore, therapeutic agents must overcome the permeability barrier, evade protecting biological drugs, such as peptides and proteins from enzymatic degradation, have an acceptable taste to patients, and be easily administered without being easily swallowed by accident (Sudhakar, Kuotsu & Bandyopadhyay, 2006).
Creation and application of mucoadhesive polymeric systems (gels, films, microcapsules) with immobilized enzymes could help to overcome some of these challenges. Over the last few decades, there were many examples of the development of lysozyme-loaded forms with used polymeric matrix of natural or synthetic origin, such as siliceous mesocellular foams (Sridhar et al., 2014), ethylene vinyl alcohol copolymers (Muriel-Galet, Talbert, Hernandez-Munoz, Gavara & Goddard, 2013), electrospun chitosan nanofiber (Park, Kim, Park, Jang, Min & Kim, 2013), glycerol diglycidyl ether cross-linked oxidized starch (Zhao et al., 2015), carboxymethyl chitosan-poly(amidoamine) dendrimer (Zhang, Zhao, Wen, Zhu, Yang & Yao, 2013), cellulose and polyacrylamide (Datta, Armiger & Ollis, 1973), calcium carbonate/carboxymethyl cellulose (Lu, Zhang, Ma, Song & Gu, 2012), keratin sponge (Kurimoto, Tanabe, Tachibana & Yamauchi, 2003), chitosan (Bucatariu, 2013), silica nanotubes and nanotubes (Ding, Shao, Liu, Xiao & Chen, 2005; Xiao, Tao, Zou & Chen, 2008), zeolite (Chang, Huang, Lin, Chiu & Tsai, 2006), polystyrene (Wu & Daeschel, 2007), and others. We also consider this direction as promising and thus aimed the current study to develop and characterize mucoadhesive films with incorporated lysozyme based on gelatin/sodium carboxymethyl cellulose as perspective antimicrobial preparation.
The proposed system based on gelatin/CMC in comparison with the other polymeric systems with included lysozyme that have already been reported in the literature (Bucatariu, 2013; Kurimoto, Tanabe, Tachibana & Yamauchi, 2003) has the following advantages: 1) better biocompatibility; 2) higher permeability; 3) increased hydrophilic character; 4) faster biodegradability; 5) absence of toxicity; 6) suitable form and size of the film; 7) higher mucoadhesive properties; and 8) low cost.
2. Material and Methods
2.1. Materials
Lysozyme (activity 40000 U/mg, M.m. ≈ 14.4 kDa) and Micrococcus lysodeikticus ATCC №4698 were purchased from Sigma. Sodium carboxymethyl cellulose (Na-CMC) was obtained from FMC Biopolymer. Gelatin type B and the other chemicals, used in this work, were of analytical grade from local sources.
2.2. Assay of lysozyme hydrolytic activity
The lysozyme activity was measured using the turbidimetric assay based on following the method described (Gorin, Wang & Papapavlou, 1971) and preparation data sheet. One unit will produce a change in A450 of 0.001 per minute at pH 6.24 at 25 °C, using a suspension of Micrococcus lysodeikticus as substrate, in a 2.6 ml reaction mixture (1 cm light path). Suspension of Micrococcus lysodeikticus and lysozyme solution were prepared in 0.1 M sodium phosphate buffer (pH 6.2).
Lytic activity was calculated as follows:
where ΔA450 – the decrease in optical density for 1 min.
F – dilution factor.
C – protein concentration.
0.001– decrease in optical density, which corresponds to 1 unit of activity.
Self-sedimentation of cells was measured and considered as a control value.
2.3. Preparation of gelatin/Na-CMC mucoadhesive films with lysozyme
Known amounts of gelatin solution and Na-CMC were dissolved separately in water. The gelatin solution and Na-CMC solution were mixed, giving two final polymeric concentrations 15.0 % w/v and 0.75 % w/v, respectively.
The lysozyme-loaded gelatin/Na-CMC films were prepared by mixing 5 ml of 4 % lysozyme solution, 1 ml glycerol and 9 ml gelatin/Na-CMC solution. The obtained solution was poured into a petri dish (9 cm in diameter) and dried at 25 °C for 48 h. Then, circles of 0,64 cm2 were cut from each film. Films were packed and then irradiated at incremental doses (10, 17, 28 kGy) of gamma radiation emitted by a 60Coradiation source. Then the films stored at not more than 50% of relative humidity and 4°C.
2.4. Characterization of mucoadhesive films
Thickness, weight, time of dissolution in water, and flatness of mucoadhesive films were characterized using a Mututoyo pocket thickness gauge (Mututoyo Mtc. Co. Ltd., Tokyo, Japan), electronic balance, and stopwatch, respectively. Random sampling of films was done from each batch, and different evaluations were performed in triplicate (n = 5).
2.5. Determination of extracted lysozyme percentage
An accurately weighed 50 mg of lysozyme-loaded films were crushed and dissolved in 250 ml in water by stirring for 3 h using magnetic stirrer. The resulting solutions were then centrifuged using Heraeus Labofuge 400 R.
The suitably diluted filtrate was assayed using a UV-visible spectrophotometer (Shimadzu/UV-1700, Japan) at 280 nm. The percentage of extracted lysozyme was calculated using the formula:
2.6. Determination of mucoadhesive properties of films
Mucoadhesive properties of films were determined by one of the methods (Woertz, Preis, Breitkreutz & Kleinebudde, 2013) on a TA.XT2 Texture analyzer instrument. The polymeric film was fixed on the mobile rod of device and brought in contact with wetted mucosal tissue. The load on rod (2 MPa) and the retention time (60 s) were fixed. Next, at an angle of 90°, the breaking off the rod of device with sample of polymeric film fixed on it, from the mucosal tissue, was accomplished. The device automatically determines force of adhesion. The experiment was conducted at room temperature (23 °C) with five trials.
As a model of mucosal tissue surface, a porcine small intestine segment was used. For the experiment, samples with area of 5 sm2, morphologically homogeneous and without obvious physical defects were excised.
2.7. Surface morphology studies
Selected samples of mucoadhesive films containing the lysozyme were investigated for surface morphology by scanning electron microscopy. SEM samples were sputter coated with Au/Pd using an Edwards Auto 306 (Milan, Italy) electron-beam evaporator and was examined using a scanning electron microscope (Zeiss EVO 50XVP) at 10 kV accelerating voltage.
2.8. In vitro release study
The in vitro release profile of lysozyme from the mucoadhesive films was determined at 37 °C using a shaking water bath. A fixed mass of lysozyme-loaded film was suspended in 3 ml of distilled water. Then nine samples were put in a shaking water bath for 180 min with constant temperature of 37 °C. Film is not removed from the solution before measuring lysozyme activity. It is dissolving gradually and releasing enzyme. As a control we used the film without lysozyme (placebo). We used minimal water volume for modelling conditions similar to human organism. Hydrolytic activity of lysozyme was determined at 5, 15, 30, 45, 60, 75, 90, 120, and 180 min according to item 2.2.
2.9. Effect of pH and temperature on the activity of free and released lysozyme
The effect of pH on free and released from films lysozyme was studied by assessing the activity of preparations at different pH values (3.0–10.0) using the following buffers: citric-phosphate acetate (3.0–6.0), sodium phosphate (pH 7.0–8.0), glycine-sodium hydroxide (9.0–10.0). Lysis reaction has been conducted at 25°C in the buffer solutions mentioned above for pH-profile obtaining. Previously enzyme has been released from the film by dissolving it at 37°C. The film without lysozyme (placebo) was used as a control.
The effect of temperature on free and released from films lysozyme was determined by changing the reaction medium temperature in the range from 20 to 80 °C according to item 2.2.
2.10. Thermostability of free and released lysozyme
The thermostability of the free and released from mucoadhesive films lysozyme was determined by measuring the residual enzymatic activity at 80 °C in 0.1 M sodium phosphate buffer (pH 6.2) for 300 min. After a 30 min time interval, samples were taken and assayed for their enzymatic activity. Each set of experiments was carried out in triplicate. The thermal inactivation constants were calculated from the slope of logarithmic value of residual activity vs. time plot by the linear regression method.
2.11. pH-stability of free and released lysozyme
The pH-stability of the free and released from mucoadhesive films lysozyme was determined by measuring the residual enzymatic activity at pH 5.5 in 0.1 M sodium phosphate buffer for 120 min. Lysis reaction has been conducted at 25°C in the above buffer solution. Previously enzyme has been released from the film by dissolving it at 37°C. As a control we used the film without lysozyme (placebo). After 15min, samples were taken and assayed for enzymatic activity. Each set of experiments was carried out in triplicate.
2.12. Storage stability
Storage stability of free and enzyme in the films was investigated by determining the residual activity after storing the enzyme at intervals of up to 3 years. Samples (1.0 g of lysozyme films) were stored at 4 °C. Activity assay of released lysozyme was performed at 14 days, and then 30 days, intervals.
2.13. Antimicrobial effect of lysozyme films
The antimicrobial activity of the lysozyme film was examined against Staphylococcus aureus ATCC 25923 F-49, Pseudomonas aeruginosa 415, Escherichia coli 055 K59912/4, and Candida albicans ATCC 885 – 653 according to the inhibition zone assay (Lian, Ma, Wei & Liu, 2012). Fifteen milliliters of molten nutrient agar were inoculated with a 100 μl of bacterial culture (colony count of 107 cfu/ml). One sterile film disk (0.64 sm2) with lysozyme was placed at the center of the bacterial lawn. The agar plates were then incubated at 37 °C for one week in an appropriate incubation chamber. All experiments were carried out under sterile conditions. The diameter of the inhibition zone (mm), surrounding the test sample, was measured each day and was used as an indicator of antimicrobial effectiveness of the lysozyme-loaded film. The experiment was carried out in triplicate.
3. Results and discussion
3.1. Lysozyme inclusion
Gelatin and sodium carboxymethyl cellulose, the mucoadhesive polymers for medical purposes, were selected as carriers for lysozyme. These polymers are characterized by high hydrophilicity, and the large number of available functional groups capable of forming ionic or hydrogen bonds with biologically active substances. (Laffleur, 2014; Veis, 1964). Because of these properties, gelatin and sodium carboxymethyl cellulose are promising for non-covalent immobilization, with a lesser degree of affecting the structure of the enzyme protein globule.
Previously (Dekina, Romanovska & Gromovoy, 2011), we had investigated the behavior of lysozyme molecules inclusion in solutions of gelatin and sodium carboxymethyl cellulose by matrix assisted laser desorption/ionization (MALDI). The results confirmed the presence of lysozyme in an aqueous solution in the monomeric and oligomeric forms. It was also shown for the first time that the lysozyme inclusion by non-covalent interactions in the gelatin solution and Na- CMC leads to the dissociation of the oligomeric structure of the enzyme.
Preliminary experiments on animals showed high bioavailability and therapeutic efficacy in the treatment of artificially induced stomatitis on rats during buccal administration of lysozyme. To adapt the lysozyme concentration in our films to humans, we used the conversion factors of animal doses to human equivalent doses (HED) based on body surface area for extrapolating doses between species according to the FDA “Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers” (FDA, 2005).
HED was calculated as follows:
Lysozyme entrapment efficiency in all films is listed in Table 1. As one could see from there, the best results were observed in the samples 4 and 5 at a weight ratio lysozyme: gelatin 1: 3.5 and 1: 5, respectively.
Table 1.
Composition of the mucoadhesive polymer films
| № | Component content (mg/films) | Water content (%) | Lysozyme entrapment efficiency (%) | |||
|---|---|---|---|---|---|---|
| Lysozyme (weight ratio of lysozyme: gelatin) | Gelatin | Na-CMC | Glycerol | |||
| 1 | 2 (1:1) | 2 | 0.1 | 0.005 | 10 | 30 |
| 2 | 2 (1:2.5) | 4.0 | 0.2 | 50 | ||
| 3 | 2 (1:3) | 5.5 | 0.25 | 85 | ||
| 4 | 2 (1:3.5) | 6.7 | 0.34 | 100 | ||
| 5 | 2 (1:5) | 10 | 0.5 | 100 | ||
3.2. Properties of mucoadhesive films with lysozyme
The main characteristics of mucoadhesive films obtained as a result of enzyme inclusion into polymeric matrix based on gelatin with addition of Na-CMC are shown in Table 2.
Table 2.
Characteristics of mucoadhesive films with lysozyme.
| Property, units | Value ±SD* |
|---|---|
| Hydrolytic activity of one film, unit | 75480±1250 |
| Lysozyme content in one film, mg | 2.0 |
| Area, cm2 | 0.8±0.1 |
| Thickness, mm | 0.35±0.02 |
| Average weight of the film, mg | 8±0.02 |
| Visual characteristics of the film | transparent, smooth, light yellow |
| Time of film dissolution in water, physiological saline, min at 37 °C | 18±0.5 |
| Water content in film, % | 10.0±1.7 |
| Activity after sterilization by γ-irradiation (10–28 kGy) | 100 % |
| Mucoadhesive force, Pa | 4380±250 |
SD, mean-square deviation, n = 3.
As clearly shown from Table 2, the mechanical stability of the films was good. This is important for buccal delivery system. Time of the film dissolution in water and physiological solution is similar, 17.5 and 18.5 min at 37 °C, respectively.
After sterilization by γ-radiation at the doses of 10, 17, 28 kGy the included enzyme retains 100 % of its biological activity (Table 2). This indicates the existence of radio protective properties of gelatin for lysozyme. The impact of high energetic rays between 10 and 100 kGy on gels and solutions of gelatin was studied earlier (Voigt, 1986). It was shown that physical and biopharmaceutical properties of gelatin depend on the content of glycerol and water. We suggest that the low water content in the films causes radioprotective properties of gelatin for the lysozyme.
Mucoadhesive force of gelatin/Na-CMC combination in films is 4380±250 Pa (Table 2), which is comparable to the values of adhesion for the most commonly used adhesive polymers. Various mechanisms of mucoadhesion were considered in the recent reviews of the mucoadhesive forms (Carvalho, Bruschi, Evangelista & Gremião, 2010; Khurana, Madhav & TANGRI, 2011). These mechanisms include electronic, adsorption, wetting, diffusion, and fracture theories. But the chemical and physical basis of mucoadhesion is not well understood yet, and adhesion of gelatin/Na-CMC films can be explained by several theories.
Results of SEM show some irregularity of the gelatin/Na-CMC surface with entrapped lysozyme (Fig. 1b). As can be seen from the selected photographs, polymeric system contains enzyme agglomerates of various size but not greater than 3 microns in diameter (if we accept that the particles are spherical).
Fig. 1.
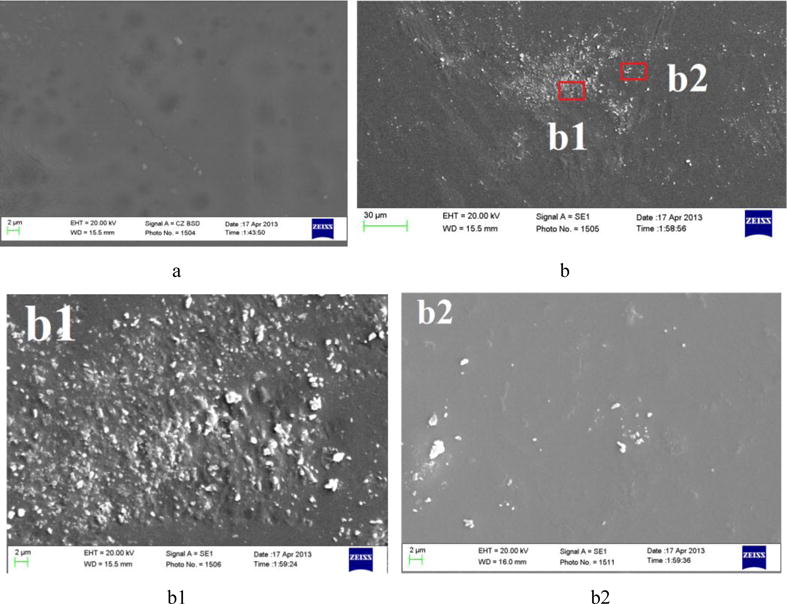
Scanning electron micrographs of the surface of gelatin/Na-CMC film (a) and gelatin/Na-CMC film with included lysozyme (b – 30 μm, b1, b2 – 2 μm).
Fig. 2 shows the ratio of elements on the surface of mucoadhesive films with and without lysozyme detected by SEM. Carbon, oxygen, and nitrogen are the most common elements on the surface of mucoadhesive films both with and without lysozyme; their accumulated ratio 96.05% and 98.06% respectively. Location of lysozyme on the surface and in the layer of polymeric film provides a gradual release of the enzyme, which is confirmed by further research.
Fig. 2.
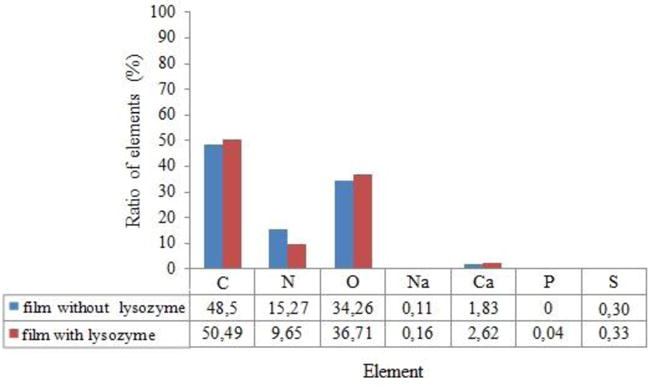
Ratio of elements (%) on the surface of mucoadhesive films by SEM at n=9.
3.3. Lysozyme release
The amount of lysozyme released from the film matrix is illustrated in Fig. 3. When lysozyme-containing composite films were placed in the Na-phosphate buffer solution or distilled water, the films were swollen as a result of the diffusion of water molecules into the polymeric film structure, leading to the release of incorporated lysozyme into aqueous environment from polymeric matrix. The rate of lysozyme release and its completeness showed that the 75 % of the enzymatic activity of included lysozyme is reached after 30 min of incubation. Maximum hydrolytic activity is reached after 90 min of incubation and maintained at a high level for 3 hours.
Fig. 3.
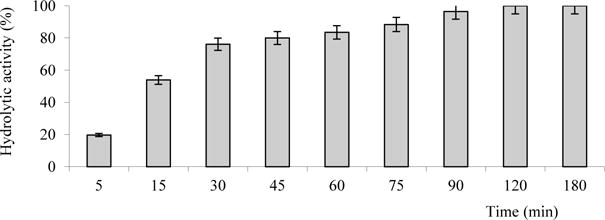
Release of lysozyme from mucoadhesive film at 37 °C and pH 6.2.
3.4. Effect pH and temperature
The effect of pH on the activity of the free and released lysozyme was determined in the pH range from 3.0 to 10.0 at 37°C. As shown in Fig. 4, the optimum pH of the free and released lysozyme is the same (5.5 – 6.5 unites). However, the released enzyme was characterized by extended pH-profile of hydrolytic activity in the acidic (by 0.5 units.) and alkaline (1.0 unit) regions. Released enzyme has more gradual decline in activity at acidic values of pH (Fig. 3) due to the stabilizing effect of the matrix.
Fig. 4.
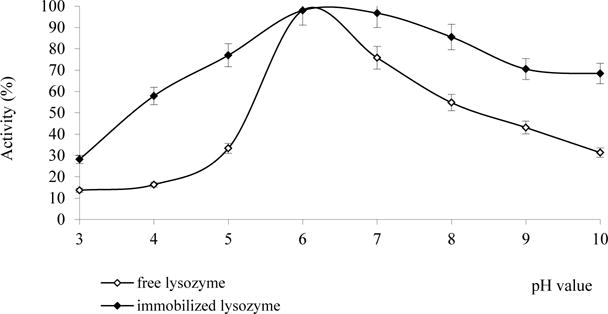
The effect of pH on the activity of free and released lysozyme at 25 °C.
Charge state of lysozyme and its activity can be changed depending on the pH of the solution. The polymeric matrix modifies the microenvironment of the enzyme, thus preventing it from sudden fluctuations in pH and lysozyme is active over a wide pH range.
The effect of temperature on the activity of free and released lysozyme was investigated in the temperature range from 20°C to 80°C at pH 6.2. The relative activity expressed in percentage of the maximum activity is presented in Fig. 5 as a function of temperature. As shown in Fig. 5, the maximum of activity for both free and released lysozyme is obtained at 55°C. However, the activity of the released enzyme in the temperature range of 60–80 °C is lower for 10–20 % than that of free lysozyme, which is possible due to the influence of partial denaturation changes of gelatin matrix.
Fig. 5.
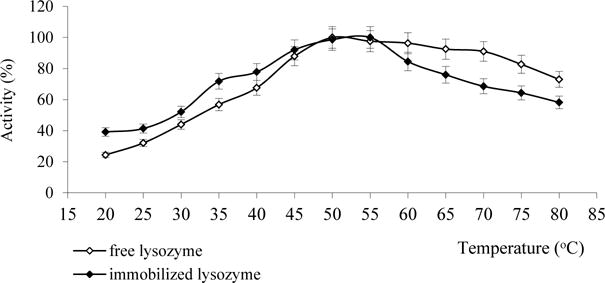
The effect of temperature on the activity of free and released lysozyme at pH 6.2.
The Fig. 6 shows the effect of heating time on the activity of free and released lysozyme. Study of the thermal stability of free and released lysozyme demonstrates a 1.7-fold decrease in the constants of thermal inactivation of released enzyme at 80°C, so for free lysozyme Kin is of 9.2·10−3 min−1, for released - 5.3·10−3 min−1. Decrease in thermal stability at 80°C is also associated with changes in denaturation gelatin matrix.
Fig. 6.
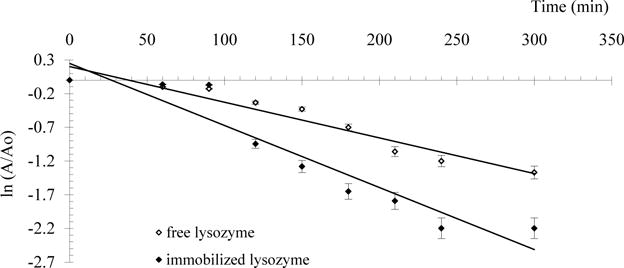
Thermal stability of free and released lysozyme at 80 °C and pH 6.2.
A/A0 – residual lysozyme activity.
3.4. pH-stability of free and released lysozyme
An inflammation area is characterized by a pH around 5.5 (Reis, 2002). Therefore, it is important for developed preparation to be stable at weakly acidic pH environment. The activity of both released and free lysozyme significantly decreased with the lapse of time, but activity of the released enzyme decreases more smoothly. Thus, after incubation of 30 minutes, lysozyme from mucoadhesive film is 24% more active than the free enzyme (Fig. 7). We suggest that the lysozyme is stabilized by polymers in the buffer solution due to interactions with them.
Fig. 7.
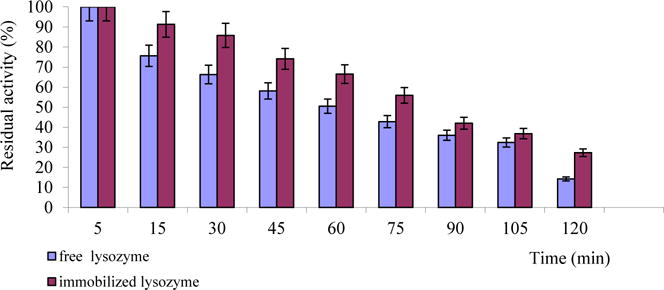
Stability of free and released lysozyme at pH 5.5 and 37 °C.
3.5. Storage stability
Stability of lysozyme-loaded films was studied during a period of 3 years. We compared the lysozyme stability in a buffer solution because in this form the enzyme has been used in clinical practice. The films were stored with the temperature of 4°C. The free enzyme lost hydrolytic activity within 14–17 days in buffer solution, but enzyme from mucoadhesive film was highly stable – residual hydrolytic activity after 36 month of storage is 95–100 % (Fig. 8).
Fig. 8.
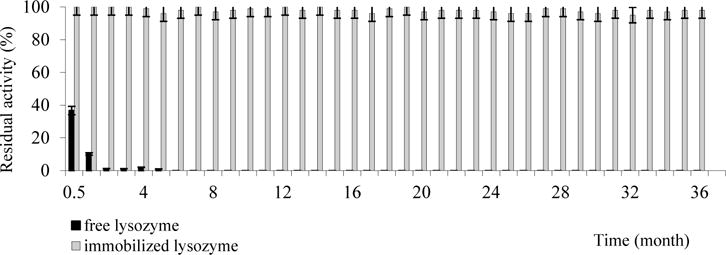
Storage stability of free and lysozyme released from mucoadhesive film at 5 °C.
3.5. Antimicrobial action of lysozyme-loaded mucoadhesive films
Results of the antimicrobial experiments had shown that mucoadhesive polymeric films with lysozyme influenced the growth of all test strains studied after 24 h incubation at 37 °C (Table 3). Maximal bacteriostatic action was noted for Staphylococcus aureus (diameter of the growth inhibition zone = 1.7 cm). Activity against three other strains was somewhat smaller but still high (diameter of the growth inhibition zone = 1.1–1.3 cm). The obtained results indicate that lysozyme diffuses into the agar gel from the polymeric films and inhibits microbial growth.
Table 3.
Antimicrobial effect of mucoadhesive films
| Test-strain of the microorganism | Diameter of the growth inhibition zone, cm, ±SD* | |
|---|---|---|
| Film with lysozyme | Control film (sample without lysozyme) | |
| Staphylococcus aureus ATCC 25923 F-49 | 1.7 ± 0.15 | 0.0 |
| Pseudomonas aeruginosa 415 | 1.1 ± 0.10 | |
| Escherichia coli 055 K59912/4 | 1.2 ± 0.13 | |
| Candida albicans ATCC 885 – 653 | 1.3 ± 0.13 | |
SD, mean-square deviation, Р<0.05, n=3
In addition, we have also shown, that inclusion of lysozyme in gelatin/CMC allows obtaining a preparation with full entrapment of protein and 100% retention of activity after release. Mucoadhesive films with lysozyme could be stored for 3 years at 4° C without loss of enzyme activity. This medical form of entrapped lysozyme can be can be a prospective treatment also for certain ulcers, measles, some skin diseases, and post operation infections as antibacterial agent.
4. Conclusion
The results of our study indicate a possibility to prepare highly stable mucoadhesive form of lysozyme (residual hydrolytic activity after 36-month storage is 95–100%). The enzyme was incorporated into gelatin/sodium carboxymethyl cellulose films. The experimental results showed good mucoadhesive properties of films and retention of physicochemical properties of the enzyme after release from the film. The film with lysozyme was effective in inhibiting the growth of Staphylococcus aureus ATCC 25923 F – 49, Pseudomonas aeruginosa 415, Escherichia coli 055 K59912/4, Candida albicans ATCC 885 – 653.
Acknowledgments
The authors gratefully thank Mr. Stephen Capuzzi for his kind help with editing the manuscript. E.M. also acknowledges the financial support of NIH (grants GM66940 and GM096967).
Footnotes
The authors declare no conflict of interests.
References
- Bachali S, Jager M, Hassanin A, Schoentgen F, Jollès P, Fiala-Medioni A, Deutsch JS. Phylogenetic Analysis of Invertebrate Lysozymes and the Evolution of Lysozyme Function. Journal of Molecular Evolution. 2002;54(5):652–664. doi: 10.1007/s00239-001-0061-6. [DOI] [PubMed] [Google Scholar]
- Bu HF, Wang X, Zhu YQ, Williams RY, Hsueh W, Zheng X, Rozenfeld RA, Zuo XL, Tan XD. Lysozyme-Modified Probiotic Components Protect Rats against Polymicrobial Sepsis: Role of Macrophages and Cathelicidin-Related Innate Immunity. The Journal of Immunology. 2006;177(12):8767–8776. doi: 10.4049/jimmunol.177.12.8767. [DOI] [PubMed] [Google Scholar]
- Bucatariu F, Ghiorghita CA, Mihai M, Simon F, Dragan ES. Pepsin and lysozyme immobilization onto daisogel particles functionalized with chitosan cross-linked multilayers. Revista de Chimie. 2013;64(3):334–337. [Google Scholar]
- Carvalho FC, Bruschi ML, Evangelista RC, Gremião MPD. Mucoadhesive drug delivery systems. Brazilian Journal of Pharmaceutical Sciences. 2010;46:1–17. [Google Scholar]
- Chang YK, Huang RZ, Lin SY, Chiu SJ, Tsai JC. Equilibrium study of immobilized lysozyme on the extrudate-shaped NaY zeolite. Biochemical Engineering Journal. 2006;28(1):1–9. [Google Scholar]
- Datta R, Armiger W, Ollis DF. Lysis ofMicrococcus lysodeikticus by lysozyme covalently immobilized on cellulose and polyacrylamide. Biotechnology and Bioengineering. 1973;15(5):993–1006. [Google Scholar]
- Dekina S, Romanovska I, Gromovoy TY. Influence of polymers on lysozyme molecules association. Biopolymers and Cell. 2011;27(6):442–445. [Google Scholar]
- Ding HM, Shao L, Liu RJ, Xiao QG, Chen JF. Silica nanotubes for lysozyme immobilization. Journal of Colloid and Interface Science. 2005;290(1):102–106. doi: 10.1016/j.jcis.2005.04.029. [DOI] [PubMed] [Google Scholar]
- FDA. Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER); 2005. Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. [Google Scholar]
- Ferrari R, Callerio C, Podio G. Antiviral Activity of Lysozyme. Nature. 1959;183(4660):548–548. doi: 10.1038/183548a0. [DOI] [PubMed] [Google Scholar]
- Gorin G, Wang SF, Papapavlou L. Assay of lysozyme by its lytic action on M. lysodeikticus cells. Analytical Biochemistry. 1971;39(1):113–127. doi: 10.1016/0003-2697(71)90467-2. [DOI] [PubMed] [Google Scholar]
- Helal R, Bader G, Melzig MF. Stimulation of lysozyme release by selected microbial preparations. Die Pharmazie - An International Journal of Pharmaceutical Sciences. 2012;67(6):564–566. [PubMed] [Google Scholar]
- Iacono VJ, MacKay BJ, DiRienzo S, Pollock JJ. Selective Antibacterial Properties of Lysozyme for Oral Microorganisms. Infection and Immunity. 1980;29(2):623–632. doi: 10.1128/iai.29.2.623-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollès P, Jollès J. What’s new in lysozyme research? Molecular and Cellular Biochemistry. 1984;63(2):165–189. doi: 10.1007/BF00285225. [DOI] [PubMed] [Google Scholar]
- Karle H, Hansen NE, Killmann SA. Intracellular Lysozyme in Mature Neutrophils and Blast Cells in Acute Leukemia. 1974 [PubMed] [Google Scholar]
- Kawamura T, Aoki H, Ono K, Mizuki H, Yanagisawa S, Hanai Y, Shimizu M. Clinical evaluation of the anti-inflammatory enzyme lysozyme (Leftose) for the treatment of ambulatory postoperative cases in oral and maxillo-facial surgery. ORAL THERAPEUTICS AND PHARMACOLOGY. 1988;7(1):28–33. [Google Scholar]
- Khurana S, Madhav NS, TANGRI P. Mucoadhesive drug delivery: Mechanism and methods of evaluation. International Journal of Pharma and Bio Sciences. 2011;2(1):458–467. [Google Scholar]
- Kurimoto A, Tanabe T, Tachibana A, Yamauchi K. Keratin sponge: Immobilization of lysozyme. Journal of Bioscience and Bioengineering. 2003;96(3):307–309. doi: 10.1016/s1389-1723(03)80199-8. [DOI] [PubMed] [Google Scholar]
- Laffleur F. Mucoadhesive polymers for buccal drug delivery. Drug Development and Industrial Pharmacy. 2014;40(5):591–598. doi: 10.3109/03639045.2014.892959. [DOI] [PubMed] [Google Scholar]
- Lian ZX, Ma ZS, Wei J, Liu H. Preparation and characterization of immobilized lysozyme and evaluation of its application in edible coatings. Process Biochemistry. 2012;47(2):201–208. [Google Scholar]
- Lu Z, Zhang J, Ma Y, Song S, Gu W. Biomimetic mineralization of calcium carbonate/carboxymethylcellulose microspheres for lysozyme immobilization. Materials Science and Engineering: C. 2012;32(7):1982–1987. doi: 10.1016/j.msec.2012.05.027. [DOI] [PubMed] [Google Scholar]
- Morioka T, Nishimura M, Matsumura T. Anti-inflammatory Effect of Lysozyme Paste on Experimental Gingivitis Induced by Various Chemical Mediators in Guinea Pig. Journal of Periodontology. 1970;41(6):341–348. doi: 10.1902/jop.1970.41.6.341. [DOI] [PubMed] [Google Scholar]
- Muriel-Galet V, Talbert JN, Hernandez-Munoz P, Gavara R, Goddard JM. Covalent Immobilization of Lysozyme on Ethylene Vinyl Alcohol Films for Nonmigrating Antimicrobial Packaging Applications. Journal of Agricultural and Food Chemistry. 2013;61(27):6720–6727. doi: 10.1021/jf401818u. [DOI] [PubMed] [Google Scholar]
- Ogundele MO. A novel anti-inflammatory activity of lysozyme: modulation of serum complement activation. Mediators of Inflammation. 1998;7(5):363–365. doi: 10.1080/09629359890893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T. Clinical significance of serum lysozyme in viral hepatitis. Nihon Shokakibyo Gakkai zasshi = The Japanese journal of gastro-enterology. 1977;74(6):755–764. [PubMed] [Google Scholar]
- Park JM, Kim M, Park HS, Jang A, Min J, Kim YH. Immobilization of lysozyme-CLEA onto electrospun chitosan nanofiber for effective antibacterial applications. International Journal of Biological Macromolecules. 2013;54(0):37–43. doi: 10.1016/j.ijbiomac.2012.11.025. [DOI] [PubMed] [Google Scholar]
- Petkar SR. International Research Journal of Science and Engineering Applications 2014 [Google Scholar]
- Reis RL, Cohn D, editors. Polymer based systems on tissue engineering, replacement and regeneration. Springer Science & Business Media; 2002. [Google Scholar]
- Rymuszka A, S AK, Studnicka M, Sieroslawska A, Bownik A. Immunomodulating effect of dimerized lysozyme [KLP-602] on cellular and humoral defence mechanisms in fish after suppression induced by selected pesticides. Acta Poloniae Toxicologica. 2000;08(1) [Google Scholar]
- Sankar V, Hearnden V, Hull K, Juras DV, Greenberg MS, Kerr AR, Lockhart PB, Patton LL, Porter S, Thornhill M. Local drug delivery for oral mucosal diseases: challenges and opportunities. Oral Diseases. 2011;17:73–84. doi: 10.1111/j.1601-0825.2011.01793.x. [DOI] [PubMed] [Google Scholar]
- Smart JD. Drug delivery using buccal-adhesive systems. Advanced Drug Delivery Reviews. 1993;11(3):253–270. [Google Scholar]
- Sridhar M, Reddy GK, Hu N, Motahari A, Schaefer DW, Thiel SW, Smirniotis PG. Preparation, characterization and lysozyme immobilization studies on siliceous mesocellular foams: Effect of precursor chemistry on pore size, wall thickness and interpore spacing. Microporous and Mesoporous Materials. 2014;190(0):215–226. [Google Scholar]
- Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery — A promising option for orally less efficient drugs. Journal of Controlled Release. 2006;114(1):15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Veis A, editor. The macromolecular chemistry of gelatin. New York: Academic Press; 1964. [Google Scholar]
- Voigt R, Werchan D. Radiation-induced effects on the properties of gelatin. Pharmazie. 1986;41(2):120–123. [PubMed] [Google Scholar]
- Woertz C, Preis M, Breitkreutz J, Kleinebudde P. Assessment of test methods evaluating mucoadhesive polymers and dosage forms: An overview. European Journal of Pharmaceutics and Biopharmaceutics. 2013;85(3, Part B):843–853. doi: 10.1016/j.ejpb.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Wu Y, Daeschel MA. Lytic Antimicrobial Activity of Hen Egg White Lysozyme Immobilized to Polystyrene Beads. Journal of Food Science. 2007;72(9):M369–M374. doi: 10.1111/j.1750-3841.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- Xiao QG, Tao X, Zou HK, Chen JF. Comparative study of solid silica nanoparticles and hollow silica nanoparticles for the immobilization of lysozyme. Chemical Engineering Journal. 2008;137(1):38–44. [Google Scholar]
- Zhang X, Zhao J, Wen Y, Zhu C, Yang J, Yao F. Carboxymethyl chitosan-poly(amidoamine) dendrimer core–shell nanoparticles for intracellular lysozyme delivery. Carbohydrate Polymers. 2013;98(2):1326–1334. doi: 10.1016/j.carbpol.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen Y, Li W, Lu M, Wang S, Chen X, Shi M, Wu J, Yuan Q, Li Y. Controlled uptake and release of lysozyme from glycerol diglycidyl ether cross-linked oxidized starch microgel. Carbohydrate Polymers. 2015;121(0):276–283. doi: 10.1016/j.carbpol.2015.01.002. [DOI] [PubMed] [Google Scholar]


