Abstract
Introduction
Extrahepatic biliary malignancies are often diagnosed at an advanced stage. We compared patients with unresectable perihilar cholangiocarcinoma (PHCC) and gallbladder cancer (GBC) who underwent a palliative procedure versus an aborted laparotomy.
Methods
Seven hundred seventy-seven patients who underwent surgery for PHCC or GBC between 2000 and 2014 were identified. Uni- and multivariable analyses were performed to identify factors associated with outcome.
Results
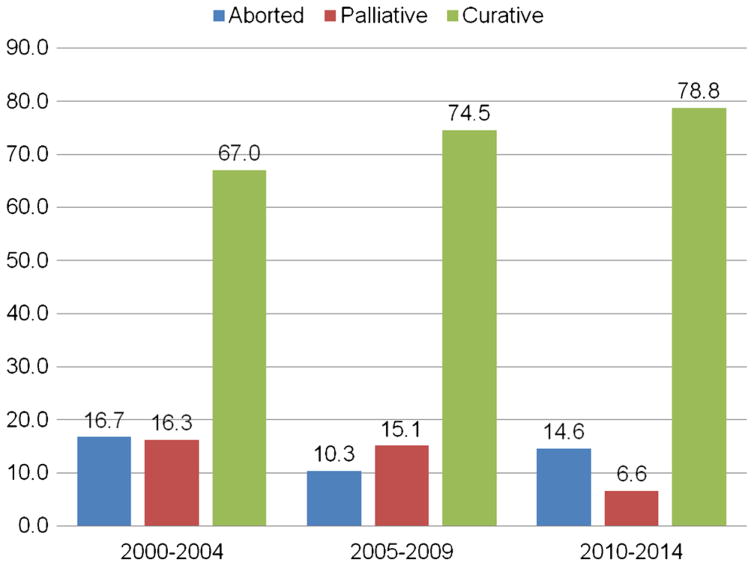
Utilization of preoperative imaging increased over time (CT use, 80.1 % pre-2009 vs. 90 % post-2009) (p <0.001). The proportion of the patients undergoing curative-intent resection also increased (2000–2004, 67.0 % vs. 2005–2009, 74.5 % vs. 2010–2014, 78.8 %; p =0.001). The planned surgery was aborted in 106 (13.7 %) patients and 94 (12.1 %) had a palliative procedure. A higher incidence of postoperative complications (19.2 vs. 3.8 %, p =0.001) including deep surgical site infections (8.3 vs. 1.1 %), bleeding (4.8 vs. 0 %), bile leak (6.0 vs. 0 %) and longer length of stay (7 vs. 4.5 days) were observed among the patients who underwent a palliative surgical procedure versus an aborted non-therapeutic, non-palliative laparotomy (all p <0.05). OS was comparable among the patients who underwent a palliative procedure (8.7 months) versus an aborted laparotomy (7.8 months) (p =0.23).
Conclusion
Increased use of advanced imaging modalities was accompanied by increased curative-intent surgery. Compared with patients in whom surgery was aborted, patients who underwent surgical palliation demonstrated an increased incidence of postoperative morbidity with comparable survival.
Keywords: Palliation, Surgical, Extrahepatic, Biliary, Malignancy
Introduction
Extrahepatic biliary malignancies represent up to 3 % of all cancers in the USA with a dramatic rise in the incidence of these malignancies noted over the last several decades.1,2 Despite recent advances in diagnostic tools, perioperative therapy, and surgical approach, prognosis following resection of these malignancies remains poor. Given their diffuse and sclerotic nature, extrahepatic biliary tumors tend to invade local structures and a subset has an increased propensity for distant metastasis. As a consequence, many patients are either diagnosed at advanced stages of disease when curative resection is no longer feasible or are found to have unresectable or metastatic disease at the time of surgery.2,3 For example, in a recent report of patients with hilar cholangiocarcinoma, only 36 % of patients were amenable to surgery at the time of diagnosis due to metastatic or locally advanced disease.4 Under such circumstances, rather than cure, efforts are often aimed at palliating symptoms of biliary obstruction including jaundice, pruritis, nausea, and weight loss. However, procedures for biliary drainage including percutaneous transhepatic cholangiography (PTC) and endoscopic retrograde cholangiography (ERC) are invasive and complications following their use may compromise further management and quality of life.1,2,4
Currently, there is disagreement about what constitutes the most appropriate method for palliation. Non-operative management is typically recommended among patients with a life expectancy of less than 6 months who present with malignant obstructive jaundice, while the best course of treatment among patients found to have unresectable disease at the time of surgery is debated.5–8 Data evaluating utilization patterns and outcomes of palliative surgery are scarce, with most reports coming from small cohorts at single centers. As such, these reports are limited and may not be generalizable. With an increasing number of patients diagnosed with extrahepatic malignancies each year, data on the use of palliative surgical procedures may help inform the management of these difficult-to-treat patients. Given this, the aim of the current study was to analyze trends in operative approach among patients undergoing non-curative operations for extrahepatic biliary malignancies. Specifically, using a large, multi-institutional cohort of patients, we sought to compare short- and long-term outcomes of patients with unresectable perihilar cholangiocarcinoma (PHCC) and gallbladder cancer (GBC) who underwent a palliative procedure versus an aborted non-therapeutic, non-palliative exploratory laparotomy.
Methods
Data Sources and Patient Population
Patients presenting with PHCC or GBC between January 1, 2000, and December 31, 2014, were identified using the Extrahepatic Biliary Malignancy Consortium database from 2000 to 2014. Collected at and maintained by 10 academic centers in the USA (Johns Hopkins University, Baltimore, MD; Emory University, Atlanta, GA; Stanford University, Stanford, CA; University of Wisconsin, Milwaukee, WI; Ohio State University, Columbus, OH; Washington University, St. Louis, MO; Vanderbilt University, Nashville, TN; New York University, New York, NY; University of Louisville, Louisville, KY; Wake Forest University, Winston-Salem, NC), the Extrahepatic Biliary Malignancy Consortium database records sociodemographic and clinicopathologic characteristics for all patients presenting with a primary extrahepatic biliary malignancy. Specifically, sociodemographic variables including age, sex, and race, as well as clinicopathologic characteristics such as the American Society of Anesthesiology (ASA) physical classification score, presence of preoperative comorbidity, preoperative imaging, preoperative serum CA 19-9, preoperative peak serum bilirubin, and type of cancer were recorded from each patient record. Tumor size, tumor grade, margin status, presence of nodal disease and invasion of adjacent structures were determined using the final histopathology report. Additionally, operative details including the type and extent of surgery, completion of the procedure, as well as the nature of the procedure (curative vs. palliative) were also recorded for each patient. Palliative procedures included biliary bypass and cholecystectomy, both of which have been previously reported as palliative procedures to help improve quality of life.9–11 Perioperative morbidity was classified according to the Clavien-Dindo classification system while other short-term perioperative outcomes recorded for each patient included index hospitalization length of stay (LOS), perioperative mortality, and 30-day readmission.12 Overall survival (OS) was calculated from the date of surgery to the date of death or last follow-up, as appropriate. The institutional review board of each participating institution approved this study.
Statistical Analysis
Continuous variables were described as means with standard deviation or medians with interquartile range (IQR), while categorical variables were reported as whole numbers and proportions. For ease of interpretation, the patients were categorized into one of three groups based on year of diagnosis: 2000–2004, 2005–2009, and 2010–2014.13 Differences in patient, disease, and treatment-specific characteristics were compared among these groups using Pearson’s chi-squared test or a Kruskal-Wallis test, as appropriate. OS was estimated using the Kaplan-Meier method, and differences in OS were compared between patient groups using the Mantel-Haenszel test. Uni- and multivariable Cox proportional hazard regression analyses were performed to identify clinicopathologic characteristics predictive of poor postoperative survival. All variables with a corresponding p < 0.20 on univariable analysis were entered into the multivariable model. Results from multivariable analysis were presented as hazard ratios (HR) with corresponding 95 % confidence intervals (95 % CI). All analyses were performed using STATA version 13.0 (StataCorp, College Station, TX), and p < 0.05 (two tailed) was used to define statistical significance.
Results
Baseline Sociodemographic and Clinicopathologic Characteristics
A total of 777 patients who underwent surgery for hilar cholangiocarcinoma (n = 328, 42.2 %) or gallbladder carcinoma (n = 449, 57.8 %) between January 1, 2000 and December 31, 2014 were identified (Table 1). The median age was 66.6 years (IQR 57.6–73.1) and a majority of the patients were female (n = 429, 55.2 %). Nearly two thirds of patients presented with an ASA score of 3 or 4 (n = 364, 64.2 %). The median CA 19-9 among all patients was 63.8 (18.0–281.0) U/mL, while the median peak and final preoperative bilirubin for all patients was 1.6 (0.6–8.4) and 0.9 (0.5–2.3) mg/dL, respectively. Preoperative clinical jaundice was observed in 350 patients (48.4 %). Preoperatively, 405 patients had no biliary drainage or stent (52.9 %), while 191 (25.0 %) underwent endoscopic drainage, 95 (12.4 %) were drained percutaneously, and 74 (9.7 %) underwent both types of drainage. Neoadjuvant therapy was administered to 43 patients; 30 (3.9 %) patients received preoperative chemotherapy and 13 (1.7 %) patients received neoadjuvant radiotherapy.
Table 1.
Trends in indication and outcomes after surgery for hilar cholangio- and gallbladder carcinoma
| Variable | 2000–2004 (n = 203, 26.1 %) |
2005–2009 (n = 271, 34.9 %) |
2010–2014 (n = 303, 39.0 %) |
p value | Total (n = 777) |
|---|---|---|---|---|---|
| Median age (years) | 65.0 (55.3–72.9) | 66.2 (58.3–72.9) | 68.0 (59.0–73.8) | 0.102 | 66.6 (57.6–73.1) |
| Gender | 0.429 | ||||
| Female | 120 (59.1) | 146 (53.9) | 163 (53.8) | 429 (55.2) | |
| Male | 83 (40.9) | 125 (46.1) | 140 (46.2) | 348 (44.8) | |
| ASA | <0.001 | ||||
| 1–2 | 56 (50.0) | 80 (36.9) | 67 (28.2) | 203 (35.8) | |
| 3–4 | 56 (50.0) | 137 (63.1) | 171 (71.9) | 364 (64.2) | |
| Clinical jaundice | 93 (52.8) | 134 (52.8) | 123 (42.0) | 0.017 | 350 (48.4) |
| Type of extrahepatic malignancy | 0.154 | ||||
| Gallbladder cancer | 113 (55.7) | 148 (54.6) | 188 (62.1) | 449 (57.8) | |
| Hilar cholangiocarcinoma | 90 (44.3) | 123 (46.4) | 115 (38.0) | 328 (42.2) | |
| Biliary drainage or stent | 0.03 | ||||
| None | 101 (50.5) | 131 (49.4) | 173 (57.7) | 405 (52.9) | |
| Endoscopic | 44 (22.0) | 72 (27.2) | 75 (25.0) | 191 (25.0) | |
| Percutaneous | 37 (18.5) | 33 (12.5) | 25 (8.3) | 95 (12.4) | |
| Endoscopic and percutaneous | 18 (9.0) | 29 (10.9) | 27 (9.0) | 74 (9.7) | |
| Imaging | |||||
| CT | 153 (80.1) | 244 (90.7) | 276 (91.1) | <0.001 | 673 (88.2) |
| MRI | 48 (25.1) | 94 (35.1) | 161 (53.5) | <0.001 | 303 (39.9) |
| PET | 9 (4.7) | 38 (14.2) | 43 (14.2) | 0.002 | 90 (11.8) |
| Preoperative chemo | 2 (1.0) | 9 (3.35) | 19 (6.3) | 0.009 | 30 (3.9) |
| Preoperative radiation | 1 (0.5) | 6 (2.3) | 6 (2.0) | 0.310 | 13 (1.7) |
| Diagnostic laparoscopy | 48 (23.6) | 59 (21.8) | 104 (34.3) | 0.002 | 211 (27.2) |
| Aborted operation | 34 (16.8) | 28 (10.3) | 44 (14.5) | 0.112 | 106 (13.7) |
| Reason for abortion of operation | 0.068 | ||||
| Locally advanced disease | 6 (17.7) | 10 (35.7) | 6 (13.6) | 22 (20.8) | |
| Presence of metastases | 28 (82.4) | 18 (64.3) | 38 (86.4) | 84 (79.3) | |
| T-stage hilar | 2b (2a–2b) | 2b (2a–3) | 2b (2a–2b) | 0.191 | 2b (2a–3) |
| T-stage gallbladder | 3 (2–3) | 3 (2–3) | 3 (2–3) | 0.540 | 3 (2–3) |
| Lymph node metastases | 57 (45.2) | 85 (42.9) | 107 (45.7) | 0.833 | 249 (44.6) |
| Distant disease | 44 (21.7) | 48 (17.7) | 51 (16.8) | 0.362 | 143 (18.4) |
| Palliative operation | 33 (16.3) | 41 (15.1) | 20 (6.6) | 0.001 | 94 (12.1) |
| Reason for palliation | 0.218 | ||||
| Locally advanced disease | 21 (63.6) | 20 (48.8) | 14 (70.0) | 55 (58.5) | |
| Presence of metastases | 12 (36.4) | 21 (51.2) | 6 (30.0) | 39 (41.5) | |
| Extent of palliative surgery | 0.425 | ||||
| Bile duct resection | 4 (13.3) | 5 (35.7) | 5 (31.3) | 14 (18.9) | |
| Cholecystectomy | 19 (63.3) | 20 (71.4) | 8 (50.0) | 47 (63.5) | |
| Other | 7 (23.3) | 3 (10.7) | 3 (18.8) | 13 (17.6) | |
| Length of stay (days), mean ± SD | 7 (5–11) | 8 (6–12) | 6 (4–10) | <0.001 | 7 (5–11) |
| Any complication | 74 (49.0) | 132 (51.8) | 154 (50.8) | 0.865 | 360 (50.8) |
| No. of complications | 0 (0–1) | 1 (0–2) | 1 (0–1) | 0.540 | 1 (0–1) |
| Major complication | 31 (15.3) | 71 (26.2) | 76 (25.1) | 0.010 | 178 (77.1) |
| Perioperative mortality | 6 (3.1) | 16 (6.1) | 11 (3.8) | 0.241 | 33 (4.4) |
| Readmissions | 36 (21.4) | 57 (24.8) | 79 (29.4) | 0.166 | 172 (25.8) |
| Adjuvant chemotherapy | 57 (44.9) | 123 (54.2) | 142 (53.2) | 0.206 | 322 (51.9) |
| Adjuvant radiation | 46 (37.4) | 84 (37.8) | 57 (22.4) | <0.001 | 187 (31.2) |
At the time of surgery, diagnostic laparoscopy was performed in 211 (27.2 %) patients. Among all patients who underwent surgery, the planned surgery was aborted in 106 (13.7 %) due to the presence of either locally advanced disease (n = 22, 20.8 %) or metastatic disease (n = 84, 79.3 %). In contrast, 94 (12.1 %) patients who had unresectable disease underwent a palliative surgical procedure (cholecystectomy, n = 47, 63.5 %; bile duct resection, n = 14, 18.9 %).
In the study cohort, 123 patients had distant disease. Disease was located in the liver in 33 (27.7 %) of these patients, while 53 (44.5 %) had peritoneal carcinomatosis and 11 (9.2 %) had both. The remaining 22 patients (18.5 %) had distant disease elsewhere. Among the 35 patients with metastatic disease diagnosed by laparoscopy, 6 patients had disease located in the liver, 22 had peritoneal carcinomatosis, 6 had both liver and peritoneal carcinomatosis, and 1 patient had disease located elsewhere. Four of these patients then underwent palliative resection. In contrast, the remaining 88 (71.5 %) patients were found to have metastatic disease at the time of laparotomy. Among these 88 patients, 27 had disease located in the liver, 31 had peritoneal carcinomatosis, 5 had both liver and peritoneal carcinomatosis, and 21 had disease located elsewhere. Thirty-five (39.7 %) patients with distant disease diagnosed by laparotomy went on to have a palliative-intent resection, while the remaining operations were aborted.
Trends in Patient, Disease, and Operative Characteristics Over Time
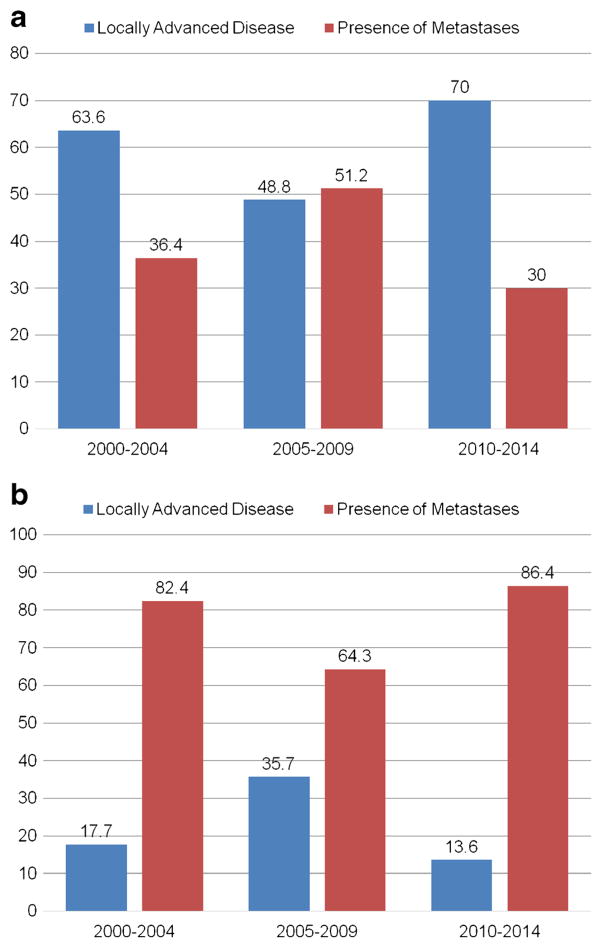
To compare trends in disease presentation and treatment over time, the patients were divided into three categories based on the year of diagnosis. Marked differences were noted among these three patient groups. For example, the proportion of the patients with an ASA score of 3 or 4 undergoing surgery was noted to increase over the study time, with 71.9 % (n = 171) of the patients having an ASA score of 3 or 4 between 2010–2014 compared with 50.0 % (n = 56) and 63.1 % (n = 137) of the patients between 2000–2004 and 2005–2009, respectively (p < 0.001). Of note, the use of preoperative imaging also increased over the study period. Compared with 80.1 % (n = 153) of the patients between 2000–2004 who had a pre-operative computed tomography (CT) scan, over 90 % (n = 276, 91.1 %) of patients underwent a preoperative CT scan in the last 5 years of the study (p < 0.001). Similarly, the proportion of patients undergoing preoperative magnetic resonance imaging (MRI) increased from 25.1 % (n = 48) between 2000–2004 to 53.5 % (n = 161) between 2010–2015 (p < 0.001). Patients undergoing surgery in the last 5 years of the study were also proportionally more likely to have received neoadjuvant therapies; specifically, the proportion increased from 1.0 % (n = 2) between 2000–2004 to 6.3 % (n = 19) between 2010–2014 (p = 0.009). A similar trend in the receipt of neoadjuvant radiation therapy was not observed (p = 0.310). Interestingly, while the proportion of patients undergoing a curative-intent resection increased from 67.0 % before 2005 to 78.8 % in the last 5 years of the study, the proportion of patients undergoing a palliative procedure decreased from 16.3 % between 2000–2004 to 6.6 % between 2010–2014 (p = 0.001). Of note, the number of aborted procedures (in which the surgery was ended due to the presence of unresectable disease upon surgical exploration of the abdomen) did not change over time (Figs. 1, 2). Although the proportion of patients presenting with preoperative jaundice decreased from 52.8 % between 2000–2004 to 42.0 % between 2010–2014 (p = 0.017), T-stage and the proportion of the patients presenting with lymph node metastases and distal disease remained the same (all p > 0.05).
Fig. 1.
Aborted, palliative, and curative-intent operations stratified by year of procedure (p = 0.001)
Fig. 2.
Reason for (a) palliative and (b) aborted, non-therapeutic, non-palliative laparotomy stratified by year of procedure
Trends in Postoperative Outcomes Over Time
Among all the patients identified, the total LOS was noted to decrease from an average of 7 days (IQR 5–11) before 2005 to 6 days (4–10) after 2009 (p < 0.001). However, a similar trend in postoperative morbidity or mortality was not noted. In fact, the overall incidence of postoperative complications, the average number of postoperative complications and postoperative mortality, were comparable across all time periods examined (p > 0.05). Of note, patients who underwent a palliative procedure had worse postoperative outcomes compared with patients who had an aborted non-therapeutic, non-palliative exploratory laparotomy (Table 2). Specifically, a higher incidence of major postoperative complications (19.2 vs. 3.8 %, p = 0.001), including deep surgical site infections (8.3 vs. 1.1 %, p = 0.025), bleeding (4.8 vs. 0 %, p = 0.039), and bile leak (6.0 vs. 0 %, p = 0.020) were observed among patients who underwent a palliative surgical procedure. Similarly, the median LOS was higher among patients who underwent a palliative procedure (7 days (IQR 5–10) vs. 4.5 days (IQR 2–7); p < 0.001). Readmission rates and perioperative mortality, however, were not different between groups (p > 0.05).
Table 2.
Comparison between aborted resection and palliative resection
| Variable | N (%) | Aborted (n = 106) |
Palliative surgery (n = 94) |
p value |
|---|---|---|---|---|
| Perioperative mortality | 10 (5.1) | 4 (3.8) | 6 (6.7) | 0.349 |
| Complications | 76 (38.0) | 32 (30.2) | 44 (46.9) | 0.031 |
| Minor complication | 54 (27.0) | 28 (26.4) | 26 (27.7) | 0.843 |
| Major complication | 22 (11.0) | 4 (3.8) | 18 (19.2) | 0.001 |
| No. of complications | 0 (0–1) | 0 (0–1) | 1 (0–1) | 0.010 |
| Clavien-Dindo grade (median, range) | – | I (I–II) | II (I–IIIa) | 0.012 |
| Specific complications | ||||
| Superficial surgical site infection | 12 (7.0) | 5 (5.7) | 7 (8.3) | 0.495 |
| Deep surgical site infection | 8 (4.7) | 1 (1.1) | 7 (8.3) | 0.025 |
| Intra-abdominal infection | 4 (2.3) | 2 (2.3) | 2 (2.4) | 0.962 |
| Bleeding | 4 (2.3) | 0 | 4 (4.8) | 0.038 |
| Bile leak | 5 (2.9) | 0 | 5 (6.0) | 0.020 |
| Anastomotic leak | 1 (0.6) | 0 | 1 (1.2) | 0.305 |
| New post-op ascites | 3 (1.8) | 1 (1.15) | 2 (2.4) | 0.533 |
| Reoperation | 5 (2.5) | 2 (1.9) | 3 (3.23) | 0.554 |
| Peak post-op bilirubin | 1.9 (0.8–5.4) | 1.8 (0.8–4.9) | 2 (0.8–6.2) | 0.627 |
| Length of stay (days) | 6 (3–8) | 4.5 (2–7) | 7 (5–10) | <0.001 |
| Readmission | 55 (29.4) | 32 (31.7) | 23 (26.7) | 0.460 |
| Time to readmission | 17.5 (7–37) | 14.5 (7.5–37.5) | 24.5 (7–37) | 0.481 |
| Location of readmission | ||||
| Participating center | 53 (98.2) | 31 (96.9) | 22 (100) | 0.403 |
| Other | 1 (1.9) | 1 (3.1) | 0 | |
Trends in OS and Factors Associated with OS
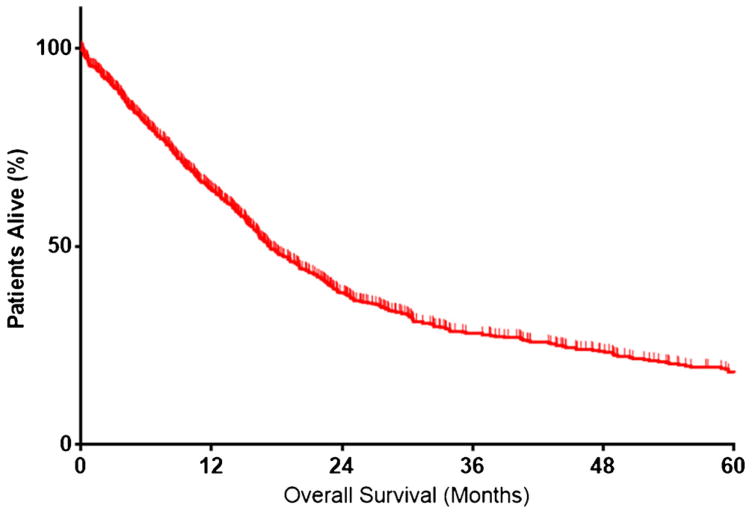
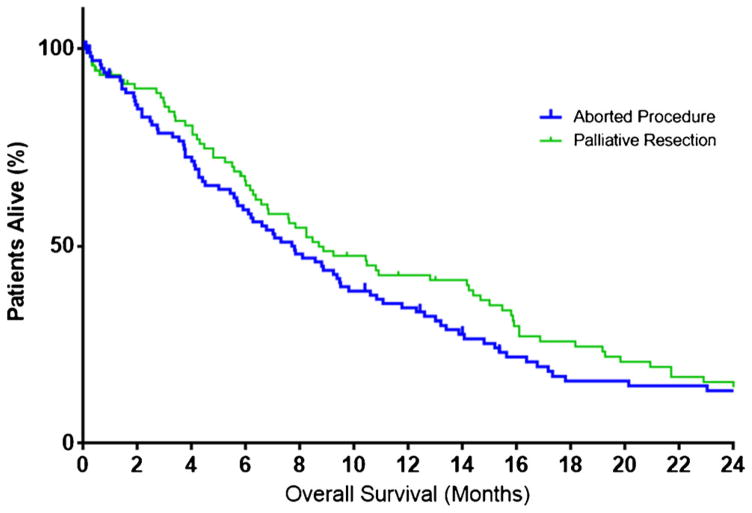
The median OS among all patients was 17.3 months (IQR 8.2–43.7, Fig. 3) with 1-year OS being 64.1 % (95 % CI 60.4–67.6). Of note, OS increased across the time periods examined, varying from 15.5 months (IQR 6.9–41.0) among patients undergoing surgery between 2000–2004 to 19.2 months (IQR 10.1–53.1) among patients undergoing surgery after 2009 (p = 0.069). Similarly, estimates for 1-year OS increased from 58.2 % (95 % CI 50.8–64.9) to 69.9 % (95 % CI 63.7–75.3) across the study period (p < 0.001). In contrast, median OS among patients who underwent a palliative procedure or a procedure that was aborted was 8 months (IQR 4.0–16.1) and was comparable among patients who underwent a palliative procedure (8.7 months) versus patients who had an aborted non-therapeutic, non-palliative exploratory laparotomy (7.8 months) (p = 0.23) (Fig. 4).
Fig. 3.
Overall survival
Fig 4.
Overall survival stratified by receipt of palliative procedure versus aborted, non-therapeutic, non-palliative laparotomy (p = 0.23)
Cox proportional hazard regression was performed to identify potential risk factors for a worse OS. Increasing patient age (HR 1.01, 95 % CI 1.00–1.03, p = 0.063), a higher peak preoperative bilirubin level (HR 1.02, 95 % CI 1.00–1.04, p = 0.131), and a positive diagnostic laparoscopy (HR 1.54, 95 % CI 1.12–2.12, p = 0.008) were associated with a worse OS (Table 3). After adjusting for these competing risk factors on multivariable analysis, a higher peak preoperative bilirubin (HR 1.02, 95 % CI 1.00–1.04, p = 0.045) and a positive diagnostic laparoscopy (HR 1.52. 95 % CI 1.09–2.13, p = 0.015) were noted to be independently associated with a worse overall survival.
Table 3.
Univariable and multivariable Cox regression model of overall survival after resections with non-curative intent
| Variable name | Univariable analysis
|
Multivariable analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | p value | HR | 95 % CI | p value | |
| Age | 1.01 | 1.00–1.03 | 0.063 | 1.01 | 0.99–1.03 | 0.215 |
| Male gender | 0.91 | 0.67–1.23 | 0.542 | |||
| Race | ||||||
| White | Ref | – | – | |||
| Black | 1.00 | 0.56–1.78 | 0.999 | |||
| Other | 1.15 | 0.73–1.82 | 0.541 | |||
| BMI | 0.99 | 0.96–1.02 | 0.367 | |||
| ASA score 3–4 | 1.14 | 0.78–1.66 | 0.501 | |||
| CA 19-9 | 1.00 | 1.00–1.00 | 0.940 | |||
| Peak bilirubin | 1.02 | 1.00–1.04 | 0.131 | 1.02 | 1.00–1.04 | 0.045 |
| Last bilirubin | 1.01 | 0.98–1.04 | 0.453 | |||
| Biliary drainage or stent | ||||||
| None | Ref | – | – | |||
| Endoscopic | 1.17 | 0.82–1.67 | 0.398 | |||
| Percutaneous | 1.28 | 0.79–2.08 | 0.317 | |||
| Endoscopic and percutaneous | 1.01 | 0.62–1.65 | 0.953 | |||
| Preoperative chemotherapy | 0.86 | 0.35–2.10 | 0.739 | |||
| Preoperative radiation | 1.50 | 0.47–4.71 | 0.492 | |||
| Diagnostic laparoscopy | 1.54 | 1.12–2.12 | 0.008 | 1.52 | 1.09–2.13 | 0.015 |
| Palliative resection | 0.84 | 0.62–1.14 | 0.274 | |||
| Reason for palliation | ||||||
| Locally advanced disease | Ref | – | – | |||
| Presence of metastases | 1.15 | 0.85–1.57 | 0.365 | |||
Discussion
Extrahepatic biliary malignancies represent a heterogeneous group of malignancies accounting for 3 % of all cancers within the USA.1,2 Given their aggressive nature and propensity for early metastasis, less than a third of patients are amenable to cure.14–18 Given this, palliative surgical resection is often the only option to relieve symptoms of biliary obstruction including jaundice, pruritis, nausea, and weight loss.19 Data evaluating the patterns of use and prognosis following palliative surgery remain limited with most data collected at single, specialized centers.5–8 The current study is important in that it represents one of the largest studies to assess the patterns of use and trends of non-curative surgery for extrahepatic biliary malignancies. Using a multicentric cohort of 777 patients, we noted a decreasing trend in the use of palliative surgery with an increasing number of curative-intent resections being performed over the study time period. Furthermore, the current study noted an increase in the use of imaging modalities for preoperative assessment/planning with the number of patients undergoing a preoperative CT or MRI scan increasing with time. Perhaps of greater interest, postoperative clinical outcomes were also noted to improve with time as overall survival was better over time. Specifically, patients who underwent surgery before 2004 demonstrated an OS of 15.5 months compared with an OS of 19.2 months among those undergoing surgery after 2009.
The observed increased trend in the number of curative-intent resections being performed is likely multifactorial and may be a consequence of improvements in diagnostic imaging and surgical technique in recent years. Studies assessing the efficacy of diagnostic imaging for biliary cancers have demonstrated that newer imaging modalities such as MRCP and PET scans can achieve an accuracy of up to 84.9 and 77.9 %, respectively, in assessing T and N staging, as well as a sensitivity of 78 % in detecting portal vein invasion and a sensitivity ranging from 58 to 73 % in detecting hepatic artery invasion.20,21 In the current study, we noted the use of CT, MRI, and PET for preoperative planning increased, respectively, from 80.1, 25.1, and 4.7 % before 2005 to 91.1, 53.5, and 14.2 % in the years following 2009. As such, the increased use of MRI and PET scans may have contributed to the greater proportion of the patients being identified with resectable disease and a greater proportion of patients amenable to curative resection. Of note, patients did not, however, present with an earlier stage of disease, as T-stage, nodal metastases and distant disease status were equal among the three time periods (Table 1). Only clinical jaundice declined over time from 52.8 % (n = 93) between 2000–2004 to 42.0 % (n = 123) between 2010–2014 (p = 0.017). The increase in curative resections in a population in which the stage of the disease was unchanged may also indicate a trend towards a more aggressive surgical approach over time. Furthermore, the proportion of aborted non-therapeutic, non-palliative exploratory laparotomy procedures was not observed to change over time. This suggests that advancements in diagnostic imaging still cannot fully delineate whether all extrahepatic biliary tract tumors are resectable based on preoperative cross-sectional imaging, and imaging remains inadequate at diagnosing low-volume disease such as peritoneal carcinomatosis. Due to the aggressive nature of many extrahepatic biliary malignancies, it is also possible that disease may spread during the time interval between imaging and operation. In order to limit the number of non-therapeutic laparotomies, we recommend recent (within 4 weeks) imaging as well as increased utilization of laparoscopy, whenever possible, to diagnose the extent of disease. Although the data did show a significant increase in the use of laparoscopy over time (23.6 to 34.3 %; p = 0.002), as the majority of the patients underwent laparotomies, even in the most recent tercile.
Another interesting finding of the current study was a decreasing LOS among the patients following surgery despite an increasing trend in the proportion of patients with a high ASA score (ASA score III or IV) undergoing resection. Specifically, LOS was noted to decrease from 7 days before 2005 to 6 days after 2009 while the numbers of the patients with an ASA score of III or IV increased from 50.0 % in 2004 to over 71 % after 2010. These observed differences are likely due to advances in surgical technique and improvements in the perioperative management of patients. For example, recent studies have reported an increased utilization of portal vein reconstruction with favorable outcomes, which in turn allows for the resection of more challenging tumors.13,22,23 Additionally, intraoperative and postoperative practices such as restricted fluid resuscitation strategies and enhanced recovery pathways have facilitated a better perioperative recovery and an overall decreased risk for complications.21–23 Further highlighting improvement in perioperative practices was our finding of a decreased number of patients who underwent a palliative procedure over time. Specifically, the proportion of patients undergoing palliative surgery decreased from 16.3 % during the first 5 years to 6.6 % over the last 5 years. In contrast, the proportion of patients undergoing a non-operative biliary decompression and, in particular, the proportion of patients undergoing an endoscopic biliary decompression increased over time (Table 1). Of note, patients who underwent a palliative surgical procedure demonstrated an increased incidence of postoperative complications with major complications such as bleeding and bile leaks more often noted following surgery. While endoscopic palliation with self-expanding metal stents remains a good treatment option for patients with preoperatively identified unresectable disease, our data demonstrated comparable postoperative bilirubin levels among the patients who underwent surgical biliary decompression suggesting that this approach is an effective palliative surgical option.24 Results from the current study, as well as previous reports, highlight the potential benefits of biliary decompression among patients with unresectable disease. Surgical palliation did come at a cost, however, as these patients had an increased risk of complications and a longer LOS.
In addition to short-term perioperative clinical outcomes, the current study also sought to compare long-term clinical outcomes among patients with biliary cancers. The median overall survival for all patients was 17.3 months and was noted to be lower among patients undergoing non-curative-intent surgery. Perhaps of greater interest, median OS among patients who underwent a palliative surgery was 8.7 months compared with 7.8 months for patients in whom surgery was aborted due to metastatic or locally advanced disease. Consistent with the results of the current study, Conner et al. in a review of patients with hilar cholangiocarcinoma, as well as Ercan and colleagues in a separate study of patients with gallbladder cancer, demonstrated comparable long-term survival among patients undergoing surgical palliation versus a non-therapeutic laparotomy.25,26 It is also important to note that, according to recent literature, median survival in patients receiving chemotherapy without surgical resection for locally advanced or metastatic biliary tract cancers ranged from 8 to 12 months depending upon the type of chemotherapy used. Therefore, palliative resection does not appear to provide a survival benefit in comparison to medical treatment alone.27,28
Results of the current study should be interpreted with the following limitations. First, the data used in the current analysis were collected at 10 large, academic centers each with their own patient case mix, clinical practices, and protocols. As such, differences among centers could not be controlled for and may have resulted in some residual confounding. However, the use of a large, multicentric cohort of patients facilitated more generalizable results and an adequate sample size to assess trends over time. Second, we were unable to account for any selection bias given the retrospective nature of the study. For example, patients who underwent a palliative resection may have been more amenable to surgery compared with patients who had an aborted non-therapeutic, non-palliative laparotomy. Since this is a retrospective study, we were unable to determine all the specific circumstances related to the surgeon’s decision to pursue a palliative procedure at the time of surgery, or specific information on the type of palliative procedure performed. For this reason, we were unable to make data-driven comparisons between those groups of patients undergoing surgical biliary bypass versus endoscopic or percutaneous biliary drainage.
Conclusion
In conclusion, the current study noted an increase in the number of patients undergoing curative-intent surgery for gallbladder carcinomas and hilar cholangiocarcinomas over time. The observed increase in curative surgery was associated with an increased use of advanced imaging modalities preoperatively, which may have led to better identification of patients with resectable disease and therefore a decrease in surgical palliation that was observed over time. Compared with patients in whom surgery was aborted, patients who underwent a surgical palliation demonstrated an increased incidence of postoperative morbidity with comparable survival. These data should help inform decisions around intraoperative management of patients with unresectable PHCC or GBC.
Footnotes
This study was presented as a poster presentation at the 2016 Gastrointestinal Cancers Symposium held on January 21–23, 2016, Moscone West Building, San Francisco, CA.
Compliance with Ethical Standards
The institutional review board of each participating institution approved this study.
Funds/Conflict of Interest
None
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Fact Sheets by Cancer. 2015 Aug 9; Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 3.Wakabayashi H, Ishimura K, Hashimoto N, Otani T, Kondo A, Maeta H. Analysis of prognostic factors after surgery for stage III and IV gallbladder cancer. Eur J Surg Oncol. 2004;30(8):842–846. doi: 10.1016/j.ejso.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Lepage C, Capocaccia R, Hackl M, Lemmens V, Molina E, Pierannunzio D, Sant M, Trama A, Faivre J, Grouph E-W. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999–2007: Results of EUROCARE-5. Eur J Cancer. 2015 doi: 10.1016/j.ejca.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 5.Goenka MK, Goenka U. Palliation: Hilar cholangiocarcinoma. World J Hepatol. 2014;6(8):559–569. doi: 10.4254/wjh.v6.i8.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor MC, McLeod RS, Langer B. Biliary stenting versus bypass surgery for the palliation of malignant distal bile duct obstruction: a meta-analysis. Liver Transpl. 2000;6(3):302–308. doi: 10.1053/lv.2000.5196. [DOI] [PubMed] [Google Scholar]
- 7.Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet. 1994;344(8938):1655–1660. doi: 10.1016/s0140-6736(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 8.Andersen JR, Sorensen SM, Kruse A, Rokkjaer M, Matzen P. Randomised trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundice. Gut. 1989;30(8):1132–1135. doi: 10.1136/gut.30.8.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SE, Kim KS, Kim WB, Kim IG, Nah YW, Ryu DH, Park JS, Yoon MH, Cho JY, Hong TH, Hwang DW, Choi DW Korean Association of H-B, Pancreas S. Practical guidelines for the surgical treatment of gallbladder cancer. J Korean Med Sci. 2014;29(10):1333–1340. doi: 10.3346/jkms.2014.29.10.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pradeep R, Kaushik SP, Sikora SS, Bhattacharya BN, Pandey CM, Kapoor VK. Predictors of survival in patients with carcinoma of the gallbladder. Cancer. 1995;76(7):1145–1149. doi: 10.1002/1097-0142(19951001)76:7<1145::aid-cncr2820760708>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham SC, Choti MA, Bellavance EC, Pawlik TM. Palliation of hepatic tumors. Surg Oncol. 2007;16(4):277–291. doi: 10.1016/j.suronc.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amini N, Spolverato G, Kim Y, Pawlik TM. Trends in Hospital Volume and Failure to Rescue for Pancreatic Surgery. J Gastrointest Surg. 2015;19(9):1581–1592. doi: 10.1007/s11605-015-2800-9. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara S, Miyakawa S, Takada T, Takasaki K, Nimura Y, Tanaka M, Miyazaki M, Nagakawa T, Kayahara M, Horiguchi A. Status of surgical treatment of biliary tract cancer. Dig Surg. 2007;24(2):131–136. doi: 10.1159/000101901. [DOI] [PubMed] [Google Scholar]
- 15.de Aretxabala X, Roa I, Hepp J, Maluenda F, Mordojovich G, Leon J, Roa JC. Early gallbladder cancer: is further treatment necessary? J Surg Oncol. 2009;100(7):589–593. doi: 10.1002/jso.21389. [DOI] [PubMed] [Google Scholar]
- 16.Kayahara M, Nagakawa T. Recent trends of gallbladder cancer in Japan: an analysis of 4,770 patients. Cancer. 2007;110(3):572–580. doi: 10.1002/cncr.22815. [DOI] [PubMed] [Google Scholar]
- 17.Guglielmi A, Ruzzenente A, Campagnaro T, Pachera S, Valdegamberi A, Nicoli P, Cappellani A, Malfermoni G, Iacono C. Intrahepatic cholangiocarcinoma: prognostic factors after surgical resection. World J Surg. 2009;33(6):1247–1254. doi: 10.1007/s00268-009-9970-0. [DOI] [PubMed] [Google Scholar]
- 18.de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L, Ferrone CR, Zhu AX, Bauer TW, Walters DM, Gamblin TC, Nguyen KT, Turley R, Popescu I, Hubert C, Meyer S, Schulick RD, Choti MA, Gigot JF, Mentha G, Pawlik TM. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140–3145. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 19.Muller BG, De Aretxabala X, Gonzalez Domingo M. A review of recent data in the treatment of gallbladder cancer: what we know, what we do, and what should be done. Am Soc Clin Oncol Educ Book. 2014:e165–170. doi: 10.14694/EdBook_AM.2014.34.e165. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez JA, Newman KD, Eichelberger MR, Nauta RJ. The papillary-cystic neoplasm of the pancreas. An increasingly recognized clinicopathologic entity. Arch Surg. 1990;125(11):1502–1505. doi: 10.1001/archsurg.1990.01410230098017. [DOI] [PubMed] [Google Scholar]
- 21.Gurusamy KS, Li J, Sharma D, Davidson BR. Cardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database Syst Rev. 2009;(4):CD007338. doi: 10.1002/14651858.CD007338.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Gurusamy KS, Li J, Vaughan J, Sharma D, Davidson BR. Cardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database Syst Rev. 2012;5:CD007338. doi: 10.1002/14651858.CD007338.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y, Ejaz A, Gani F, Wasey JO, Xu L, Frank SM, Pawlik TM. Crystalloid administration among patients undergoing liver surgery: Defining patient- and provider-level variation. Surgery. 2016;159(2):389–398. doi: 10.1016/j.surg.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 24.Mukai T, Yasuda I, Nakashima M, Doi S, Iwashita T, Iwata K, Kato T, Tomita E, Moriwaki H. Metallic stents are more efficacious than plastic stents in unresectable malignant hilar biliary strictures: a randomized controlled trial. J Hepatobiliary Pancreat Sci. 2013;20(2):214–222. doi: 10.1007/s00534-012-0508-8. [DOI] [PubMed] [Google Scholar]
- 25.Connor S, Barron E, Redhead DN, Ireland H, Madhavan KK, Parks RW, Garden OJ. Palliation for suspected unresectable hilar cholangiocarcinoma. Eur J Surg Oncol. 2007;33(3):341–345. doi: 10.1016/j.ejso.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Ercan M, Bostanci EB, Cakir T, Karaman K, Ozer I, Ulas M, Dalgic T, Ozogul Y, Aksoy E, Akoglu M. The rationality of resectional surgery and palliative interventions in the management of patients with gallbladder cancer. Am Surg. 2015;81(6):591–599. [PubMed] [Google Scholar]
- 27.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010 Apr 8;362(14):1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 28.Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, Nagino M, Kondo S, Nagaoka S, Funai J, Koshiji M, Nambu Y, Furuse J, Miyazaki M, Nimura Y. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010 Aug 10;103(4):469–74. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]