Abstract
Introduction
Multiple airway protective mechanisms are impacted with Parkinson’s disease (PD), including swallowing and cough. Cough serves to eject material from the lower airways, and can be produced voluntarily (on command) and reflexively in response to aspirate material or other airway irritants. Voluntary cough effectiveness is reduced in PD however it is not known whether reflex cough is affected as well. The goal of this study was to compare the effectiveness between voluntary and reflex cough in patients with idiopathic PD.
Methods
Twenty patients with idiopathic PD participated. Cough airflow data were recorded via facemask in line with a pneumotachograph. A side delivery port connected the nebulizer for delivery of capsaicin, which was used to induce cough. Three voluntary coughs and three reflex coughs were analyzed from each participant. A two-way repeated measures analysis of variance was used to compare voluntary versus reflex cough airflow parameters.
Results
Significant differences were found for peak expiratory flow rate (PEFR) and cough expired volume (CEV) between voluntary and reflex cough. Specifically, both PEFR and CEV were reduced for reflex as compared to voluntary cough.
Conclusion
Cough PEFR and CEV are indicative of cough effectiveness in terms of the ability to remove material from the lower airways. Differences between these two cough types likely reflect differences in the coordination of the respiratory and laryngeal subsystems. Clinicians should be aware that evaluation of cough function using voluntary cough tasks overestimates the PEFR and CEV that would be achieved during reflex cough in patients with PD.
Keywords: airway protection, cough, Parkinson’s disease, reflex cough, voluntary cough
Introduction
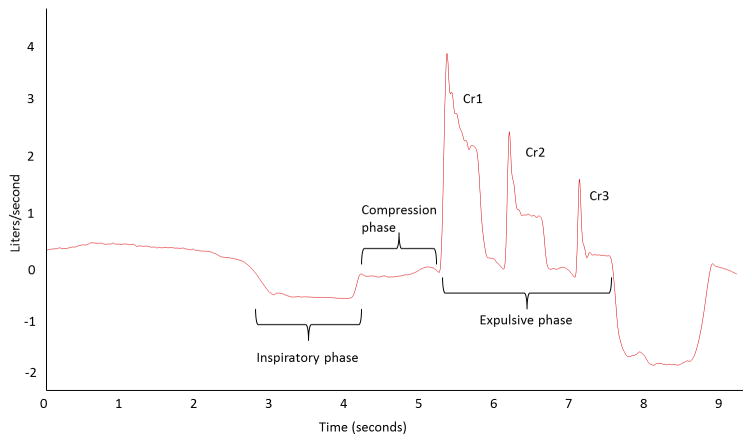
The cough motor pattern comprises a highly coordinated sequence of respiratory and laryngeal muscle contractions resulting in a high rate of expiratory airflow. The cough pattern is responsible for the shearing forces utilized to eject material from the airway. The cough neural network is activated by stimulation of various airway afferents (reflex or induced cough) or alternatively by cortically mediated voluntary neural activation [1–3]. Both reflex and voluntary cough mechanisms initiate a sequence of similar cough motor behaviors, including the inspiratory, compression, and expulsive phases (Figure 1).
Figure 1.
Cough airflow waveform for a cough epoch containing 3 cough re-accelerations (Cr1, Cr2, and Cr3) in the expulsive phase.
Cough has long been studied as an important airway defense behavior, with more recent literature drawing attention to the relationship between cough and swallowing function. A “weak” voluntary cough is associated with increased likelihood of dysphagia in multiple patient populations [4, 5]. In Parkinson’s disease (PD), objective measures of reduced voluntary cough expiratory airflow parameters are strongly correlated to penetration and aspiration of bolus material during swallowing [6, 7]. These cough impairments may be attributed to bradykinesia affecting the abdominal muscles, chest-wall rigidity, reduced central neural drive, and/or poor laryngeal valving, all of which are associated with PD [8, 9].
Fewer empirical studies have investigated reflex cough function in PD. Fontana and colleagues found a reduction in peak abdominal EMG activity for both reflex and voluntary cough in PD when compared to healthy control participants [10]. These researchers observed that EMG activity rise time was slower for PD versus healthy age-matched control participants for both voluntary and reflexive cough, and that for the PD participants, the rate of EMG rise was further reduced for reflex as compared to voluntary cough. The authors hypothesized that the effects on EMG activity would correspond to increased time to reach peak airflow, and overall reduced airflow for voluntary and reflex cough in PD (it is important to note that these hypotheses were not tested in that study). Leow and colleagues also studied reflex cough in PD using citric acid as the tussigenic stimulus. These researchers focused on sensory thresholds associated with cough production in PD, and did not provide information on the expiratory airflows associated with reflex PD cough production [11].
There is evidence that both reflex and voluntary cough airflows are impaired with neurologic injury, including traumatic brain injury (TBI) [12] and stroke [13]. To date there has been no direct comparison of airflow measures between voluntary and reflex cough in PD. The performance of a volitional cough will be restrained by the ability to comprehend the task, initiate the task, and execute the task in the requisite coordinated fashion. This sequence could prove difficult in the PD population which is known to have difficulty with the initiation and coordination of voluntary motor tasks. In contrast, reflex cough has traditionally been considered a brainstem mediated response, and it may be that the more automatic nature of the task will be less affected by sequencing and coordination deficits. The goal of this study was to compare airflow parameters between reflex cough and voluntary cough in patients with idiopathic PD. We hypothesized that voluntary cough airflow measures, including peak airflow rate and total expired air, would be reduced, and cough compression phase duration prolonged as compared to reflex cough in PD. Further, we hypothesized that the time sequence of the cough produced (i.e., whether first or second) would influence these differences. Specifically, we hypothesized that the first cough produced would be significantly different between the two cough types, however the differences would not extend to the second cough produced. Understanding reflex and voluntary cough in PD could potentially impact prevention of aspiration pneumonia and counseling of at risk patients.
Methods
Approval for this study was granted from the University of Florida (UF) Health Science Center Institutional Review board. Twenty participants with idiopathic PD (14 males and 6 females, age M = 68.3 years, SD = 7.33) were recruited from the UF Center for Movement Disorders and Neurorestoration based on consecutive referral to the Speech-Language Pathology service over a three-month time period. The diagnosis of idiopathic PD was determined by a University of Florida neurologist fellowship trained in Movement Disorders and by use of the UK Brain Bank Criteria [14]. All participants provided written informed consent.
Exclusionary criteria were: 1) other neurological disorders (e.g., multiple sclerosis, stroke, brain tumor); 2) history of head, neck, or lung cancer; 3) history of chronic respiratory disorders or diseases (e.g. chronic obstructive pulmonary disease (COPD), asthma, emphysema); 4) history of smoking in the last five years; 5) uncontrolled hypertension; 6) difficulty complying due to neuropsychological or cognitive dysfunction.
Equipment
Cough airflow data were recorded via facemask in line with an antibacterial filter attached to a pneumotachograph (MLT 1000; ADInstruments, Inc). A side delivery port with a one-way inspiratory valve between the facemask and pneumotachograph allowed for nebulizer connection. The nebulizer was a DeVilbiss T-piece (DeVilbiss Healthcare, Inc.) connected to a dosimeter (Koko Dosimeter) that delivered aerosolized solution during inspiration with delivery duration of 2 seconds. The pneumotachograph input differential pressure change to the digital spirometer (ADInstruments, Inc), was digitized (PowerLab, ADInstruments, Inc) and recorded (LabChart 7, ADInstruments, Inc) for analysis. A 3-L syringe was used to calibrate airflow and volume.
Cough was induced using capsaicin ((E)-N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methyl-6-nonenamide) dissolved in a vehicle solution vehicle solution consisting of 80% physiological saline, and 20% ethanol. The capsaicin solution was diluted to concentrations of 50, 100, and 200 μm. The vehicle solution alone (80% physiological saline, 20% ethanol) was administered as a control aerosol (0 μM). For the purposes of this study, only coughs produced at the highest capsaicin concentration (200 μm) were included for comparison with voluntary cough. The rationale for this was based on our preliminary work in healthy adults showing 200 μm to be a supra-threshold level of capsaicin where reflex cough was consistently elicited. Because the study aimed to compare airflow parameters from reflex versus voluntary cough, it was appropriate to use only the supra-threshold level of capsaicin.
Procedures
All procedures were completed in a quiet clinic room with the participant seated upright in a chair. The facemask was held in place over the participant’s nose and mouth by the experimenter or an assistant. First, 30 seconds of tidal breathing were recorded in order for participants to acclimate to the facemask. For reflex cough testing, participants were told that they would “inhale different levels, or concentrations, of a vapor that may or may not make you feel like you need to cough,” with the instruction to “cough if you need to cough into the facemask.” Three presentations of each capsaicin concentration (including the control solution) were administered in a randomized block design in order to control for participant anticipation of inhaling the vapor. The dosimeter was programmed to deliver each single breath inhalation upon detection of the beginning of the inspiratory phase of tidal breathing. Participants were instructed to continue inhaling as the capsaicin vapor was delivered. Airflow was continuously recorded throughout the task, and the facemask was kept in place until at least 2 coughs were produced, or for up to 30 seconds following presentation of the capsaicin solution. Participants rested for at least 90 seconds between each capsaicin presentation, and water was available for sipping between trials.
In order to maintain consistent dead air space and facemask distance from the pneumotachograph between voluntary and reflex cough testing, the same equipment set up as in RC testing was used for voluntary cough (VC), however the nebulizer placed at the one-way valve did not contain any solution. To elicit a voluntary cough, participants were instructed to “cough as though something went down the wrong pipe”. In our clinical and research experience, this set of instructions results in production of more than one voluntary cough [15]. For comparison to reflex cough, which necessarily produces more than one cough, it was important that the voluntary cough be sequential in nature. In order to avoid any residual sensation from capsaicin exposure, voluntary coughs were always completed at the beginning of the study, followed by the reflex cough procedure described in the previous paragraph.
Data measurement and analysis
All airflow measures were recorded and saved to desktop computer (Dell Inspiron) using LabChart 7 (ADInstruments) software. Measurement of cough airflow for both RC and VC was completed for the first (cr1) and second cough (cr2) produced with a cough epoch. A cough epoch was defined as all cough expulsive events associated with a single inhalation. Airflow measures included:
Cough inspired volume (CIV; Liters)
Compression phase duration (CPD; seconds)
Peak expiratory flow rate (PEFR; L/s)
Peak expiratory flow rate rise time (PEFRT; seconds)
Cough volume acceleration (CVA; L/s/s)
Cough expired volume (CEV; Liters)
Total coughs produced (CrTot)
Two repeated measures analyses of variance were completed. First, because the amount of air inspired prior to a cough epoch (CIV), and total number of coughs produced (CrTot) directly influence cough expiratory airflow variables [7, 16] these variables were entered into a RM ANOVA with within subject factor cough type (VC or RC) in order to test for differences that may influence cough expulsive phase variables. Second, cough compression and expulsive phase variables (CPD, PEFR, PEFRT, CVA and CEV) were entered into RM ANOVA with within subject factors cough type (VC or RC) and cough number (Cr1 or Cr2). Post-hoc analysis was performed using Tukey’s HSD.
Results
There were 20 participants who consented to study participation, and all were studied “on” PD medication. One participant was later excluded due to a change in neurologic diagnosis, and four patients were also excluded who did not cough when administered 200 μm capsaicin. These patients were not included in the statistical analysis. A complete analysis was performed on 15 participants, and their demographic information including sex, age, and disease staging according to Hoehn & Yahr (H&Y) score are summarized in Table 1. Descriptive statistics for RC and VC airflow parameters (CPD, PEFR, PEFRT, CVA, and CEV) for Cr1 and Cr2 are in Table 2.
Table 1.
Participant demographic information.
| Participant | Age (years) | Sex | Hoehn & Yahr score |
|---|---|---|---|
| 1 | 68 | M | 2 |
| 2 | 70 | M | 2 |
| 3 | 71 | M | 3 |
| 4 | 69 | M | 2.5 |
| 5 | 62 | M | 2.5 |
| 6 | 49 | M | 2 |
| 7 | 63 | M | 2 |
| 8 | 65 | F | 2.5 |
| 9 | 70 | M | 2 |
| 10 | 72 | M | 2 |
| 11 | 76 | M | 2.5 |
| 12 | 81 | F | 2.5 |
| 13 | 66 | M | 2 |
| 14 | 77 | M | 3 |
| 15 | 67 | F | 2 |
| Mean: 68.4 | 12 M; 3 F | Median: 2 |
Table 2.
Descriptive statistics, including mean and standard error, for the dependent variables according to cough type (reflex or voluntary) and cough number (1 and 2).
| Cough number | Dependent variable | Reflex cough | Voluntary cough | ||
|---|---|---|---|---|---|
| Mean | Standard error | Mean | Standard error | ||
| Total coughs in epoch | 4.66 | 0.52 | 3.93 | 0.36 | |
| Cough inspired volume | 0.76 | 0.10 | 0.89 | 0.11 | |
| Cough 1 | CPD (seconds) | 0.71 | 0.28 | 0.32 | 0.07 |
| PEFR (L/s) | 2.40 | 0.12 | 3.23 | 0.39 | |
| PEFRT (seconds) | 0.05 | 0.01 | 0.05 | 0.00 | |
| CVA (L/s/s) | 55.22 | 5.92 | 61.80 | 5.04 | |
| CEV (Liters) | 0.51 | 0.05 | 0.66 | 0.10 | |
| Cough 2 | CPD (seconds) | 0.18 | 0.05 | 0.14 | 0.04 |
| PEFR (L/s) | 1.61 | 0.11 | 2.27 | 0.20 | |
| PEFRT (seconds) | 0.03 | 0.002 | 0.03 | 0.002 | |
| CVA (L/s/s) | 57.40 | 5.37 | 70.31 | 7.00 | |
| CEV (Liters) | 0.22 | 0.03 | 0.32 | 0.04 | |
Note: CPD is compression phase duration; PEFR is peak expiratory flow rate; PEFRT is peak expiratory flow rise time; CVA is cough volume acceleration; CEV is cough expired volume.
Results of the first RM ANOVA model showed no significant effect for CIV or CrTot according to cough type (F (2,13) = 2.369, p = 133). The second RM ANOVA showed a significant effect for cough type (F (5,10) = 3.906, p = .032, η2 = .661) and for cough number (F(5,10) = 12.649, p < .001, η2 = .863), but no significant interaction effect (F (5,10) = 2.235, p = .131) on the dependent variables. Results of the univariate tests for the dependent variables revealed that for cough number, all measures were significantly different with the exception of CVA. For cough type, only PEFR (F (1) = 10.670, p = .006, η2 = .433) and CEV (F (1) = 4.860, p = .045, η2 = .258) were significantly different between reflex and voluntary cough (Figure 2).
Figure 2.
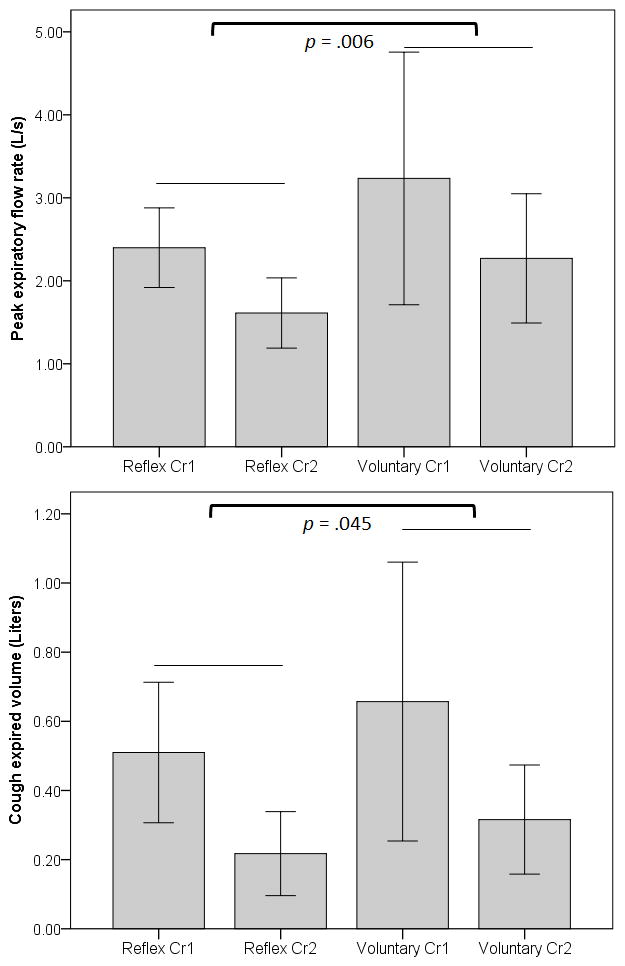
Significant differences for peak expiratory flow rate (PEFR; top graph) and cough expired volume (bottom graph). Bars are means, and whisker bars are 1 standard error.
Discussion
The goal of this study was to compare reflex and voluntary cough airflows in PD. The a-priori hypothesis that voluntary cough airflow parameters would be reduced versus reflex cough parameters in this PD cohort was based on anticipated difficulty with the initiation and execution of the voluntary cough task. However, the results failed to support this hypothesis. The results instead revealed that cough airflow parameters, including PEFR and CEV were reduced for reflex cough as compared to voluntary cough. These differences were present for both the first and second cough (Cr1 and Cr2) in the epoch, and this also contrasted to our a priori hypothesis. The main effect for cough number was not surprising, and was consistent with previous research that observed airflows associated with Cr1 were greater than subsequent coughs in an epoch [7, 17].
Cough expired volume (CEV) has been shown to have a strong positive relationship to PEFR in healthy adults [7]. It was therefore not surprising that CEV was reduced for reflex cough versus voluntary cough given the concomitant reduction of PEFR. Both PEFR and CEV during cough are influenced by the amount of air in the lungs at cough initiation [16], total coughs produced in an epoch [7], and the coordination between the laryngeal and respiratory subsystems [18, 19]. While we did not directly measure total lung volume at cough initiation, our data showed no significant differences between voluntary and reflexive coughing in terms of cough inspired volume. Because participants began each cough task from a resting tidal breathing pattern, we expect that potential differences in total lung volume were minimal between the tasks. We observed no significant differences between the total number of coughs produced per epoch between RC and VC. We believe it is possible that the differences in PEFR and CEV found between reflex and voluntary cough were related to differences in the function and coordination between the respiratory and laryngeal subsystems.
Respiratory function in Parkinson’s disease is characterized by reduction in maximal voluntary ventilation [20], reduced peak expiratory flow [8, 20, 21], delay in reaching PEF during a forced vital capacity task and reduced inspiratory and expiratory pressure generating capacities [8]. These changes have been largely attributed to bradykinesia, increased rigidity, and difficulty with coordinated contraction of upper airway and chest wall muscles in PD [22]. Because cough is an overlaid function of the respiratory system, it is not surprising that studies have shown differences in cough function for patients with PD. Specifically with regard to the comparison of RC and VC, Fontana and colleagues showed the rate of rise and maximum amplitude of abdominal electromyographic (EMG) activity was reduced in reflex cough compared to voluntary cough in patients with PD compared to healthy control participants [10]. While they did not measure airflow in that study, it is reasonable to speculate that expiratory muscle (abdominal) EMG activity would relate to the rate of expiratory airflow [23]. The results of our current study could be hypothesized to reflect, in part, differences in the rate and amplitude of expiratory muscle activation between RC and VC cough.
The coordination of the respiratory system with adequate laryngeal function is an important factor in the production of cough. Patients with PD exhibit glottal insufficiency and slowing in the rate of vocal fold movement secondary to increased rigidity of the intrinsic laryngeal muscles. Phase asymmetry during phonation is also a common phenomenon [9]. Laryngeal valving during cough is an active process [18, 19, 24] that involves precise timing of onset and offset between the abductor muscle, posterior cricoarytenoid (PCA) and adductors, thyroaryteniod (TA) and lateral cricoarytenoid (LCA) [25]. The rate and volume of expiratory airflow is impacted by the laryngeal resistance to airflow during the compression phase, and the rate and amplitude of vocal fold (VF) abduction during the expulsive phase. If laryngeal resistance is reduced secondary to glottal insufficiency, tracheal pressure will likely also be reduced, because tracheal pressure during cough results from adequate laryngeal resistance to expiratory airflow [26]. As well, if the antagonistic abductor/adductor muscles are not precisely timed, leading to excessive co-contraction during the expulsive phase of cough, the resulting glottal configuration may be inadequate to achieve high rate and volume of expiratory airflow. Poliaceck and colleagues found differences in the burst duration of TA and PCA muscles between two types of induced cough in a cat model. These differences lead to varying degrees of overlap in the contraction of the TA and PCA muscles [18]. We hypothesize that differences for the cough tasks related to laryngeal function in the current study reflect differences in degree of co-contraction between adductor and abductor muscles. Specifically, it is possible that the degree of glottal opening was restricted during the reflex cough due to prolonged co-contraction of adductor muscles during the expulsive phase.
Additionally, the important role of neural control for reflex and voluntary cough must be considered. Our understanding of reflex cough has evolved from a brainstem-mediated reflex, to now include cortical input influencing both the excitation and inhibition of reflex cough [27–30]. The urge-to-cough, a respiratory sensation preceding reflex cough, is an additional variable that is known to influence the total number of reflex coughs produced [31]. Along with the fact that healthy adults can volitionally modulate reflexive cough, we cannot definitively eliminate the possible effect of voluntary modulation of reflex cough by participants that could influence the results for reflex coughing. We made every attempt to remind participants to simply “cough if you need to cough” and utilized the “C2” reflex cough criteria used by others for establishing a reflex cough threshold in previous studies (e.g., Dicipinigaitis et al., 2009) [32]. Given the complexity of cough across sensory and motor systems, and the central and peripheral nervous systems necessitates additional research to fully understand the mechanisms underlying results of this study. Current studies in our laboratory are ongoing to address these important questions.
Conclusions
Results of this study revealed that the rate and volume of airflow was reduced during the expulsive phase of reflex cough as compared to voluntary cough in patients with PD. Clinically, reflex cough is critical for clearing aspirate and endogenously produced material from the lower airway. Because PEFR is considered a marker for cough effectiveness, counseling patients with PD to produce an epoch of voluntary coughs in response to any urge-to-cough (regardless of whether or not a reflex cough was triggered) may aid in expelling unwanted material from the airway. Clinicians should be aware that evaluation of cough function using voluntary cough tasks overestimates the PEFR and CEV that would be achieved during reflex cough in patients with PD. Given the complexity of both peripheral and central control of cough, there is much additional research to be done in order to fully understand the respiratory, laryngeal, and neural mechanisms underlying the results of this study, and how the results relate to typical aging versus PD. Because aspiration pneumonia is among the leading causes of death in PD, continuing to better understand the mechanism(s) leading to deficits in airway protection is critical for improving management of these patients.
Acknowledgments
Dr. Hegland reports no disclosures or conflicts of interest related to this work. Dr. Hegland’s work is sponsored in part by the American Heart Association and BAE defense systems.
Dr. Troche reports no disclosures or conflicts of interest related to this work. Dr. Troche’s research is supported in part by an NIH (NCATS) CTSA through the University of Florida (UL1TR000064 and KL2TR000065).
Ms. Brandimore reports no disclosures related to this study. Ms. Brandimore’s work is supported in part by a pre-doctoral fellowship through the Department of Veterans Affairs.
Dr. Davenport reports no disclosures or conflicts of interest related to this work. Dr. Davenport’s research is supported by NIH and BAE defense systems. He also has financial interest in Aspire Products, LLC.
Dr. Okun reports no disclosures or conflicts of interest related to this work. Dr. Okun serves as a consultant for the National Parkinson Foundation, and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. Dr. Okun has previously received honoraria, but in the past >36 months has received no support from industry. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Okun has participated in CME activities on movement disorders in the last 36 months sponsored by PeerView, Prime, and by Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry sponsored trials over the years but has not received honoraria.
References
- 1.Bolser DC, Davenport PW. Functional organization of the central cough generation mechanism. Pulmonary pharmacology & therapeutics. 2002;15(3):221–5. doi: 10.1006/pupt.2002.0361. [DOI] [PubMed] [Google Scholar]
- 2.Widdicombe JG. Neurophysiology of the cough reflex. The European respiratory journal. 1995 Jul;8(7):1193–202. doi: 10.1183/09031936.95.08071193. [DOI] [PubMed] [Google Scholar]
- 3.Eccles R. Central mechanisms IV: conscious control of cough and the placebo effect. Handbook of experimental pharmacology. 2009;(187):241–62. doi: 10.1007/978-3-540-79842-2_12. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder MF, Daniels SK, McClain M, Corey DM, Foundas AL. Clinical and cognitive predictors of swallowing recovery in stroke. Journal of rehabilitation research and development. 2006 May-Jun;43(3):301–10. doi: 10.1682/jrrd.2004.12.0154. [DOI] [PubMed] [Google Scholar]
- 5.Mann G. MASA: The mann assessment of swallowing ability. Clifton, NY: Thomson Learning Inc; 2002. [Google Scholar]
- 6.Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008 Sep;23(3):297–301. doi: 10.1007/s00455-007-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hegland KW, Troche MS, Davenport PW. Cough expired volume and airflow rates during sequential induced cough. Frontiers in physiology. 2013;4:167. doi: 10.3389/fphys.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hovestadt A, Bogaard JM, Meerwaldt JD, van der Meche FG, Stigt J. Pulmonary function in Parkinson’s disease. Journal of neurology, neurosurgery, and psychiatry. 1989 Mar;52(3):329–33. doi: 10.1136/jnnp.52.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanson DG, Gerratt BR, Ward PH. Cinegraphic observations of laryngeal function in Parkinson’s disease. The Laryngoscope. 1984 Mar;94(3):348–53. doi: 10.1288/00005537-198403000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Fontana GA, Pantaleo T, Lavorini F, Benvenuti F, Gangemi S. Defective motor control of coughing in Parkinson’s disease. American journal of respiratory and critical care medicine. 1998 Aug;158(2):458–64. doi: 10.1164/ajrccm.158.2.9705094. [DOI] [PubMed] [Google Scholar]
- 11.Leow LP, Beckert L, Anderson T, Huckabee ML. Changes in chemosensitivity and mechanosensitivity in aging and Parkinson’s disease. Dysphagia. 2012 Mar;27(1):106–14. doi: 10.1007/s00455-011-9347-z. [DOI] [PubMed] [Google Scholar]
- 12.Lee SC, Kang SW, Kim MT, Kim YK, Chang WH, Im SH. Correlation Between Voluntary Cough and Laryngeal Cough Reflex Flows in Patients With Traumatic Brain Injury. Archives of physical medicine and rehabilitation. 2013 Aug;94(8):1580–3. doi: 10.1016/j.apmr.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Ward K, Seymour J, Steier J, et al. Acute ischaemic hemispheric stroke is associated with impairment of reflex in addition to voluntary cough. The European respiratory journal. 2010 Dec;36(6):1383–90. doi: 10.1183/09031936.00010510. [DOI] [PubMed] [Google Scholar]
- 14.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. Journal of neurology, neurosurgery, and psychiatry. 1992 Mar;55(3):181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hegland KW, Okun MS, Troche MS. Sequential Voluntary Cough and Aspiration or Aspiration Risk in Parkinson’s Disease. Lung. 2014 May 3; doi: 10.1007/s00408-014-9584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JA, Aliverti A, Quaranta M, et al. Chest wall dynamics during voluntary and induced cough in healthy volunteers. The Journal of physiology. 2012 Feb 1;590(Pt 3):563–74. doi: 10.1113/jphysiol.2011.213157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris RS, Lawson TV. The relative mechanical effectiveness and efficiency of successive voluntary coughs in healthy young adults. Clin Sci. 1968 Jun;34(3):569–77. [PubMed] [Google Scholar]
- 18.Poliacek I, Stransky A, Jakus J, Barani H, Tomori Z, Halasova E. Activity of the laryngeal abductor and adductor muscles during cough, expiration and aspiration reflexes in cats. Physiological research/Academia Scientiarum Bohemoslovaca. 2003;52(6):749–62. [PubMed] [Google Scholar]
- 19.Sant’Ambrogio G, Kuna ST, Vanoye CR, Sant’Ambrogio FB. Activation of intrinsic laryngeal muscles during cough. American journal of respiratory and critical care medicine. 1997 Feb;155(2):637–41. doi: 10.1164/ajrccm.155.2.9032206. [DOI] [PubMed] [Google Scholar]
- 20.Polatli M, Akyol A, Cildag O, Bayulkem K. Pulmonary function tests in Parkinson’s disease. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2001 Jul;8(4):341–5. doi: 10.1046/j.1468-1331.2001.00253.x. [DOI] [PubMed] [Google Scholar]
- 21.Monteiro L, Souza-Machado A, Valderramas S, Melo A. The Effect of Levodopa on Pulmonary Function in Parkinson’s Disease: A Systematic Review and Meta-Analysis. Clin Ther. 2012 May;34(5):1049–55. doi: 10.1016/j.clinthera.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 22.de Bruin PF, de Bruin VM, Lees AJ, Pride NB. Effects of treatment on airway dynamics and respiratory muscle strength in Parkinson’s disease. The American review of respiratory disease. 1993 Dec;148(6 Pt 1):1576–80. doi: 10.1164/ajrccm/148.6_Pt_1.1576. [DOI] [PubMed] [Google Scholar]
- 23.Lasserson D, Mills K, Arunachalam R, Polkey M, Moxham J, Kalra L. Differences in motor activation of voluntary and reflex cough in humans. Thorax. 2006 Aug;61(8):699–705. doi: 10.1136/thx.2005.057901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Britton D, Yorkston KM, Eadie T, et al. Endoscopic assessment of vocal fold movements during cough. The Annals of otology, rhinology, and laryngology. 2012 Jan;121(1):21–7. doi: 10.1177/000348941212100105. [DOI] [PubMed] [Google Scholar]
- 25.Poletto CJ, Verdun LP, Strominger R, Ludlow CL. Correspondence between laryngeal vocal fold movement and muscle activity during speech and nonspeech gestures. J Appl Physiol (1985) 2004 Sep;97(3):858–66. doi: 10.1152/japplphysiol.00087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaker R, Dua KS, Ren J, Xie P, Funahashi A, Schapira RM. Vocal cord closure pressure during volitional swallow and other voluntary tasks. Dysphagia. 2002 Winter;17(1):13–8. doi: 10.1007/s00455-001-0096-2. [DOI] [PubMed] [Google Scholar]
- 27.Lee PC, Cotterill-Jones C, Eccles R. Voluntary control of cough. Pulmonary pharmacology & therapeutics. 2002;15(3):317–20. doi: 10.1006/pupt.2002.0365. [DOI] [PubMed] [Google Scholar]
- 28.Hutchings HA, Morris S, Eccles R, Jawad MS. Voluntary suppression of cough induced by inhalation of capsaicin in healthy volunteers. Respiratory medicine. 1993 Jul;87(5):379–82. doi: 10.1016/0954-6111(93)90052-2. [DOI] [PubMed] [Google Scholar]
- 29.Hegland KW, Bolser DC, Davenport PW. Volitional control of reflex cough. J Appl Physiol (1985) 2012 Jul;113(1):39–46. doi: 10.1152/japplphysiol.01299.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Troche MS, Brandimore AE, Godoy J, Hegland KW. A framework for understanding shared substrates of airway protection. Journal of Applied Oral Science. doi: 10.1590/1678-775720140132. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davenport PW, Vovk A, Duke RK, Bolser DC, Robertson E. The urge-to-cough and cough motor response modulation by the central effects of nicotine. Pulmonary pharmacology & therapeutics. 2009 Apr;22(2):82–9. doi: 10.1016/j.pupt.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dicpinigaitis PV. Clinical cough III: measuring the cough response in the laboratory. Handbook of experimental pharmacology. 2009;(187):297–310. doi: 10.1007/978-3-540-79842-2_15. [DOI] [PubMed] [Google Scholar]