Abstract
BACKGROUND
Given observational findings that physical activity reduces breast cancer risk, improves survival, and improves quality of life in breast cancer survivors, a need has been identified for randomized controlled trials that testthe efficacy of exercise on biological mechanisms associated with breast cancer survival. The primary aims of the Yale Exercise and Survivorship Study were to 1) determine the feasibility of recruiting breast cancer survivors into a randomized controlled trial of the effects of exercise on biological markers and/or mechanisms associated with survival, 2) compare the effectiveness of various recruitment strategies on accrual rates and baseline characteristics, and 3) report adherence to the exercise trial.
METHODS
Seventy-five postmenopausal breast cancer survivors self-referred into the trial or were recruited through the Connecticut Tumor Registry and randomly assigned to an exercise (n = 37) or usual-care (n = 38) group. The exercise group participated in 150 min/wk of supervised gym-based and home-based aerobic exercise for 6 months. The usual-care group was instructed to maintain current physical activity level.
RESULTS
A total of 75 women (an accrual rate of 9.5%) were randomized to the trial. Rates of accrual were higher for women who self-referred into the study (19.8%) compared with women recruited via the cancer registry (7.6%); however, demographic, physiologic, and prognostic characteristics did not differ between the 2 recruitment strategies. On average, exercisers increased moderate- intensity to vigorous-intensity aerobic exercise by 129 minutes per week compared with 44 minutes per week among usual-care participants (P < .001). Women in the exercise-intervention group increased their average pedometer steps by 1621 steps per day compared with a decrease of 60 steps per day among women in the usual-care group (P < .01).
CONCLUSIONS
Findings from this study will provide useful information for investigators who are conducting exercise trials in cancer populations, clinicians who are treating women diagnosed with breast cancer, and exercise professionals who are developing community-based exercise programs for cancer survivors.
Keywords: physical activity, fitness, survival, recurrence, death, body fat, obesity, weight
With a predicted 178,000 incident cases in 2007, breast cancer is the most frequently diagnosed cancer among American women.1 Because of the combined influences of increased incidence and improved survival, there are now an estimated 2.3 million breast cancer survivors in the United States alone,2 comprising the largest group (about 40%) of female cancer survivors.3 During and after breast cancer treatment, many survivors experience unpleasant side effects such as weight gain, nausea, fatigue, myalgias and/or arthralgias, osteoporosis, and decreased cardiovascular function.4,5 Primary and adjuvant breast treatments are associated with acute and long-term morbidity, and interventions to improve functional ability and quality of life are needed.
Physical activity has been shown to be effective in improving physical functioning,6,7 in reducing treatment-related fatigue,8–11 and in improving quality of life9,12–16 in breast cancer survivors and may also be effective in preventing weight gain.9,17 Physical activity has also been shown to reduce the risk of primary breast cancer,18–20 and recent observational evidence suggests that approximately 2–3 hr/wk of moderate-intensity aerobic exercise, such as brisk walking, may reduce the risk of recurrence and death from breast cancer.21 The mechanisms thought to confer this reduced risk include the effect of exercise on insulin,22 insulin-like growth factors (IGFs),23 sex hormones,24 and adiposity.25 Despite these and other well documented benefits of physical activity, a breast cancer diagnosis is typically followed by a decrease in physical activity and a large proportion of breast cancer survivors do not participate in recommended amounts of physical activity,26,27 suggesting that intervention is necessary in this population.28
Approximately 23 published, randomized trials have examined the effect of exercise after a breast cancer diagnosis on physical and emotional functioning and overall quality of life.29–39 The majority of these studies have shown that physical activity is safe after a diagnosis of breast cancer and is associated with improvements in physical and emotional quality of life. However, although these findings are positive, most of these studies had methodological limitations. Most of the studies recruited small samples (eg, fewer than 50 participants), used convenience-sampling approaches, and recruited cancer survivors into non-randomized trials of short duration (eg, less than 3 months) at doses lower than the recommended level of physical activity. Furthermore, a majority of these studies did not examine the effect of exercise on innovative outcomes, such as biological mechanisms associated with prognosis. Thus, the feasibility of recruiting a diverse sample of breast cancer survivors into high-quality studies, that is, randomized controlled exercise trials of recommended amounts of physical activity for longer study durations, is unknown. Few of the published exercise trials in breast cancer survivors have described the recruitment strategies used in their trials and compared such strategies on accrual rates, reasons for nonparticipation, and baseline demographic, physiologic, and prognostic characteristics. Understanding the strengths and limitations of various recruitment strategies on the feasibility of recruiting a diverse sample of breast cancer survivors is important for future trials and has implications for designing community, hospital, and clinic exercise programs. Lastly, few trials have reported adherence results in sufficient detail to show that such trials are feasible in cancer populations. If exercise trials in breast cancer survivors who are participating in recommended levels of physical activity are not feasible, then we cannot expect to see a benefit of exercise on biological mechanisms associated with breast cancer prognosis or on breast cancer survival.
Thus, given the recent observational finding that physical activity improves breast cancer survival, we conducted the Yale Exercise and Survivorship (YES) Study to examine the feasibility of recruiting 75 breast cancer survivors into a 6-month randomized controlled trial of exercise on biological markers and/or mechanisms associated with survival. The purposes of this article are 1) to describe the recruitment process and to compare various recruitment strategies on accrual rates and baseline characteristics, and 2) to report adherence to the exercise trial. The YES Study is novel in that it addressed some of the methodological limitations of past studies by using a randomized study design, by using a population-based recruitment strategy, by prescribing recommended levels of physical activity, by making a high quality assessment of physical activity and adherence, by using a 6-month study duration and a 18-month postrandomization follow-up to assess maintenance of behavior change, and by obtaining more conclusive data on the effect of exercise on biological mechanisms that may mediate the potential effect of exercise on survival. We believe findings from the YES Study will provide useful information for investigators who are conducting exercise trials in cancer populations, clinicians who are treating women diagnosed with breast cancer, and those who are developing community-based exercise programs for cancer survivors.
MATERIALS AND METHODS
All study procedures, including written informed consent, were reviewed and approved by the Yale University School of Medicine Human Investigation Committee.
Study Participants
The YES Study included postmenopausal women who had been diagnosed 1–10 years ago with stage 0 (in situ) to stage IIIA breast cancer and who had completed adjuvant treatment (with the exception of hormonal therapy) at least 6 months before enrollment. We chose to recruit only postmenopausal women because of the potential modifying effect of menopausal status on hormones. Women younger than 40 years of age were excluded from the study because of potential differences in the etiology of breast cancer among very young women, whereas women older than 75 years were excluded because of the high likelihood of significant comorbidity as well as possible feasibility and safety issues involved in conducting an exercise program in elderly women. Women with stages IIIB to IV were excluded because of the severity and poorer overall prognosis of these tumors.1 To observe a maximal and independent effect of exercise on biological outcomes, only those women who were sedentary or who reported low physical activity levels at baseline (<90 minutes per week of moderate- to vigorous-intensity recreational physical activity) and were not currently participating in an exercise or weight-loss diet program were eligible. Smokers, diabetics, and women with a history of invasive cancer before the breast cancer diagnosis were excluded because of the potential effect of these factors on biological outcomes of interest. All women resided in Connecticut; however, women had to be willing to travel to an exercise facility in New Haven 3 times per week over the 6-month study period.
Recruitment
We used 2 strategies to recruit women into our exercise trial, the Rapid Case Ascertainment Shared Resource, a population-based strategy, and self-referral.
Rapid Case Ascertainment Shared Resource (RCA)
Breast cancer survivors who were diagnosed through Yale-New Haven Hospital were identified via RCA (a field arm of the Connecticut Tumor Registry, a National Cancer Institute Surveillance, Epidemiology, and End Results site) of the Yale Comprehensive Cancer Center. RCA provided YES staff with the names of Connecticut women diagnosed with breast cancer by any Yale-affiliated physician in the past 10 years (years 1994 to 2005). A total of 1072 names of women diagnosed by Yale-affiliated physicians were obtained, as well as their pathology reports (patient’s name, birth date, contact information, patient’s physician). YES staff then contacted each patient’s physician by letter to request permission to contact the participant and to obtain the physician’s consent for the participant to begin a moderate-intensity aerobic exercise program. Physicians responded to our request by mailing or faxing to us their signature of consent for us to contact the participant and consent for her to exercise. An invitation letter was then mailed to the participant to tell her about the study and inform her that the study manager would contact her within a few days to solicit her interest and eligibility.
Recruitment packets included an invitation letter, a brochure describing the YES Study, and a pre-stamped, preaddressed postcard that participants could use to indicate interest in the study, interest in similar future studies, or to request not to be contacted again by study staff. One week after mailing the recruitment packet, study staff telephoned each potential participant who had either indicated interest on the postcard, or who had not returned the postcard, to discuss the study. If the woman was interested, a telephone screening questionnaire was completed to determine eligibility. If eligible, a potential participant was scheduled for a baseline visit at Yale University the next week.
Self-Referral
The population of potential participants for the YES Study also included a small number of women who learned about the study through the media and contacted study staff directly. Specifically, the principal investigator was interviewed at least 8 times between 2004 and 2006, when we were actively recruiting participants (2 television news programs and 6 newspaper interviews). We also purchased advertisement space in 1 local newspaper. The advertisement was published for 1 week in September 2004.
After learning of the study, potential participants contacted us. These women were screened by the same eligibility criteria as those recruited via RCA. Interested and eligible study participants were then scheduled for a baseline visit at Yale University.
For women recruited via RCA or self-referral who were not interested or ineligible for the study, a brief demographic questionnaire (age, ethnicity, education level, reason for nonparticipation) was completed.
Baseline Visit
The baseline visit, completed at Yale University for all participants who were interested and eligible, provided an opportunity for potential participants to meet study staff in person, learn more about the study, and sign informed consent forms. Interviewer-administered questionnaires were used to collect information including demographics, medical history, readiness to change (the transtheoretical model), and current and prior physical activity (see Measures). A clinic visit was then scheduled at Yale-New Haven Hospital for the following week, and the participant was given a 7-day physical activity log, a pedometer, a 7-day pedometer log, and a packet of psychosocial questionnaires (see Measures) to complete at home and then bring to the clinic visit.
Clinic Visit
Physical measurements were collected during a 1-hour appointment at the General Clinical Research Center (GCRC) at Yale-New Haven Hospital. Study staff reviewed the packet of take-home questionnaires and physical activity and pedometer logs so that any missing or unclear responses could be clarified by the participant. A nurse performed a fasting blood draw and measured blood pressure and resting heart rate, and study staff measured the participant’s height, weight, and waist and hip circumferences. A whole-body dual energy x-ray absorptiometry (DEXA) scan was completed to assess total body fat and bone mineral density.
Randomization
After completion of all baseline measures, each participant was randomly assigned with equal probability to either the exercise or usual-care group. Randomization was performed by using a random number generation, and group assignment was placed in a sealed envelope, which was opened by the study coordinator at the time of randomization. Exercise group participants met with study staff within the week after randomization to begin the exercise program, and usual-care participants were told that they would be asked to complete a follow-up clinic visit at 6 months.
Follow-up Data Collection
For each participant, the same data that were collected at the baseline visit were collected in a similar manner at 6 months postrandomization by staff blinded to the participant’s group.
Measures
Physical Activity
Physical activity was assessed at baseline and 6 months by using an interview-administered Physical Activity Questionnaire (PAQ),40 a 7-day physical activity log, and a 7-day pedometer log. Adherence to the intervention among exercise group participants was assessed with 7-day physical activity logs weekly.
Physical Activity Questionnaire (PAQ)
To determine eligibility, staff used a validated physical activity questionnaire to interview participants on their current (past 6 months) recreational activity level. For each activity reported, participants were asked to specify the average frequency of the activity (eg, days per week [d/wk) and the average duration of the activity (minutes per session [min/session]). Hours per week spent at different intensities (light, moderate, vigorous) of recreational activities were then computed by classifying each type of activity according to intensity and associated MET level (light, <3 METS; moderate, 3–6 METs; or vigorous, >6METs) using Ainsworth’s Compendium of Physical Activities.41
Seven-Day Physical Activity Log (7-Day PAL)
The 7-Day PAL40 is a validated measure that was completed by all participants before randomization and at the 6-month follow-up visit and was also used to measure adherence in the exercise group. When completing the log, women recorded the type and duration of any recreational activity performed on each day, along with (among exercise group participants) their corresponding heart rate. Hours per week spent in moderate-to-vigorous intensity aerobic activity were determined by using Ainsworth’s Compendium of Physical Activities.41
Seven-Day Pedometer Log
The 7-Day Pedometer Log42 provides a comprehensive indicator of all walking performed each day and was completed by participants before randomization and at the 6-month follow-up visit. Participants were given a digital pedometer and taught how to properly use it to measure their daily steps. Participants wore the pedometer from when they awoke in the morning until they went to bed in the evening.
Demographics & Medical History
Information on demographics, relevant medical history, health habits, and comorbidities was collected via an interviewer-administered questionnaire at the baseline visit. The questionnaire included questions pertaining to smoking and alcohol intake habits, reproductive history, and family history of cancer. Comorbidities assessed included current or past occurrence of high blood pressure, angina, heart disease, arthritis, and other conditions that could affect a participant’s ability to adhere to the intervention or the safety of performing regular aerobic exercise. Information on disease stage, hormone-receptor status, histological grade, recommended therapy and evidence of completion, and surgery was provided by participants at baseline and 6 months, and this information was later confirmed by the participant’s physician and review of medical records.
Anthropometrics
Height and weight were measured at baseline and at 6 months. Participants were weighed on a digital scale in light clothing and without shoes; measurements were rounded up to the next 0.1 kg. Height without shoes was measured by using a stadiometer, rounding up to the next 0.5 cm. Circumference measurements were taken at the waist (minimum circumference), umbilicus, and hips (greatest circumference). All measurements were taken twice in succession and averaged for data entry. DEXA Scans. A DEXA scan was completed for each participant at baseline and at 6 months. The DEXA measurements were made with a Hologic scanner (Hologic QDR 1500; Hologic, Waltham, Mass) that uses a constant potential x-ray source and a k-edge filter (cerium) to achieve a congruent beam of stable dual-energy radiation.43 All DEXA scans were evaluated by a radiologist blinded to the intervention group of the participant.
Food Frequency Questionnaire
All participants completed a 120-item, validated, food-frequency questionnaire at baseline and at 6 months.44 Although participants were advised to maintain their current dietary habits, we measured their dietary habits to control for any changes in diet over the 6-month time period.
Exercise Intervention
The YES Study exercise intervention consisted of a combined supervised training program at a local health club and a home aerobic training program. Participants exercised in small groups of 2–5 participants at the health club during designated sessions 3 times per week and were instructed to exercise 2 d/wk on their own, either at the health club or in their neighborhood or home. Because of our goal to examine the efficacy of exercise on biological outcomes, we wanted to maintain high control over adherence, including directly observing exercise sessions and their intensity and duration.
At the initial health club exercise meeting, a YES exercise physiologist outlined the program goals, instructed the participant on the use of the heart rate monitor, and introduced the participant to the study materials. Participants received a binder containing educational worksheets and exercise logs. The worksheets, adapted from the Physical Activity for Total Health Study,40 included topics such as overcoming barriers and enlisting social support, injury prevention, choosing footwear for exercise, and a list of local parks and fitness areas. After reviewing the materials, the exercise physiologist and participant set initial goals for the first week of physical activity. Participants were asked to perform 3 15-minute sessions during Week 1, building to 5 30-minute moderate-intensity sessions by Week 5.
During the 6-month intervention, each participant was taught exercise techniques and principles during the thrice weekly health club meetings with a YES exercise physiologist. The intervention consisted primarily of walking, an activity preferred by most women42 and breast cancer survivors,27 although participants could choose to meet the exercise goal through swimming, aerobics, other forms of activity, or a combination of different activities. Activities that did not involve sustained aerobic effort, such as weight lifting and yoga, could be performed but did not count toward the exercise goal for each week.
The goal of the intervention was to perform 30 minutes of moderate-to-vigorous aerobic exercise, 5 days a week, at 60%–80% of maximal heart rate reserve, in accordance with the American College of Sports Medicine’s current physical activity recommendation for adults.45 Participants wore heart-rate monitors during each workout to enable them to accurately self-monitor exercise intensity. After each exercise session, participants recorded the type, duration, perceived intensity of activity, and average heart rate during exercise (as calculated by the heart rate monitor) in physical activity logs. Participants returned logs to the exercise physiologists at the end of each week.
Tools used to enhance adherence included 1) personalized and written feedback on baseline data with determination of an individualized exercise program, 2) self-monitoring via the physical activity logs, pedometers, and heart rate monitors, 3) realistic goals, 4) initial use of individual in-person instruction to teach concepts and techniques, 5) weekly and/or monthly counseling to facilitate overcoming barriers and answer questions, 6) directly observing participants exercise, 7) group participant-support during weekly exercise sessions, and 8) quarterly newsletters with a focus on the benefits of physical activity, upcoming events (eg, fun runs and walks), and issues related to breast cancer survivorship.
Usual Care Group
Because of the many documented health benefits of physical activity, instructing study participants to avoid exercise would be unethical. Therefore, participants assigned to usual care were asked to maintain their current physical activity levels; if a participant wanted to exercise, however, then she was told that she could, but our exercise program and training materials could not be offered to her until the end of the study (at 6 months). After their 6-month end-of-study assessment, women in the usual-care group were given the exercise handouts and pedometer to keep and shown how to use these materials to mimic the intervention on their own if they wished. They were also offered 3 supervised training exercise sessions at the health club. Usual-care group participants also received quarterly newsletters that focused on issues related to breast cancer survivor-ship but did not mention or promote physical activity. At the end of their participation in the study, all participants received individualized results of those procedures and tests that had clinical relevance.
Statistical Analyses
Participants were grouped according to the intention-to-treat procedure, in which all randomized participants were grouped according to their intervention assignment at randomization, regardless of compliance or adherence to the study. We used Student t test and chi-square analyses to assess any baseline differences between 1) women recruited via the Rapid Case Ascertainment Shared Resource or self-referral, and 2) women randomized to exercise or usual care. The 7-Day Physical Activity Log was the primary measure used to compare physical activity at baseline and at 6-months between the exercise and usual-care groups. A secondary measure of physical activity included the 7-day pedometer log. Adherence, assessed from the 26 weekly, 7-day physical activity logs kept by women randomized to exercise, was defined as the average minutes per week of moderate-intensity aerobic exercise performed from baseline to 6 months. Good adherence was defined as meeting 80% of the exercise prescription (or 120 minutes of the 150 minutes per week goal). Logs not returned by participants throughout the 26-week intervention were assigned 0 minutes per week of physical activity.
Power Considerations
Our goal was to recruit 75 participants, which included a potential 10% dropout rate, into the study. This was the sample size calculated to detect at least a 2% and a 10% absolute difference between groups in change from baseline to 6 months for percentage body fat and fasting insulin levels, respectively. These effect sizes were based on sizes observed in a randomized controlled exercise trial in women at high risk for breast cancer.42,46
RESULTS
Recruitment
Recruitment began in March 2004 and was completed in January 2006; the recruitment process is shown in Figure 1. A total of 1072 pathology reports with patient contact information and a listed Yale-affiliated physician were received from RCA. Of these, physician consent was obtained for 763 (71%) women. A recruitment packet was mailed to all 763 women for whom physician consent to contact and exercise was obtained; 101 of these women opted out of the screening call by returning the postcard indicating that they were not interested in the study. Staff contacted the remaining 662 women by telephone within a week of the mailing of the recruitment packet.
FIGURE 1.
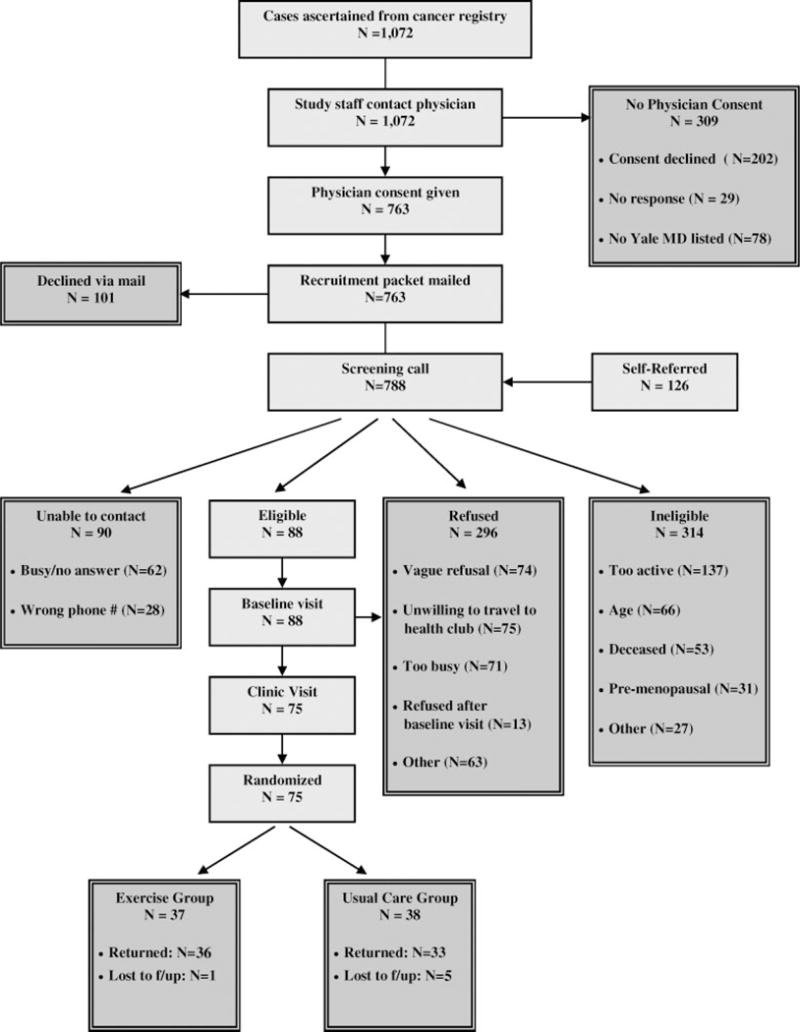
Flow of participants through the YES study.
In addition, 126 women contacted the study staff during the recruitment period after hearing about the YES Study primarily through the media. The principal investigator was interviewed at least 8 times between 2004 and 2005, when we were actively recruiting participants (2 television news programs and 6 newspaper interviews). We also purchased advertisement space in 1 local newspaper. The advertisement was published for 1 week in September 2004. Of the 126 women who self-referred into the study, 9% heard about the study from 1 of 2 television news interviews, 42% heard about the study from 1 of 6 newspaper interviews, 4% heard about the study at a local American Cancer Society Relay for Life event, 3% were referred by a Yale-affiliated physician, and 2% heard about the study via the Internet. The remaining 40% of women who self-referred into the study could not recall how they heard of the study.
Of the 788 screening calls conducted, 90 women could not be reached, either because the telephone was not answered or voice messages left by staff were not returned (n = 62) or because the telephone number listed on the pathology report was incorrect or out of service (n = 28). The average number of telephone calls to complete a successful screening call were 2.7 (range = 1 to 8 telephone calls).
Baseline visits were completed with 88 women, and 75 women attended the clinic visit, completed all baseline paperwork, and were randomized to either exercise (n = 37) or usual care (n = 38).
Fifty of the 75 women randomized into the trial were recruited via RCA; the remaining 25 of the 75 women randomized were recruited via self-referral (Table 1). Among the 50 women recruited via RCA, the average number of days from the date RCA provided us with the participant’s name to randomization was 90 days (range = 33 to 277 days). Among the 25 women who self-referred into the study, the average number of days from their contacting us to randomization was 44 days (range = 17 to 150 days). Combing both RCA and self-referred participants, the average number of days to recruit participants into the YES Study was 73 days.
TABLE 1.
Effect of Recruitment Strategy on Accrual Rate, Recruitment Duration and Costs in the YES Study
| Cancer registry | Self-referred | Total | |
|---|---|---|---|
| No. randomized | 50 | 25 | 75 |
| Accrual rate* | 7.6% | 19.8% | 9.5% |
| Mean (range) of recruitment duration† | 90 (33–277) | 44 (17–250) | 73 (17–277) |
| Recruitment costs | $4600 | $294 | $4894 |
Accrual rate is number randomized divided by number screened (662 and 126 for cancer registry and self-referred, respectively).
Recruitment duration for cancer registry is date RCA provided study staff with name of participant to date of randomization. For self-referred, recruitment duration is from date the participant contacted us to date of randomization.
Although the YES Study in total accrued 9.5% of those who were screened for interest and eligibility (ie, 75 of 788 women), accrual was higher among women who self-referred into the study (25 of 126 women or 19.8%) compared with women recruited via RCA (50 of 662 or 7.6%). Reasons for nonparticipation, however, were similar between the 2 recruitment strategies. Costs associated with recruitment were $294 (for 1 newspaper advertisement) for the self-referral strategy and $4600 for RCA (primarily for the identification of women diagnosed with breast cancer at Yale-New Haven Hospital). Case identification was accomplished by field staff who survey hospital pathology departments and tumor registries. Documentation of each pathology report was photocopied, and appropriate demographic information was collected on an RCA face sheet. Data entry of cases was verified weekly, and cases were screened against a regularly updated local copy of the Connecticut Tumor Registry (to flag second or previous primary cancers) and screened against the RCA database (to flag repeat acquisitions). Lastly, we observed similar baseline demographic, physiologic, and prognostic characteristics between the 2 recruitment strategies.
Sample Characteristics
Baseline characteristics are based on the full YES sample of 75 participants (see Table 2). We observed no statistically significant differences between women randomized to exercise versus usual care. The average age of study participants was 55.8 years. The majority of participants were non-Hispanic White and highly educated. Average time since diagnosis for participants was 3.5 years. Eleven percent of women were diagnosed with stage 0 (in situ) breast cancer, 41% with stage I, 36% with stage IIA or IIB, and 12% with stage IIIA. All study participants had completed radiation and/or chemotherapy. Body mass index (BMI) ranged from 19.7 to 54.5 with a mean BMI of 30.2 (standard deviation [SD] = 6.7). Participants also had, on average, a high percentage of body fat, with a mean of 40.3% (SD = 6.3).
TABLE 2.
Baseline Characteristics of Randomized Participants in the YES Study (N = 75)*
| Characteristics | Exercisers mean ± SD or % |
Usual care mean ± SD or % |
|---|---|---|
| No. | 37 | 38 |
| Age, y | 56.5 (9.5) | 55.1 (7.7) |
| Ethnicity | ||
| Non-Hispanic White | 84% | 84.0% |
| African American | 16% | 11% |
| Asian/Pacific Islander | 0% | 3% |
| Education (%) | ||
| High school graduate | 16% | 22% |
| Some school after HS | 24% | 30% |
| College graduate+ | 60% | 41% |
| Time since diagnosis, y | 3.6 (2.2) | 3.3 (2.6) |
| Disease stage (%) | ||
| In situ | 11% | 11% |
| Stage I | 54% | 27% |
| Stage II | 27% | 46% |
| Stage IIIA | 8% | 16% |
| Type of surgery, % | ||
| Lumpectomy | 70% | 54% |
| Unilateral mastectomy | 19% | 27% |
| Bilateral mastectomy | 11% | 18% |
| Treatment, % | ||
| None | 5% | 14% |
| Radiation only | 41% | 24% |
| Chemotherapy only | 19% | 19% |
| Radiation and chemotherapy | 35% | 43% |
| Hormone therapy | ||
| No | 43% | 30% |
| Tamoxifen | 30% | 30% |
| Aromatase inhibitors | 27% | 40% |
| BMI, kg/m2 | 30.4 (6.0) | 30.1 (7.4) |
| % Total body fat, DEXA | 41.3 (6.4) | 39.4 (5.9) |
| Physical Activity Questionnaire, min/wk recreational exercise | 13.0 (24.0) | 12.0 (20.0) |
| Daily Activity Log, min/wk recreational exercise | 30.0 (41.1) | 11.3 (24.8) |
| Pedometer steps/d | 5145 (2,312) | 5342 (2,744) |
BMI indicates body mass index; DEXA, dual-energy x-ray absorptiometry.
No statistically significant differences between exercise and usual care groups at baseline.
Participants reported a mean of 12 minutes per week (SD = 22) of moderate- to vigorous-intensity recreational activity in the physical activity questionnaire, and 68% of participants reported no activity. Participants recorded a mean of 21 minutes per week (SD = 37) of moderate- to vigorous-intensity recreational activity on the 7-Day Physical Activity Log, with 66% reporting no activity. All participants wore pedometers during these 7 days; mean steps per day during this week was 5244 (SD = 2522), with only 3 participants achieving the “active lifestyle” goal of 10,000 or more steps per day.47
Adherence
Adequate adherence was observed in the YES study (see Tables 3 and 4). Shown on their 7-Day Physical Activity Log, completed at baseline and 6-months, exercisers, on average, increased moderate-to-vigorous intensity recreational activity from 30 minutes per week at baseline to 162 minutes per week at 6 months (P < .001) compared with smaller increases among usual-care participants (11 minutes per week at baseline to 56 minutes per week at 6 months). Sixty-one percent of exercisers reported at least 150 minutes per week of moderate-intensity aerobic exercise at 6-months compared with only 9% of usual care participants. Furthermore, women randomized to exercise increased their pedometer steps per day, on average, by 1621 steps (0.9 of a mile) compared with a decrease of 60 steps per day among women randomized to usual care.
TABLE 3.
Physical Activity Levels, Mean ± SD (Range) by YES Study Participants at Baseline (N = 75) and 6 Months (N = 67)
| Variable | Exercisers
|
Usual care
|
P | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | Change | Baseline | 6 Months | Change | ||
| 7-Day Physical Activity Log* | 30.0±41.1 (0–90) | 161.7±114.7 (0–637) | 129.0±117.9 (−5–637) | 11.3±24.8 (0–90) | 55.6±101.9 (0–325) | 44.3±89.4 (−60–325) | .0018 |
| % of Subjects reporting | |||||||
| ≥150 min/wk | 0% | 61% | 31% | 0% | 9% | 9% | |
| ≥120 min/wk | 0% | 61% | 45% | 0% | 12% | 9% | |
| ≥90 min/wk | 0% | 75% | 59% | 0% | 25% | 22% | |
| ≥60 min/wk | 28% | 81% | 76% | 9% | 32% | 28% | |
| ≥30 min/wk | 39% | 81% | 76% | 12% | 41% | 37% | |
| < 30 min/wk | 100% | 100% | 100% | 100% | 100% | 100% | |
| Pedometer, steps/day | 5083±2313 (702–10,927) | 6738±2958 (1165–13,956) | 1621±2108 (−1683–5799) | 5580±2750 (644–10,934) | 5537±3352 (1116–14,148) | −60±2341 (−4615–5304) | .0040 |
Minutes per week of moderate-intensity aerobic activity.
TABLE 4.
Adherence From Baseline to 6-Months in the YES Exercise Intervention (N = 37)
| Baseline to 6 months | |
|---|---|
| Min/wk of moderate-intensity aerobic activity | |
| Mean SD | 122.8 ± 52.4 |
| Range | 0–216 |
| % of goal | 81 |
| % of Subjects adhering to | |
| ≥150 min/wk (100%) | 33% |
| ≥120 min/wk (80%) | 56% |
| ≥90 min/wk (60%) | 75% |
| ≥60 min/wk (40%) | 89% |
| ≥30 min/wk (20%) | 94% |
| < 30 min/wk (0%) | 100% |
Exercise group participants also completed physical activity logs each of the 26 weeks of the exercise program. On average, exercise group participants performed an average of 123 minutes per week (SD = 52) of moderate- to vigorous-intensity recreational activity. However, only thirty-three percent were performing at least 150 minutes per week of moderate-intensity aerobic activity over the 6 months, and a total of 56% were completing at least 80% of this goal (120 minutes per week). Regarding the weekly goals of thrice-weekly supervised exercise sessions at the health club and twice-weekly unsupervised sessions on their own, women participated in 67% of the supervised exercise sessions and 96% women reported exercising on their own 2 other days of the week. Lastly, women randomized to exercise wore the heart rate monitors for 94% of the exercise sessions (98% during the gym-based sessions and 90% during the home sessions), and exercised on average at 76% of their maximal heart rate.
Retention
Among the 75 women randomized, complete 6-month data was available for 67 women (89%); 34 women randomized to exercise and 33 women randomized to usual care. At their 6-month clinic visit, participants completed a participation-satisfaction questionnaire developed by Courneya and colleagues48 and exercise program questionnaire developed by us. The participant-satisfaction questionnaire asked participants how they felt about the exercise or usual-care program, plans for future exercise, and their thoughts about participating in the trial.
For questions focused on aspects of the study design and procedures that might have influenced them to comply with the study and to adhere to the exercise intervention (on a 7-point Likert scale with 1 = not at all, 3 = somewhat, and 7 = very much), the 37 women randomized to exercise, on average, felt that getting to the health club was somewhat of a barrier to their participation (3.1 + 2.1) but that the trainers were very supportive of their participation (6.2 + 1.6). Women randomized to exercise also felt that their participation in the exercise program was helped by having access to a health club (5.5 + 1.9), having a trainer to tell them what exercises to do (4.9 + 2.2), and knowing that a trainer was expecting them to attend the session and checking their progress (5.6 + 2.0). Keeping an exercise log (5.6 + 1.7), seeing other participants complete the exercise (4.2 + 2.2), and attending group exercise sessions (3.8 + 2.3) also helped their participation in the exercise program. However, being given incentives such as mugs or T-shirts (2.3 + 2.0) and getting exercise equipment (eg, running shoes coupon, heart rate monitor, pedometer) (4.0 + 2.3) did not help or only somewhat helped their participation in the program. When asked about how many supervised exercise sessions they would have preferred to be required to attend, women randomized to exercise, on average, reported 2.6 + 0.6 sessions per week. By using a 5-point Likert scale with 1 = strongly agree, 3 = neutral, and 5 = strongly disagree, when asked whether their participation in the study was time consuming (2.8 + 1.5), a waste of time (4.9 + 0.3), self-rewarding (1.6 + 0.9), something they would consider doing again (1.8 + 1.1), useful for breast cancer research (1.3 + 0.6), and restricting because they could not start a weight-loss diet program (3.8 + 1.3), responses, on average, among women randomized to exercise were in favor of the exercise program we offered them. Lastly, these responses did not differ among women who adhered to the exercise program (at least 120 minutes per week of exercise) versus those who did not adhere (<120 minutes per week of exercise).
At the 6-month clinic visit, women randomized to usual care also completed a participation satisfaction questionnaire. By using a 4-point Likert scale with 1 = not at all and 4 = very well, women randomized to usual care, on average, understood that they had a 50/50 chance of being in the usual care group (3.9 + 0.3). By using a 5-point Likert scale with 1 = strongly agree, 3 = neutral, and 5 = strongly disagree, women randomized to usual care, on average, were neutral on whether they thought participating in the study was restricting because they could not participate in the exercise program (3.1 + 1.3).
At their 6-month clinic visit, participants also completed an exercise program questionnaire, developed by us for informing us in the design of future exercise trials in breast cancer survivors, that asked about their ideal exercise program. By using a 7-point Likert scale with 1 = not at all, 4 = maybe, and 7 = very much, the majority (71%) of women randomized to exercise reported that their ideal exercise program would be access to a health club with regularly (eg, weekly) scheduled telephone counseling sessions or supervised exercise at a health club (65%). When asked about home-based exercise programs, participants were neutral with some (52%) not preferring this type of program at all and some (48%) women very much preferring it. Similar responses were observed among women randomized to usual care, with the majority (80%) of women preferring access to a health club and/or supervised exercise sessions at a health club (70%). Similarly, when asked about home-based exercise programs, women randomized to usual care were neutral with some (50%) not preferring this type of program and some (50%) women very much preferring it. We also included an “other” category where some women reported that their ideal exercise program would include a combination of structured exercise sessions (eg, Months 1–3 of a yearlong exercise trial) and access to a health club (eg, Months 4–12 of a yearlong exercise trial). Other ideal exercise programs suggested by some of the participants included exercise classes, such as pilates or yoga and a buddy system.
Adverse Events
If a women randomized to exercise experienced an adverse event during the trial, we probed her about the cause of the event and if it was related to the exercise program. Five of the 37 women randomized to exercise experienced an adverse event; 2 events were related to the study (plantar fascitis), and 3 were unrelated (swollen achilles, stress fracture in foot, and plantar fascitis) to the study. No women developed lymphedema during the study.
DISCUSSION
The YES study used 2 methods to recruit breast cancer survivors into a 6-month randomized controlled exercise trial, a population-based strategy using a cancer registry and a convenience-sampling approach of self-referral. The overall accrual rate was 9.5% of women screened; however, accrual rates differed by recruitment strategy with higher rates among women who self-referred into the trial (19.8%) compared with women recruited via the cancer registry (7.6%).
Because convenience-sampling approaches (ie, self-referred) are often associated with selection bias, population-based recruitment strategies are generally the preferred recruitment method. However, findings from our study show no baseline demographic, physiologic, or prognostic differences between women recruited via the cancer registry and women who self-referred into the study. Furthermore, the self-referral strategy was less expensive than using the cancer registry and associated with recruiting women more quickly (primarily because we did not need to receive consent from their clinician to contact them nor send a letter to them describing the study before calling them to solicit interest and eligibility) and at a higher enrollment rate. However, a major strength of using a cancer registry was that we were provided with names of at least 30 women per month to approach for participation in our study. Thus, at no time during the 2 years of study recruitment was our recruitment slowed because of a lack of women calling us to learn of the study. These recruitment results provide important information for future exercise trials in cancer survivors in regard to recruitment strategies and the feasibility of conducting exercise trials under certain study timelines. A strength of our recruitment results is our ability to compare the cancer registry and self-referred recruitment strategies. To our knowledge, few, if any, published studies have examined the effects of various recruitment strategies on enrollment rates, reasons for refusal or ineligibility, duration of recruitment and associated costs, and baseline demographic, physiologic and prognostic characteristics. Although our accrual rate is slightly less than other randomized controlled exercise trials in breast cancer survivors, we recruited women into an exercise trial of longer study duration and higher dose of physical activity. Furthermore, some of the inclusion criteria consisted of physician consent to contact, being physically inactive at baseline, willingness to be randomized, ability to travel to the exercise facility, and no history of recurrence or other malignancies. These criteria, as well as the more rigorous study design, resulted in a lower recruitment rate compared with other published studies.
Most similar to our study, Courneya and colleagues examined the effect of a supervised exercise program on cardiopulmonary and quality-of-life outcomes in 53 postmenopausal breast cancer survivors.48 In their study, 370 breast cancer survivors were assessed over 18 months for eligibility through the Alberta Cancer Registry; 53 (14%) breast cancer survivors were eventually randomized to exercise or control. Their recruitment rate was slightly higher than ours and may be a result of including an approximately 40% of women who were physically active at baseline.
Most recently, a study by Vallance and colleagues was published examining the effect of print materials on physical activity and quality of life in 377 breast cancer survivors.37 Higher recruitment rates of 25% were observed and are most likely because of fewer subject demands (eg, intervention consisting of reading print material vs attending thrice-weekly supervised sessions); however, physical activity, on average, increased to a significantly lesser extent in their study (89 minutes per week) compared with our study (162 minutes per week).
Although most physicians in our study were cooperative in providing consent to contact the participants, more than 20% of physicians declined consent or did not respond to our request. Another major barrier to recruitment in our study was lack of interest on the part of potential participants. Of those women who were willing to specify the reason(s) they were uninterested in the study, the most frequent issues were that women felt they were too busy or unable to travel to the health club. These problems were anticipated by the investigators; however, a supervised exercise program was ultimately chosen in an effort to directly observe and motivate participants to exercise at recommended intensities and durations. Given that one of the aims of the study was to examine the impact of exercise on biological mechanisms associated with breast cancer prognosis and survival, a focus on efficacy and high internal validity was a priority. A challenge, though, is to maintain external validity and generalize findings to the population of breast cancer survivors. We compared age, race or ethnicity, and education levels between women randomized to the trial and women who refused participation and observed no differences between the 2 groups. However, consistent with many other behavioral trials, our sample was highly educated and mostly non-Hispanic white. Lastly, whereas a common reason for nonparticipation was being too busy or an unwillingness to travel to a health club, when we asked the participants randomized to the study what their ideal exercise program would include, the majority of women reported preferring having access to a health club or supervised exercise sessions at a health club. Whether women who did not participate in the trial would report similar responses as to their ideal program is unknown.
Adequate adherence and little contamination (ie, exercise in the usual-care group) was observed in the YES study. Over 6 months, exercisers increased their moderate-to-vigorous intensity aerobic activity by 129 minutes per week compared with 44 minutes per week among usual-care participants (P = .0018). Furthermore, 61% of exercisers reported at least 150 minutes per week of moderate-intensity aerobic exercise at 6-months compared with only 9% of usual-care participants, despite being told they could begin an exercise program on their own but that our intervention would not be offered to them until after 6 months. This finding implies that recruiting women into a randomized controlled exercise trial and randomizing them to usual care does not increase physical activity to recommended levels but that weekly contact, support, and education in the form of a supervised program from an experienced exercise physiologist is necessary. Some of the other strategies we used to limit the amount of physical activity in the usual-care group included 1) limiting discussion of physical activity in the quarterly newsletters, 2) not using friends or family members of women randomized to exercise as a referral or recruitment strategy because of the risk that they could end up in different groups, 3) recruiting physically inactive women or women not participating in recommended levels; thus, there was less likelihood that they would be able to participate in recommended levels on their own, and 4) agreeing to offer them a personalized exercise program when they completed the trial.
Among women randomized to exercise, our pedometer findings of an approximate 1 mile increase per day or 7 miles per week further validates our physical activity log results, assuming that women walked at a 20-minute per mile pace or 140 minutes per week of walking. Although adherence was satisfactory in the YES study, however, not all participants completed the prescribed amount of exercise, and a small number of participants adhered poorly to the protocol. However, our measure of adherence was very conservative in that participants recorded their daily moderate-to-vigorous intensity aerobic activity in a 7-day physical activity log for 26 weeks of the study. Most published studies measure adherence by simply reporting the change in baseline to follow-up physical activity assessed from a questionnaire (similar to our results presented in Table 4a) or by reporting the number of supervised sessions completed (ie, attendance) with no mention of intensity or duration of exercise sessions or unsupervised sessions of exercise. Courneya and colleagues reported a high level of adherence in their supervised exercise intervention in breast cancer survivors, with 98.4% (44.3 of 45) of the prescribed exercise sessions completed.48 Our study, which was longer in study duration (26 weeks vs 15 weeks) with a greater duration of exercise prescribed (150 minutes per week vs 105 minutes per week) resulted in an attendance rate of 67% of supervised sessions completed and 96% of home-based sessions completed. Attendance for our supervised sessions was lower than in the Courneya and colleagues study primarily because some participants preferred to exercise for longer durations on fewer supervised days (eg, 2 supervised sessions for 45 minutes rather than 3 supervised sessions for 30 minutes), whereas other participants preferred to exercise the 5 days per week for 30 minutes per session but chose to do 3 home-based sessions and 2 supervised sessions per week, resulting in a higher home-based attendance and lower supervised attendance. Predictors of YES adherence (weekly sessions and duration) as well as adherence stratified by important covariates, including age, education, race or ethnicity, baseline BMI, disease stage, hormonal therapy use, and time since diagnosis, will be published separately. However, whether different durations or frequencies of exercise sessions affect breast cancer prognosis and survival is currently unknown.
The YES study was methodologically strong relative to many studies previously conducted in this area. The YES study used a randomized controlled trial design, exercise programs with physical activity prescriptions that met national guidelines, and detailed measurement of physical activity and adherence using validated measures. The 6-month exercise program was longer than those used in many previous studies and, therefore, is potentially more appropriate, given the slow nature of behavior change. Furthermore, we are currently collecting follow-up data on physical activity, body composition, quality of life, and hormone-use data on YES participants 1 year after their completion of the trial. More research on effective ways to help cancer survivors maintain recommended levels after the intervention ends is necessary.
Although the YES sample size is comparable to or larger than those of several earlier studies, it is still small in absolute terms, with consequent implications for compromised statistical power with regard to some of our secondary outcomes (eg, sex hormones and adipocytokines). Furthermore, our sample consists of mostly non-Hispanic white women and highly educated women.
There are a variety of issues that must be addressed in future research. First, it is unknown what type of exercise is most appropriate for breast cancer survivors and whether this differs for women who are currently undergoing treatment versus those who have completed treatment. Second, gym-based or other supervised interventions offer the opportunity to monitor adherence and progress of each participant. They also allow participants to benefit from social interaction with one another and may be ideal for those women who need extra motivation or those who lack safe venues for physical activity in their neighborhoods. Supervised interventions can pose feasibility concerns, however, including reduced participation rates and particular difficulty in recruiting women who lack time or transportation to travel to the study site. Similarly, home-based interventions provide convenience for participants but may be difficult for women who lack social support. Home-based interventions also generally lack objective measurements of adherence, although this can be partly addressed through procedures such as accelerometers and treadmill testing. A third important issue is determining when to intervene. Previous studies can be characterized into 2 groups, those that recruit women before or during breast cancer treatment, and those that recruit only women who have completed treatment. There is rationale for exercise interventions during treatment to minimize or prevent the impact of therapy such as physical deconditioning, psychological distress, fatigue, and weight gain. Exercise during radiation therapy and adjuvant chemotherapy is feasible, and women have reported improved functional ability and significantly less fatigue. Once treatment is completed, breast cancer survivors may be particularly motivated to adopt healthy behaviors.49
In conclusion, the YES study demonstrates that recruiting women into a 6-month, supervised aerobic exercise intervention study is feasible after a diagnosis of breast cancer, and women who self-referred into the trial did not differ from women recruited via a cancer registry in terms of demographic, physiologic, and prognostic characteristics. Although self-referral strategies are less expensive and quicker, if investigators are not actively promoting the study to the media, there is a risk of having slow months of recruitment. Cancer registries have a longer recruitment process and are more expensive, but investigators have more control over the recruitment process in that they have access to a list of individuals diagnosed with cancer in a specified time period. We believe findings from this study will provide useful information for investigators conducting exercise trials in cancer populations, for clinicians treating women diagnosed with breast cancer, and for developing community-based exercise programs for cancer survivors.
Acknowledgments
This supplement was sponsored by the American Cancer Society’s Behavioral Research Center and the National Cancer Institute’s Office of Cancer Survivorship.
Additional support was provided by the American Cancer Society (MRSG-04-006-01-CPPB) and the Susan G. Komen Breast Cancer Foundation (BCTR0201916). Supported in part by a General Clinical Research Center grant from the National Center of Research Resources, National Institutes of Health (Grant # M01-RR00125) awarded to Yale University School of Medicine.
The authors thank Christian Stoddard, Linda Saucier, and Andrew Wiley for their assistance. We are indebted to the participants in the Yale Exercise and Survivorship Study for their dedication.
References
- 1.American Cancer Society. Breast Cancer Facts and Figures: 2005–2006. Atlanta: American Cancer Society; 2006. [Google Scholar]
- 2.American Cancer Society. Cancer Facts and Figures: 2005. Atlanta: American Cancer Society; 2005. [Google Scholar]
- 3.National Cancer Institute. 2005 Available at URL: http://www.cancer.gov/
- 4.McInnes J, Knobf T. Weight gain and quality of life in women treated with adjuvant chemotherapy for early stage breast cancer. Oncol Nurs Forum. 2001;28:675–684. [PubMed] [Google Scholar]
- 5.Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med. 2001;344:1997–2008. doi: 10.1056/NEJM200106283442607. [DOI] [PubMed] [Google Scholar]
- 6.Drake D, Falzer P, Xistris D, Robinson G, Roberge M. Physical fitness training: outcomes for adult oncology patients. Clin Nurs Res. 2004;13:245–264. doi: 10.1177/1054773804265673. [DOI] [PubMed] [Google Scholar]
- 7.Segal R, Evans W, Johnson D, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19:657–665. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- 8.Dimeo FC, Stieglitz RD, Novelli-Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer. 1999;85:2273–2277. [PubMed] [Google Scholar]
- 9.Schwartz AL. Fatigue mediates the effects of exercise on quality of life. Qual Life Res. 1999;8:529–538. doi: 10.1023/a:1008978611274. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz AL. Daily fatigue patterns and effect of exercise in women with breast cancer. Cancer Pract. 2000;8:16–24. doi: 10.1046/j.1523-5394.2000.81003.x. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz AL, Mori M, Gao R, Nail LM, King ME. Exercise reduces daily fatigue in women with breast cancer receiving chemotherapy. Med Sci Sports Exerc. 2001;33:718–723. doi: 10.1097/00005768-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Kendall AR, Mahue-Giangreco M, Carpenter CL, Ganz PA, Bernstein L. Influence of exercise activity on quality of life in long-term breast cancer survivors. Qual Life Res. 2005;14:361–371. doi: 10.1007/s11136-004-1468-5. [DOI] [PubMed] [Google Scholar]
- 13.Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Ann Behav Med. 1999;21:171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- 14.Blanchard CM, Courneya KS, Laing D. Effects of acute exercise on state anxiety in breast cancer survivors. Oncol Nurs Forum. 2001;28:1617–1621. [PubMed] [Google Scholar]
- 15.Pinto BM, Trunzo JJ, Reiss P, Shiu SY. Exercise participation after diagnosis of breast cancer: trends and effects on mood and quality of life. Psychooncology. 2002;11:389–400. doi: 10.1002/pon.594. [DOI] [PubMed] [Google Scholar]
- 16.Kolden GG, Strauman TJ, Ward A, et al. A pilot study of group exercise training (GET) for women with primary breast cancer: feasibility and health benefits. Psychooncology. 2002;11:447–456. doi: 10.1002/pon.591. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz AL. Exercise and weight gain in breast cancer patients receiving chemotherapy. Cancer Pract. 2000;8:231–237. doi: 10.1046/j.1523-5394.2000.85007.x. [DOI] [PubMed] [Google Scholar]
- 18.Thune I, Furberg AS. Physical activity and cancer risk: dose-response and cancer, all sites and site specific. Med Sci Sports Exerc. 2001;33(6 suppl):S530–S550. doi: 10.1097/00005768-200106001-00025. discussion S609–S610. [DOI] [PubMed] [Google Scholar]
- 19.Bernstein L, Henderson BE, Hanisch R, Sullivan-Halley J, Ross RK. Physical exercise and reduced risk of breast cancer in young women. J Natl Canc Inst. 1994;86:1403–1408. doi: 10.1093/jnci/86.18.1403. [DOI] [PubMed] [Google Scholar]
- 20.Carpenter CL, Ross RK, Paganini-Hill A, Bernstein L. Lifetime exercise activity and breast cancer risk among postmenopausal women. Br J Cancer. 1999;80:1852–1858. doi: 10.1038/sj.bjc.6690610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 22.Goodwin P, Ennis M, Pritchard K, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 23.Hankinson SE, Willett Walter, Colditz G, et al. Circulating concentrations of insulin-like growth factor-1 and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 24.Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. Erratum in: N Engl J Med. 2001;344:1804. [DOI] [PubMed] [Google Scholar]
- 25.Zhang S, Folsom AR, Sellers TA, Kushi LH, Potter JD. Better breast cancer survival for postmenopausal women who are less overweight and eat less fat: The Iowa Women’s Health Study. Cancer. 1995;76:275–283. doi: 10.1002/1097-0142(19950715)76:2<275::aid-cncr2820760218>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–684. [PubMed] [Google Scholar]
- 27.Irwin ML, McTiernan A, Bernstein L, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36:1484–1491. [PMC free article] [PubMed] [Google Scholar]
- 28.Jones LW, Courneya KS. Exercise counseling and programming preferences of cancer survivors. Cancer Pract. 2002;10:208–215. doi: 10.1046/j.1523-5394.2002.104003.x. [DOI] [PubMed] [Google Scholar]
- 29.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matthews CE, Wilcox S, Hanby CL, et al. Evaluation of a 12-week home-based walking intervention for breast cancer survivors. Support Care Cancer. 2007;15:203–211. doi: 10.1007/s00520-006-0122-x. [DOI] [PubMed] [Google Scholar]
- 31.Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev. 2005;14:1672–1680. doi: 10.1158/1055-9965.EPI-04-0736. [DOI] [PubMed] [Google Scholar]
- 32.Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2008;108:279–288. doi: 10.1007/s10549-007-9602-z. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz AL, Winters-Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncol Nurs Forum. 2007;34:627–633. doi: 10.1188/07.ONF.627-633. [DOI] [PubMed] [Google Scholar]
- 34.Mutrie N, Campbell AM, Whyte F, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ. 2007;334:517. doi: 10.1136/bmj.39094.648553.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Culos-Reed SN, Carlson LE, Daroux LM, Hately-Aldous S. A pilot study of yoga for breast cancer survivors: physical and psychological benefits. Psychooncology. 2006;15:891–897. doi: 10.1002/pon.1021. [DOI] [PubMed] [Google Scholar]
- 36.Basen-Engquist K, Taylor CL, Rosenblum C, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Educ Couns. 2006;64:225–234. doi: 10.1016/j.pec.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Vallance JK, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. J Clin Oncol. 2007;25:2352–2359. doi: 10.1200/JCO.2006.07.9988. [DOI] [PubMed] [Google Scholar]
- 38.Demark-Wahnefried W, Clipp EC, Lipkus IM, et al. Main outcomes of the FRESH START trial: a sequentially tailored, diet and exercise mailed print intervention among breast and prostate cancer survivors. J Clin Oncol. 2007;25:2709–2718. doi: 10.1200/JCO.2007.10.7094. [DOI] [PubMed] [Google Scholar]
- 39.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin-like growth factors, and insulin-like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2003;12:721–727. [PubMed] [Google Scholar]
- 40.McTiernan A, Ulrich CM, Yancey D, et al. The Physical Activity for Total Health (PATH) Study: rationale and design. Med Sci Sports Exerc. 1999;31:1307–1312. doi: 10.1097/00005768-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 42.Irwin ML, Yasui Y, Ulrich CM, et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA. 2003;289:323–330. doi: 10.1001/jama.289.3.323. Comment in: JAMA. 2003;289:1778; author reply 1778. [DOI] [PubMed] [Google Scholar]
- 43.Van Loan MD, Mayclin PL. Body composition assessment: Dual energy x-ray absorptiometry for total body and regional bone mineral and soft tissue composition. Eur J Clin Nutr. 1992;46:125–130. [PubMed] [Google Scholar]
- 44.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 45.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 46.Frank LL, Sorensen BE, Yasui Y, et al. Effects of exercise on metabolic risk variables in overweight postmenopausal women: a randomized clinical trial. Obes Res. 2005;13:615–625. doi: 10.1038/oby.2005.66. [DOI] [PubMed] [Google Scholar]
- 47.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 48.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 49.Doyle C, Kushi LH, Byers T, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society Guide for informed choices. CA Cancer J Clin. 2006;56:323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]


