Abstract
Alzheimer’s disease (AD) is a uniquely human, age-related central nervous system (CNS) disorder for which there is no adequate experimental model. While well over 100 transgenic murine models of AD (TgAD) have been developed that recapitulate many of the neuropathological features of AD, key pathological features of AD such as progressive neuronal atrophy, neuron cell loss, and neurofibrillary tangle (NFT) formation have not been observed in any TgAD model to date. To more completely analyze and understand the neuropathology, altered neuro-inflammatory and innate-immune signaling pathways, and the complex molecular-genetics and epigenetics of AD, it is therefore necessary to rigorously examine short post-mortem interval (PMI) human brain tissues to gain a deeper and more thorough insight into the neuropathological mechanisms that characterize the AD process. This perspective-methods paper will highlight some important recent findings on the utilization of short PMI tissues in sporadic (idiopathic; of unknown origin) AD research with focus on the extraction and quantification of RNA, and in particular microRNA (miRNA) and messenger RNA (mRNA) and analytical strategies, drawing on the authors’ combined 125 years of laboratory experience into this investigative research area. We sincerely hope that new investigators in the field of “gene expression analysis in neurological disease” will benefit from the observations presented here and incorporate these recent findings and observations into their future experimental planning and design.
Keywords: Agonal processes, Brain death, DNA arrays, Human brain gene expression, Microbiome, miRNA arrays, miRNA-9, miRNA-34a, miRNA-146a, Post-mortem tissues, RNA instability, Run-on transcription, Sample pooling, Thanatomicrobiome
TgAD Models and AD
Several current databases contain extensive tables and information on various transgenic animal models of Alzheimer’s disease (TgAD) and closely related progressive neurodegenerative diseases [1]. Most TgAD murine models involve the age-related overexpression of (i) beta-amyloid precursor protein (βAPP), (ii) the secretases such as the beta-amyloid cleavage enzyme (BACE) or presenilin 1 (PSEN1) that catalyze amyloid beta (Aβ) peptide excision from the βAPP holoprotein, (iii) tau or hyperphosphorylated tau pathology, or (iv) various combinations of these pathological features which appear to drive the AD phenotype [1, 2]. There are currently about 102 TgAD, cerebral amyloid angiopathy, or frontotemporal dementia transgenic murine models that recapitulate some of the neuropathological features of each disease; however, key pathological features of AD such as progressive neuronal atrophy and loss and neurofibrillary tangle (NFT) formation are still not readily observed in any TgAD model [1–6]. Further, many different microRNAs (miRNAs), messenger RNAs (mRNAs) or proteins are not equally abundant in TgAD murine models when compared to human AD, have different chromosome assignments and exhibit considerably different mechanisms of transcriptional regulation. A number of recent extensive and thoughtful research reviews on the use of murine models to study progressive neurodegenerative diseases, including AD and prion diseases, and the role of cerebral ischemia as a contributor to AD and genetic dysregulation have recently appeared in the scientific literature [3–8]. Interested readers are encouraged to refer to these excellent overviews before planning experimental designs that involve the use of transgenic animals in neurodegenerative disease research. It is important to point out that no single transgenic animal model fully recapitulates the complex pathogenesis of any human neurodegenerative disease such as AD, but rather only models different aspects of the disease neuropathology and/or symptomology [2–8]. We will therefore next turn our attention to the acquisition, processing, and analysis of short post-mortem interval (PMI) human brain material which strongly complements the use of transgenic animal models in advancing our understanding of both AD and other forms of age-related neurodegenerative disease.
Human Brain Death
Because of the use of time-sensitive post-mortem human tissues for research purposes, it is constructive to understand the concept of human brain death since this is the time point marker from which all brain tissues are harvested for clinical, forensic, medical and/or scientific investigation. The post-mortem interval (PMI) of human brain tissues is defined as the death-to-brain-freezing interval; post-mortem tissues are typically harvested, dissected or sectioned, and archived and stored at − 81 °C, typically after being snap frozen in liquid nitrogen (−196 °C) or other cryogenic liquid. Currently in the Western world, “death” is defined as the termination of all biological functions that sustain a living organism [7–9]. Historically, human death was defined as the cessation of heartbeat or cardiac arrest and breathing, but that definition became problematic because heartbeat and breathing can often be artificially maintained using a combination of life support devices such as cardiac pacemakers and artificial respirators [9, 10]. It is currently considered by Westernized medical science that human death is a point in time at which the electrical activity in the brain totally ceases and when a zero-line electroencephalogram (EEG; zero-line EEG) after two consecutive time points becomes evident [9–11]. While electrical activity of the brain ceases at death, molecular-genetic processes such as gene transcription may continue for several hours past the zero line EEG time point [12, 13; see below]. Interestingly, the leading cause of death in developing countries is infectious disease, and in developed countries it is cardiovascular disease, cancer, or other diseases related to obesity and senescence [11]. Hence, depending on the source of tissues procured, pathogenic microorganisms or interceding illnesses such as cardiovascular disease, diabetes, or cancer may complicate the diagnosis of a “pure and single neurological disorder,” and this should be taken into consideration during the clinical classification of the CNS tissues analyzed [9–12].
The Thanatomicrobiome and Agonal Processes
The microbiome consists of about 1014 symbiotic microorganisms within the human body, and the major fraction of the human microbiome is located within the gastrointestinal (GI) tract [14]. Over 99 % of the microbiota in the GI tract are anaerobic bacteria with fungi, protozoa, archaebacteria, and other microorganisms making up the remainder; remarkably, the number of microbial cells in the human microbiome outnumber human host cells by about 100 to 1 [14, 15]. At the point of death, the human microbiome rapidly transforms into the thanatomicrobiome (thanatos-, Greek, death) and begins to play an important role in the decomposition of tissues. Briefly, systemic body temperature increases as former GI tract resident microbes enter the blood, lymphatic circulation, and internal organs of the deceased, including tissues of the CNS [16]. Additional elevations in body temperature, contributed in part from the cessation of blood and lymphatic circulation that normally provide CNS tissue cooling, also occurs to potentially impact the quality of harvested organ tissues and the macromolecules contained therein [16, 17]. Interestingly, DNA and protein persist for far longer than any form of RNA due to the intrinsic instability of ribonucleic acids [16–21; see below]. Agonal processes at the time of death, pertaining to, or symptomatic of agony, such as paroxysmal distress, as in “the death throes,” also contribute to tissue decomposition, including corticosteroid-release effects as the physiological stress of death ensues [14, 22]. Agonal processes include the effects of intermediary illness, fever, medications, death setting, and other stress factors associated with dying; at the point of death metabolic acidosis rapidly ensues which transiently downregulates both intracellular and extracellular pH [21]. Interestingly, intracellular pH can fall precipitously at the time of death; for example in one study blood pH in corpses fell from 7.4 to 5.1 within several hours after death; however, there are reported variations in this finding amongst individuals [11, 21, 22]. When comparing a control and AD neocortical sample, for example, multiple and interactive agonal effects can sometimes be subtracted since both control and neurological disease tissues are simultaneously undergoing the same death-associated physiological and catabolic processes [21, 22]. It needs to be pointed out that while the isolation of total RNA from post-mortem brains can represent a “physiological snapshot” of the metabolic state of the brain, disproportionate activities in the fore-mentioned death-associated agonal factors may play some contributory role to the quality of the RNA isolated and, hence, the quality of the gene expression data derived. To overcome this, simultaneous analysis of an AD-affected anatomical region (such as the hippocampal CA1 or superior temporal lobe) versus an AD-unaffected anatomical region (such as the thalamus or brain stem) within the same brain may be advantageous as different anatomical regions within the same brain appear to undergo approximately the same degree of agonal stress and post-mortem effects [16, 21]. Pooling of short PMI brain tissues for total RNA analysis may also negate some concerns of agonal processes to emphasize key features intrinsic to the AD process (see below; Fig. 1). Finally, it should be mentioned that RNA abundance, speciation, and complexity can differ significantly between specific brain cell types and tissues and, therefore, for comparative studies total RNA should be analyzed from the same anatomical region in diseased tissues and compared to the very same anatomical region in age-matched control brain tissues.
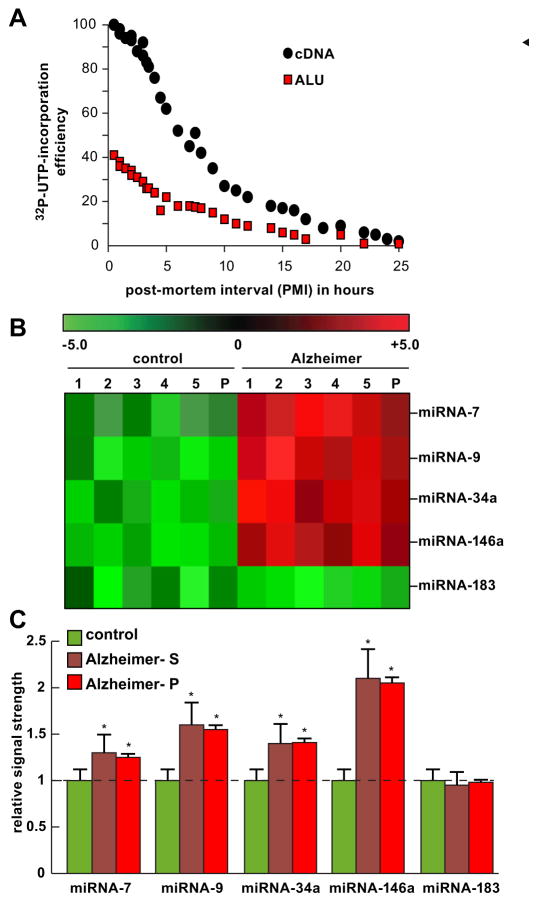
Fig. 1.
A Analysis of RNA integrity in short post-mortem interval (PMI) human brain tissues (superior temporal lobe neocortex)—relative efficiency of run-on gene transcription as a function of PMI (measured in hours) using total cDNA or ALU DNA probes. Briefly, as an index of human brain total RNA quality, run-on transcription was performed in human neocortical nuclei isolated from 0.5- to 25-h PMI human brains using [α-32P]-UTP label incorporation into newly synthesized RNA message, as assessed by probing with either total cDNA or ALU repeat DNA probes on northern dot blot membranes [12, 13]. ALU elements represent one of the most highly repetitive ~300 base pair repeats in the human genome, and the presence of transcribed ALU sequences in RNA can be used as an index of transcriptional output of newly synthesized RNA [12, 13, 23]. The efficiency of run-on gene transcription appears to decrease markedly after a 3- to 4-h PMI. Interestingly, a comparison between either total cDNA or ALU DNA probes vs 0.5- to 25-h PMI shows parallel signal decay kinetics utilizing the ALU probe, yielding approximately 30–40 % of the total cDNA signal at any given PMI. Analysis of short PMI brain using run-on gene transcription may be useful for genetic activity studies in characterizing the transcriptional competency of brain cells during normal human aging and in neurodegenerative diseases such as AD; extensive experimental details have been previously described [12, 13, 24]. B Individual and pooled analysis of samples in the human brain (superior temporal lobe neocortex)–miRNA analysis; individual age-matched control (control; N=5) or Alzheimer’s disease (Alzheimer; N=5) samples or pools of these samples (P) were analyzed using miRNA arrays (LC Sciences, Houston, TX); specific data for human miRNA-7, miRNA-9, miRNA-34a, miRNA-146a, and an unchanging (control) miRNA-183 were selected for analysis. C Compared to control, the mean signal strength of five single-AD samples (Alzheimer-S) gave comparable signal yields to a single pooled sample containing these same five single samples (Alzheimer-P); pooling of samples has the significant advantage of being more cost-effective, and individual agonal processes are more effectively controlled to emphasize AD-relevant trends (see text); pooling of samples may be further useful in the analysis of gene expression studies involving miRNA and/or mRNA in human population studies [22, 25–27]; as the colored bar graph indicates miRNA-7, miRNA-9, miRNA-34a, and miRNA-146a are significantly upregulated in AD over age-matched controls; miRNA-34a has been recently implicated in the downregulation of TREM2 expression in AD brain and an inability to effectively phagocytose Aβ peptides [28]; data are the mean±one standard deviation of that mean; *p<0.05 (ANOVA)
The Intrinsic Instability of Ribonucleic Acid (RNA)
In contrast to the deoxyribose of DNA, RNA has a ribose sugar carrying a hydroxyl group in the 2′ position (2′–OH) of the ribose ring that readily undergoes a rapid H2O-mediated hydrolysis under physiological conditions [13, 23, 29]. RNA molecules therefore rapidly depolymerize, resulting in a source of intrinsic instability in gene signaling which may give spurious results upon gene expression analysis. At least five additional features of RNA tend to mediate its instability: (i) unlike DNA, RNA depolymerizes at alkaline pH and the fluctuating pH of PMI tissues may have a bearing on intrinsic RNA longevity; (ii) RNA, typically contained in the physiological milieu of the nucleoplasm and cytoplasm, is under further destabilizing influences imparted by other nuclear and cellular ions, biological molecules, and temperature; (iii) because RNA contains uracil instead of the thymine (as in DNA) it is more difficult for the cell to discriminate between the uracil waste of deamination and the naturally occurring uracil of RNA that is rapidly recognized, degraded, and cleared from the cytoplasm via lysosomal systems; (iv) multiple, highly active endogenous enzymes that degrade RNA called ribonucleases (RNases) are abundant in the eukaryotic cytoplasm; and (v) RNA polymers have larger and deeper longitudinal grooves than DNA, making them easier to be attacked by RNase [11–13, 21, 23, 30–32]. These biophysical factors combined underscore RNA’s evolutionary role as a highly transient signal carrier from DNA to protein. We have observed empirically that human brains archived (at −81 °C) for 5 years or more have a significantly lower total RNA yield than fresh or archived tissues having storage intervals of 1 year of less. We observe in the same archived brains no significant differences in the yield of total RNA or protein per gram wet weight of AD versus control post-mortem tissues, suggesting that RNA decay rates may be comparable in both AD and control brains [13, 23]. In a related manner, the adenosine + uracil (AU) content of both miRNA (mean range 19–24 nucleotides) and mRNA (mean range 1500–5000 nucleotides) appears to have a bearing on the stability of these RNA molecules [21, 30, 31]. Many miRNA and mRNA have half-lives in the range of 1–3 h, although (i) secondary and tertiary RNA structures, (ii) adsorption to proteins or other cellular structural molecules, and (iii) storage or compartmentalization of RNA in vesicles may significantly prolong their stability and functional half-life [13, 21, 31]. A downregulated miRNA or mRNA observed in some neurological conditions may simply be due to its accelerated degradation in pathological tissues and appropriate steps should be taken to ensure this is not the case, such as the inclusion of miRNA and/or mRNA kinetic studies with each gene expression determination [13, 19, 23, 31]. The “gold standard” for RNA quality is typically an analytical spectrophotometric scan of a total RNA sample typically at 200–300 nm resulting in an electropherogram, generated for example, using a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA; Caliper Technologies, Hopkinton, MA, USA) or equivalent, from which RNA integrity numbers (RIN) can be derived. Previously, RNA integrity had been evaluated using the ratio of RNA absorbance at A260/280nm, or the 28S to 18S ribosomal RNA ratio, but due to inconsistencies, the RIN algorithm was devised, based on a combination of different features derived from the entire electrophoretic trace of the RNA sample in its electropherogram [13, 23, 32]. In this way, interpretation of RNA quality has been facilitated, is independent of sample concentration and analyst, and has become a de facto standard for RNA quality control, enabling comparison of RNA abundance and quality from different PMI samples. RIN numbers range between 1.0 (poorest quality) to 10.0 (highest quality) for total RNA extracted from human CNS cells or post-mortem tissues; in our hands, we have empirically discovered that RIN values of 8.0 or higher yield highly reproducible analytical results while RIN numbers less than 8.0 do not [13, 22, 31; unpublished observations].
Analysis of Messenger RNA-Directed Gene Expression Using Run-On Transcription
Nuclear run-on transcription is an exceptionally sensitive technique for gene expression analysis to identify genes and gene families that are being transcribed in the brain at the time of death [12, 13, 31]. In a typical experiment, intact nuclei are isolated from short PMI brain tissues and de novo RNA synthesis is analyzed utilizing the selective incorporation of [α-32P]-uridine triphosphate (32P-UTP; ~6000 Ci/mmol) into new DNA transcription products with very highly specific activities [12, 13]. The abundance and speciation of the newly generated RNA is typically analyzed (i) via hybridization to specific probes on a Northern gel or (ii) analyzed using DNA arrays, and results are quantified using pre-defined signal intensity parameters and appropriate bioinformatics algorithms [10, 13, 20, 23, 32, 33]. For example, utilizing the selective incorporation of α-32P-UTP into DNA transcription products using both neuron- and glial-specific reporters, it was found that brain tissues of up to 3–4 h PMI were highly efficient in incorporating radiolabel into new transcription products, after which there was a precipitous decline in de novo biosynthetic capacity (Fig. 1a) [10, 13, 23]. Importantly, these results have been reported to be considerably more sensitive than the use of other analytical techniques, such as RT-PCR, although they are a more time-consuming type of gene expression analysis [12, 13, 20]. In our experience, multiple experiments suggest that PMIs for human brain tissues of 3–4 h or less may be the most suitable for subsequent gene expression analysis, the analysis of miRNA and mRNA abundance, speciation, and complexity, resulting in the more meaningful gene expression data and reproducibility in AD and related neurological disease gene expression studies.
Gene Expression Analysis Using DNA and miRNA Arrays
Currently, the highest density of gene expression analysis, including miRNA and mRNA abundance, speciation, and complexity studies involve the use of mRNA (involving DNA targets) and/or DNA or miRNA arrays [12, 13, 24, 33–35]. The densities of miRNAs or expressed genes (mRNAs) analyzed is typically on the order of 2000–33,000 targets per square centimeter, allowing the interrogation of every miRNA or mRNA species in a single DNA array analysis [24, 33–35]. Briefly, total RNA, or miRNA- or mRNA-enriched fractions, isolated from short post-mortem interval (~3–4 h) AD or age-matched control tissues from the same anatomical area, are differentially labeled fluorescently using cyanine dyes (typically Cy3; λex550 nm, λem570 nm) or (Cy5; λex650 nm, λem670 nm) and are hybridized to known oligonucleotide gene targets on the gene array. After washing off of un-hybridized probes, laser scanning typically generates a pattern of expressed miRNAs and mRNAs in that tissue sample [24, 33–35]. Because the resulting fluorescent signals are in a digital format, these data can be (i) expressed in a variety of informative ways depending on the bioinformatics algorithm applied, (ii) digitized gene expression information (~3 Mb/sample) can be archived for later interactive applications and/or analysis, and (iii) multiple samples can be compared which is useful in the case of long-term analysis or the serial collection and sharing of brain gene expression data in ongoing multicenter genetic studies.
Biohazard and Ethical Issues
It should be mentioned that because AD researchers are dealing with a neurological disorder for which the origin and propagation are not well understood, it is sensible to treat any post-mortem brain tissues with neurological disease with at least a biohazard 2 BSL-2 (P2) containment, or higher [11, 36]. It has recently been suggested that in AD patients, certain aggregates of the Aβ peptide become self-propagating and that some Aβ peptide strains, like prion amyloids, appear to be serially transmissible [37]. Further, AD cases and related neurological disorders with a rapidly progressive dementia may often be misdiagnosed as a transmissible neurological disease due to the similarity to aggressive, human prion disorders such as Gerstmann-Straussler-Scheinker syndrome (GSS) and sporadic Creutzfeldt-Jakob disease (sCJD); indeed, AD can often be misdiagnosed as CJD and GSS, or vice versa, and as such extreme care should be taken in the study of any lethal and progressive human neurological disease samples [37–39]. It is the responsibility of both the provider of AD tissues and researchers to obtain ethical clearances and institutional review board authorization according to the individual requirements of the each investigational institution, university or school of medicine, and for the researchers themselves to analyze diseased neurological tissues with great care, attention, and ethical responsibility.
Concluding Remarks
No single TgAD animal model fully recapitulates the entire, complex pathogenesis of AD but rather only models one particular aspect of the disease. Identifying in vivo TgAD models that are naturally predictive for particular features of AD neuropathology can be challenging due in part to the evolutionary divergence of neurobiological and molecular genetic systems that have occurred during evolution and speciation. For example, the use of humanized TgAD mouse models is intrinsically problematical due in part to the genetic background of the mouse and the 75 million years of evolution since the mouse-human divergence, which underscores interspecies neurochemical, gene expression regulation, and neurological differences and other genetic aspects of evolutionary drift [40]. Post-mortem human CNS tissues are thereby very valuable in assessing and discovering important molecular genetic mechanisms and disease-relevant signaling pathways characteristic of the AD, a uniquely human disease. Indeed, studies on gene expression in well-controlled human brain tissues can provide a highly informative “neurochemical, neurogenetic, and neurophysiological snapshot” of gene expression patterns at the time of death, provided that short PMI tissues are selected and appropriate controls are implemented. These studies require the use of intact, high spectral quality total RNA and when done carefully, can provide very useful information as to what is happening at the level of gene expression in specific anatomical regions of the human brain in aging, health, and progressive neurological disease. Analysis of a brain region targeted by the AD process compared to a non-affected control region in the same brain, such as the brain stem or thalamus, i.e., “an internal control,” can further reduce the concern of agonal and other death-associated effects on gene expression often observed between the brains of different human individuals or populations [20, 39, 40].
Pooling of control or AD samples may be able to identify “general trends” in gene expression patterns (Fig. 1b, c) [15, 22, 26, 27]. For example, pooling of AD samples in one group and age-matched controls in another using the same anatomical region matched for age, gender, and PMI, it was recently possible to show (i) significant reductions in AD of the triggering receptor expressed in myeloid/microglial cells (TREM2) sensor-receptor, a microglial-resident glycoprotein important in the clearance of amyloid peptides from the brain’s extracellular space [28] and (ii) statistically significant individual differences in abundance and complexity of a subfamily of pathology-associated and AD-relevant miRNAs between Caucasian and African-American populations [22, 41]. Finally, for gene expression analysis using PMI tissues, it is most important that an accurate diagnosis of a neurological disorder be made and what other pathophysiological, epigenetic, microbial, or environmental disturbances may have contributed to the disease phenotype. This may be particularly critical in the diagnosis of AD as an exceedingly insidious neurological disorder, and especially in the earliest stages of the disease where a mild cognitive impairment (MCI; a discrete disease entity) is initially diagnosed [42, 43]. Indeed, extensive neuropathological examination of post-mortem brain, and especially the quantitation of senile plaque and neurofibrillary tangle densities and other inflammation-related biomarkers, is still considered by many to be the “gold standard” for the most accurate diagnosis of AD, although neuroimaging-related diagnostic technologies are rapidly advancing and contributing to the more accurate diagnosis of AD [12, 44–46]. It is important to point out that besides the rigorous quality control essential for meaningful miRNA and mRNA analysis, the integration of post-mortem neuropathological examination with pre-mortem pathological factors including familial history, lifestyle factors, drug history, neuroimaging data, and serum and CSF profiles will continue to contribute to novel biomarker discovery, the advancement of AD diagnostics, and ongoing analytical standardization [44–48].
Acknowledgments
This research was presented in part at the Society for Neuroscience 44th Annual Meeting, Washington DC, USA, 14–19 November 2014. Thanks are extended to Drs. Yuhai Zhao, Surjyadipta Bhattacharjee, Aileen Pogue, and Darlene Guillot for the helpful discussions and expert technical assistance. Research on neurotoxic metals, small non-coding RNA, microRNA, the innate-immune response, amyloidogenesis, neuroinflammation, possible viral contribution to the AD process in the Clement, Culicchia, Dua, Hill, and Lukiw laboratories using extensive neuropathological and molecular genetic analysis of postmortem brain tissues and bioinformatics analysis was supported through the Alzheimer Association, an unrestricted grant from Research to Prevent Blindness (RPB), the Louisiana Biotechnology Research Network (LBRN), and NIH grants NEI EY006311, NIA AG018031 and NIA AG038834.
Abbreviations
- AD
Alzheimer’s disease
- ALU
Alu human genomic repeated sequence (~300 base pair)
- CNS
Central nervous system
- EEG
Electroencephalogram
- mRNA
Messenger RNA
- miRNA
Micro RNA
- PMI
Post-mortem interval
- TgAD
Transgenic animal model of AD
- TREM2
Triggering receptor expressed in myeloid/microglial cells
Contributor Information
Christian Clement, Infectious Diseases, Experimental Therapeutics and Human Toxicology Lab, Southern University at New Orleans, New Orleans, LA 70126, USA.
James M. Hill, LSU Department of Ophthalmology, Louisiana State University Health Science Center, New Orleans, LA 70112, USA. LSU Neuroscience Center of Excellence, Louisiana State University Health Sciences Center, New Orleans, LA 70112, USA
Prerna Dua, Department of Health Information Management, Louisiana State University, Ruston, LA 71270, USA.
Frank Culicchia, Department of Neurosurgery, Louisiana State University Health Science Center, New Orleans, LA 70112, USA.
Walter J. Lukiw, LSU Department of Ophthalmology, Louisiana State University Health Science Center, New Orleans, LA 70112, USA. LSU Neuroscience Center of Excellence, Louisiana State University Health Sciences Center, New Orleans, LA 70112, USA. Department of Neurology, Louisiana State University Health Science Center, New Orleans, LA 70112, USA
References
- 1.http://www.alzforum.org/research-models/search?species
- 2.Duff K. Neuropsychopharmacology: the fifth generation of progress. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Am Coll Neuropsychol-Pharmacol. Lippincott, Williams, & Wilkins; Philadelphia, Pennsylvania: 2002. [Google Scholar]
- 3.Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ. Using mice to model Alzheimer’s dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet. 2014;5:88. doi: 10.3389/fgene.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley AM, Ammar ZM, Lee RH, Mitchell CS. Systematic review of the relationship between amyloid-β levels and measures of transgenic mouse cognitive deficit in Alzheimer’s disease. J Alzheimers Dis. 2014 Oct 31; doi: 10.3233/JAD-142208. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franco R, Cedazo-Minguez A. Successful therapies for Alzheimer’s disease: why so many in animal models and none in humans? Front Pharmacol. 2014;5:146. doi: 10.3389/fphar.2014.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watts JC, Prusiner SB. Mouse models for studying the formation and propagation of prions. J Biol Chem. 2014;289:19841–19849. doi: 10.1074/jbc.R114.550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pluta R, Jabłoński M, Ułamek-Kozioł M, Kocki J, Brzozowska J, Januszewski S, Furmaga-Jabłońska W, Bogucka-Kocka A, Maciejewski R, Czuczwar SJ. Sporadic Alzheimer’s disease begins as episodes of brain ischemia and ischemically dysregulated Alzheimer’s disease genes. Mol Neurobiol. 2013;48:500–515. doi: 10.1007/s12035-013-8439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pluta R, Furmaga-Jabłońska W, Maciejewski R, Ułamek-Kozioł M, Jabłoński M. Brain ischemia activates β- and γ-secretase cleavage of amyloid precursor protein: significance in sporadic Alzheimer’s disease. Mol Neurobiol. 2013;47:425–434. doi: 10.1007/s12035-012-8360-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hossain MS, Gilbert P. Concepts of death: a key to our adjustment. Illness, Crisis and Loss. 2010;18(1) [Google Scholar]
- 10.Preece P, Cairns NJ. Quantifying mRNA in postmortem human brain: influence of gender, age at death, postmortem interval, brain pH, agonal state and inter-lobe mRNA variance. Brain Res Mol Brain Res. 2003;118:60–71. doi: 10.1016/s0169-328x(03)00337-1. [DOI] [PubMed] [Google Scholar]
- 11.Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukiw WJ, LeBlanc HJ, Carver LA, McLachlan DR, Bazan NG. Run-on gene transcription in human neocortical nuclei. Inhibition by nanomolar aluminum and implications for neurodegenerative disease. J Mol Neurosci. 1998;11:67–78. doi: 10.1385/JMN:11:1:67. [DOI] [PubMed] [Google Scholar]
- 13.Cui JG, Zhao Y, Lukiw WJ. Isolation of high spectral quality RNA using run-on gene transcription; application to gene expression profiling of human brain. Cell Mol Neurobiol. 2005;25:789–794. doi: 10.1007/s10571-005-4035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhattacharjee S, Lukiw WJ. Alzheimer’s disease and the microbiome. Front Cell Neurosci. 2013;7:153. doi: 10.3389/fncel.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill JM, Clement C, Pogue AI, Bhattacharjee S, Zhao Y, Lukiw WJ. Pathogenic microbes, the microbiome, and Alzheimer’s disease (AD) Front Aging Neurosci. 2014;6:127. doi: 10.3389/fnagi.2014.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Can I, Javan GT, Pozhitkov AE, Noble PA. Distinctive thanatomicrobiome signatures found in the blood and internal organs of humans. J Microbiol Methods. 2014;106:1–7. doi: 10.1016/j.mimet.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 17.Lukiw WJ, Alexandrov PN. Regulation of complement factor H (CFH) by multiple miRNAs in Alzheimer’s disease (AD) brain. Mol Neurobiol. 2012;46:11–19. doi: 10.1007/s12035-012-8234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajagopalan LE, Malter JS. Regulation of eukaryotic messenger RNA turnover. Prog Nucleic Acid Res Mol Biol. 1997;56:257–286. doi: 10.1016/s0079-6603(08)61007-7. [DOI] [PubMed] [Google Scholar]
- 19.Sethi P, Lukiw WJ. Micro-RNA abundance and stability in human brain: specific alterations in Alzheimer’s disease temporal lobe neocortex. Neurosci Lett. 2009;459:100–104. doi: 10.1016/j.neulet.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 20.Pogue AI, Hill JM, Lukiw WJ. MicroRNA (miRNA): sequence and stability, viroid-like properties, and disease association in the CNS. Brain Res. 2014;1584:73–79. doi: 10.1016/j.brainres.2014.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donaldson AE, Lamont IL. Biochemistry changes that occur after death: potential markers for determining post-mortem interval. PLoS One. 2013;8(11):e82011. doi: 10.1371/journal.pone.0082011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukiw WJ. Variability in microRNA (miRNA) abundance, speciation and complexity amongst different human populations and potential relevance to Alzheimer’s disease (AD) Front Cell Neurosci. 2013;7:133. doi: 10.3389/fncel.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukiw WJ, Handley P, Wong L, McLachlan DRC. BC200 RNA in normal human neocortex, non-Alzheimer dementia (NAD), and senile dementia of the Alzheimer type (AD) Neurochem Res. 1992;17:591–597. doi: 10.1007/BF00968788. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, He S, Liu C, Ru S, Zhao H, Yang Z, et al. MicroRNA regulation of messenger-like noncoding RNAs: a network of mutual microRNA control. Trends Genet. 2008;24:323–327. doi: 10.1016/j.tig.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Liu Y, Kim T, Min R, Zhang Z. Gene expression variability within and between human populations and implications toward disease susceptibility. PLoS Comput Biol. 2010;6:e1000910. doi: 10.1371/journal.pcbi.1000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raj A, Rifkin SA, Andersen E, van Oudenaarden A. Variability in gene expression underlies incomplete penetrance. Nature. 2010;463:913–918. doi: 10.1038/nature08781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hulse AM, Cai JJ. Genetic variants contribute to gene expression variability. Genetics. 2013;193:95–108. doi: 10.1534/genetics.112.146779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y, Bhattacharjee S, Jones BM, Dua P, Alexandrov PN, Hill JM, et al. Regulation of TREM2 expression by an NF-κB-sensitive miRNA-34a. Neuroreport. 2013;24:318–323. doi: 10.1097/WNR.0b013e32835fb6b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Roretz C, Di Marco S, Mazroui R, Gallouzi IE. Turnover of AU-rich-containing mRNAs during stress: a matter of survival. Wiley Interdiscip Rev RNA. 2011;2:336–347. doi: 10.1002/wrna.55. [DOI] [PubMed] [Google Scholar]
- 30.http://www.genomics.agilent.com/article.jsp?pageId=2181
- 31.Higaki S, Gebhardt B, Lukiw WJ, Thompson H, Hill J. Gene expression profiling in the HSV-1 latently infected mouse trigeminal ganglia following hyperthermic stress. Curr Eye Res. 2003;26:231–238. doi: 10.1076/ceyr.26.3.231.14892. [DOI] [PubMed] [Google Scholar]
- 32.García-Martínez J, Aranda A, Pérez-Ortín JE. Genomic run-on evaluates transcription rates for all yeast genes and identifies gene regulatory mechanisms. Mol Cell. 2004;15:303–313. doi: 10.1016/j.molcel.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Aitman TJ. DNA microarrays in medical practice. BMJ. 2001;323:611–615. doi: 10.1136/bmj.323.7313.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bier FF, von Nickisch-Rosenegk M, Ehrentreich-Förster E, Reiss E, Henkel J, Strehlow R, Andresen D. DNA microarrays. Adv Biochem Eng Biotechnol. 2008;109:433–453. doi: 10.1007/10_2007_087. [DOI] [PubMed] [Google Scholar]
- 35.http://www.affymetrix.com/estore/publications/foundation.affx
- 36.http://pathology2.jhu.edu/cqi/textfiles/labsafetymanual.pdf
- 37.Watts JC, Condello C, Stöhr J, Oehler A, Lee J, DeArmond SJ, et al. Serial propagation of distinct strains of Aβ prions from Alzheimer’s disease patients. Proc Natl Acad Sci U S A. 2014;111:10323–10328. doi: 10.1073/pnas.1408900111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chitravas N, Jung RS, Kofskey DM, Blevins JE, Gambetti P, Leigh RJ, et al. Treatable neurological disorders misdiagnosed as Creutzfeldt-Jakob disease. Ann Neurol. 70:437–444. doi: 10.1002/ana.22454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.http://creutzfeldt-jakob-disease.blogspot.com/2008/08/biosafety-in-microbiological-and.html
- 40.Carninci P. Genomics: mice in the ENCODE spotlight. Nature. 2014;515:346–347. doi: 10.1038/515346a. [DOI] [PubMed] [Google Scholar]
- 41.Olson MV. Human genetic individuality. Annu Rev Genomics Hum Genet. 2012;13:1–27. doi: 10.1146/annurev-genom-090711-163825. [DOI] [PubMed] [Google Scholar]
- 42.Gerstenecker A, Mast B. Mild cognitive impairment: a history and the state of current diagnostic criteria. Int Psychogeriatr. 2014;5:1–13. doi: 10.1017/S1041610214002270. [DOI] [PubMed] [Google Scholar]
- 43.Tsolaki M. Clinical workout for the early detection of cognitive decline and dementia. Eur J Clin Nutr. 2014;68:1186–1191. doi: 10.1038/ejcn.2014.189. [DOI] [PubMed] [Google Scholar]
- 44.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson PM, Vinters HV. Pathologic lesions in neurodegenerative diseases. Prog Mol Biol Transl Sci. 2012;107:1–40. doi: 10.1016/B978-0-12-385883-2.00009-6. [DOI] [PubMed] [Google Scholar]
- 46.Adlard PA, Tran BA, Finkelstein DI, Desmond PM, Johnston LA, Bush AI, Egan GF. A review of β-amyloid neuroimaging in Alzheimer’s disease. Front Neurosci. 2014;8:327. doi: 10.3389/fnins.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zetterberg H, Lautner R, Skillbäck T, Rosén C, Shahim P, Mattsson N, Blennow K. CSF in Alzheimer’s disease. Adv Clin Chem. 2014;65:143–172. doi: 10.1016/b978-0-12-800141-7.00005-x. [DOI] [PubMed] [Google Scholar]
- 48.Bolognani F, Perrone-Bizzozero NI. RNA-protein interactions and control of mRNA stability in neurons. J Neurosci Res. 2008;86:481–489. doi: 10.1002/jnr.21473. [DOI] [PubMed] [Google Scholar]