Abstract
INTRODUCTION
Fundoplication for laryngopharyngeal disease with oesophageal dysmotility has led to mixed outcomes. In the presence of preoperative dysphagia and oesophageal dysmotility, this procedure has engendered concern in certain regards.
METHODS
This paper describes a consecutive series of laryngopharyngeal reflux (LPR) patients with a high frequency of dysmotility. Patients were selected for surgery with 24-hour dual channel pH monitoring, oesophageal manometry and standardised reflux scintigraphy.
RESULTS
Following careful patient selection, 33 patients underwent fundoplication by laparoscopy. Surgery had high efficacy in symptom control and there was no adverse dysphagia.
CONCLUSIONS
Evidence of proximal reflux can select a group of patients for good results of fundoplication for atypical symptoms.
Keywords: Fundoplication, Laryngopharyngeal reflux, Cough, Reflux scintigraphy, Dysphagia
The 2006 Montreal consensus defined gastro-oesophageal reflux disease (GORD) as a condition that develops when reflux of stomach contents causes troublesome symptoms or complications.1 Traditionally, patients have been identified with a range of extraoesophageal symptoms including cough, dysphonia, sore throat, atypical chest pain and pulmonary symptoms.2 The non-specific nature of the symptoms has made diagnosis difficult. There is often no obvious heartburn or regurgitation.3,4 The accuracy of test results have been brought into question as pH catheters (previously considered the gold standard) do not record poorly acidic or non-acid regurgitation and without dual channel catheters, the upper oesophagus remains diagnostically obscure.5 The success rates for surgery for laryngopharyngeal reflux (LPR) (predominantly chronic reflux cough) have therefore stalled at 60–70% in most series.
No unequivocal test has been available to confirm that laryngeal and pulmonary symptoms are caused by the presence of gastro-oesophageal reflux. Diagnostic issues continue. Impedance reflux technology has enabled the measurement of non-acidic and mildly acidic reflux events.6–8 However, even this does not adequately demonstrate reflux into the pharynx, with at least one study showing substantial intraobserver error.9–11 Selection of patients for surgery has been troubled by diagnostic difficulty and surgical series consequently have relatively poor outcomes. Identification of non-acidic proximal reflux by impedance may help improve this.12–14 Results can be influenced in a multifactorial fashion by the difficulty of diagnostic selection, surgical variability (especially in the occasional operator) and the many other illnesses with similar symptomatology. Fundoplication has always concerned surgeons in the presence of dysphagia and this group of patients frequently present with dysphagia among the symptoms.
Our report of a consecutive group of patients undergoing surgery for LPR predominant symptoms describes careful patient selection using reflux scintigraphy technology, 24-hour dual channel pH monitoring and an experienced multidisciplinary approach in a tertiary referral service. The uniquely evolved standardised scintigraphy is able to positively demonstrate pharyngeal or pulmonary contamination by reflux fluid.
Methods
The notes for a cohort of consecutive patients undergoing laparoscopic fundoplication during a four-year period for LPR predominant symptoms were extracted retrospectively from a prospectively populated database. The data were approved for reporting by the institutional ethics committee. Symptoms recorded were chronic cough (defined as existing for more than eight weeks’ duration, with appropriate respiratory investigation),15 dysphonia, throat clearing, sore throat, laryngospasm and clinical pulmonary aspiration as well as the typical GORD symptoms of heartburn dysphagia and regurgitation. Patients with continuing marked symptoms despite a double dose of proton pump inhibitors (PPIs) were offered surgery. Reflux scintigraphy and pH monitoring with manometry were performed. Clinical reassessment was undertaken three months following surgery using a standardised proforma (Appendix 1 – available online).
pH monitoring
Monitoring of pH was performed over 24 hours using antimony crystal dual channel catheters (Synectics Medical Inc., Stockholm, Sweden). A standard technique was employed as described elsewhere.11,12 The normal values for distal reflux were defined as pH >4, overall acid exposure <4% of total time and DeMeester composite score <14.7.14 Any proximal reflux during the upright or supine period was considered abnormal.
Manometry
Stationery manometry was performed using a water perfused catheter (Dentsleeve, Mississauga, Ontario, Canada), as described elsewhere.11,12 Oesophageal motility was graded for effectiveness using a system similar to that of Kahrilas et al16 but using four quartiles rather than three groups.
Scintigraphy
A standardised scintigraphy technique described elsewhere17,18 was employed, using technetium labelled pentetic acid in the initial erect and supine dynamic images. A delayed study was then obtained at two hours to evaluate the possibility of aspiration of refluxate into the lungs. Regions of interest were drawn over the pharynx and upper oesophagus, and time activity curves were generated for the erect and supine dynamic studies. These regions of interest were related back to the relevant background region of interest (Fig 1).
Figure 1.
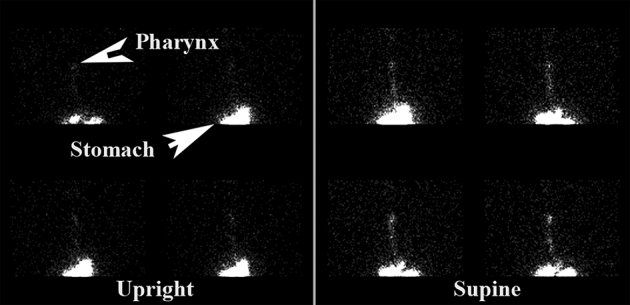
Scintigraphy showing pharyngeal contamination
Fundoplication
Fundoplication was performed by laparoscopy and crural repair was carried out posteriorly in all cases. A technique similar to the Nissen–Rossetti procedure was employed for 360° fundoplication while partial fundoplication was by the Toupet or anterior 270° technique. The fundoplication was calibrated with a 52Fr bougie (female) and a 56Fr bougie (male). Non-absorbable sutures were employed and the fundoplication was approximated using anterior sutures 1cm apart to create a short, floppy fundoplication with division of a variable number of short gastric vessels to achieve adequate lack of tension on the fundoplication. The repair was fixed to the diaphragm laterally by ‘crown’ sutures. Toupet partial fundoplication was undertaken in the standard fashion, and anterior fundoplication was performed with multiple sutures of the stomach to the oesophagus and right crus.
Statistical analysis
Non-parametric statistical methods for ordinal data were employed. Analysis of variance, the Wilcoxon signed-rank test and the Pearson correlation coefficient (two-tailed) were used to compare the data. Statistical analysis was performed with STATISTICA version 8 (StatSoft, Tulsa, OK, US). A p-value of <0.05 was considered statistically significant for the clinical, pH and manometry data owing to a higher standard deviation than the scintigraphy data, for which statistical significance was set at p<0.01. Dysphagia and quality of life scores were evaluated with the paired t-test, comparing preoperative versus early postoperative and late postoperative scores.
Selection for surgery
Patients were selected with intractable symptoms persisting on a double dose of PPIs for more than six weeks, with confirmed proximal reflux on pH study and/or scintigraphy, pulmonary aspiration or full length oesophageal reflux to the pharynx. Other diagnoses were excluded routinely using a multidisciplinary approach comprising an ear, nose and throat specialist, a respiratory physician, a chest x-ray or chest computed tomography, diagnosis and treatment of asthma, and avoidance of medication likely to precipitate cough.
Results
Over the course of the 4 years studied, fundoplication was performed in 33 patients (20 female) with a mean age of 57 years (range: 38–72 years). LPR symptoms were reported in 32 patients, 1 patient having severe heartburn and sinusitis. LPR and GORD symptoms were reported in 22 of the 33 patients. Symptoms included cough (n=25), heartburn (n=24), regurgitation (n=23), voice change (n=15), throat clearing (n=11), pulmonary aspiration (n=11), sore throat (n=10), globus (n=5) and laryngospasm (n=2). The mean duration of symptoms was 4.8 years (range: 6 months – 22 years). Dysphagia was present in 24 patients (72%) preoperatively and severe in 15 patients (45%).
Surgical symptom control
Preoperative symptoms of LPR were eliminated in 27 patients (79%) following surgery and improved in a further 4, leading to a total good outcome for 31 patients (91%). Occasional chest pain and bloating was reported in four patients. There was reduction of cough in a further patient from 15 episodes per day to 2 episodes. Another two patients showed resolution of cough after surgery while taking PPIs but reappearance occurred on stopping medication. There was no relief of LPR symptoms in one patient; however, postoperative scintigraphy showed no evidence of gastro-oesophageal reflux. Two patients were lost to follow-up.
Dysphagia
As well as preoperatively, dysphagia was assessed in the early (10 days) and late (3 months) postoperative period. Prior to surgery, dysphagia was present in 24 patients, with no dysphagia reported by the remaining 9. In the early postoperative period, worsened dysphagia was present in 5 patients, 16 had experienced no change and 4 experienced new onset of dysphagia. No dysphagia was reported by five patients and there were inadequate data for three patients. Late postoperative assessment found dysphagia improved in 19 patients, with 11 being free of dysphagia and inadequate data available for 3 patients. There was no difference between preoperative and early postoperative dysphagia (p>0.05) but late postoperative dysphagia was significantly improved (t=-4.2, 95% confidence interval: -6.4–-2.2, p<0.05). The mean preoperative and late postoperative scores for dysphagia and quality of life are shown in Table 1.
Table 1.
Comparison of mean quality of life and dysphagia scores before and after surgery
| Indicator | Preoperative score (n=33) | Postoperative score at ∼3 months (n=30) |
| GIQLI | 90 | 102 (higher score = improvement) |
| Visick score | 3 | 2 (lower score = improvement) |
| Dakkak dysphagia score | 33 | 37 (higher score = improvement) |
GIQLI = Gastrointestinal Quality of Life Index
pH monitoring
The results of distal pH studies were abnormal in all patients. The mean number of episodes of reflux in the distal oesophagus was 128 and in the upper oesophagus, it was 31. The mean acid exposure time in the distal oesophagus was 16.4% when upright and 20.4% when supine. In the proximal oesophagus, it was 1.6% when upright and 10.5% when supine. In six patients, abnormal proximal reflux was present only when supine. Conversely, 16 patients had proximal reflux when upright but not when supine. The remaining 11 patients experienced proximal reflux in both positions.
Manometry
The mean oesophageal sphincter pressure was 2.9mmHg (standard deviation: 5.0mmHg; normal: >18mmHg). Ineffective oesophageal motility (IEM) was found in 24 patients; 16 were classified as having severe IEM. There was no correlation between IEM and preoperative, early postoperative or late postoperative dysphagia because of the high overall frequency of motility disturbance among the 33 patients.
Scintigraphy
Gastro-oesophageal reflux was noted in all patients on imaging. Pulmonary aspiration was identified in 17 patients (52%) in the delayed study obtained at 2 hours after initiation of the erect and supine dynamic studies. Pharyngeal contamination with isotope was observed in 27 patients (82%). There was increasing scintigraphic activity in the pharynx over time in nine patients and this was highly predictive of pulmonary aspiration (p<0.01). Presence of isotope in the pharynx correlated strongly with total percentage of proximal acid exposure time over the 24 hours of pH monitoring (p<0.01). Pulmonary aspiration on scintigraphy correlated strongly with total proximal acid exposure on pH monitoring (p<0.01).
Discussion
Subjecting patients with LPR symptoms to scintigraphy and pH monitoring allows the prediction of successful response to fundoplication. Successful response is suggested by: a) rising pharyngeal time-activity curves or presence of pulmonary aspiration on scintigraphy; and/or b) abnormal total proximal or supine proximal acid exposure time on pH studies. The degree to which symptoms were controlled was substantially higher than that reported in other studies.19–29 Despite previous concerns regarding surgery, our study has shown improved results. This may be partly due to patient selection using standardised reflux scintigraphy and the severe nature of the reflux of the patients referred to a tertiary antireflux service. The group was highly selected and investigated intensively by multiple specialists.
Cough and LPR symptoms may be caused by direct exposure to noxious agents resulting in a decreased cough threshold,30 local inflammation,31 subcellular tight junction abnormalities, pulmonary aspiration or asthma-like symptoms. However, there is good evidence for a further mechanism, a neurally mediated cause of this symptomatology (reflex), which would not be detected by scintigraphy. This group of patients would therefore not be selected for surgery and it may be that many patients were denied effective surgery as a result. Strong correlation existed between positive results for proximal pH testing and pharyngeal and pulmonary soiling, indicating substantial laryngopharyngeal soiling.
Symptoms in the patient group in this study were caused by direct reflux damage rather than a neural reflex mechanism. The results obtained in this series are largely believed to be dependent on an experienced multidisciplinary approach to this condition in a dedicated antireflux surgical group. One cannot assume that such results would translate to a district hospital setting and general surgery. Patients with chronic cough in the community are likely to be an entirely different patient group and are therefore unlikely to benefit to the same extent.11
There was a high rate of preoperative and postoperative dysphagia, and most patients suffered severe IEM. Despite the IEM, dysphagia improved significantly following surgery. The lower oesophageal sphincter was grossly deficient and reflected the selection of severe reflux patients.
Conclusions
Acceptable results for fundoplication in LPR patients can be obtained using a multidisciplinary approach including a standardised reflux scintigraphy technique, 24-hour dual channel pH monitoring and exclusion of alternative diagnoses. Dysphagia remains as a symptom preoperatively and postoperatively. There was a high rate of IEM in our patient group. This group was highly selected and one cannot therefore assume that our results are transferable to a community population with chronic cough.
References
- 1.Vakil N, van Zanten SV, Kahrilas P et al. . The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006; : 1,900–1,9201. [DOI] [PubMed] [Google Scholar]
- 2.Koufman JA, Aviv JE, Casiano RR, Shaw GY. Laryngopharyngeal reflux: position statement of the Committee on Speech, Voice, and Swallowing Disorders of the American Academy of Otolaryngology – Head and Neck Surgery. Otolaryngol Neck Surg 2002; : 32–35. [DOI] [PubMed] [Google Scholar]
- 3.Dickman R, Kim J, Camargo L et al. . Correlation of gastroesophageal reflux disease symptoms characteristics with long-segment Barrett’s esophagus. Dis Esophagus 2006; : 360–365. [DOI] [PubMed] [Google Scholar]
- 4.Fass R, Dickman R. Clinical consequences of silent gastroesophageal reflux disease. Curr Gastroenterol Rep 2006; : 195–201. [DOI] [PubMed] [Google Scholar]
- 5.Gillison EW, Kusakari K, Bombeck CT, Nyhus LM. The importance of bile in reflux oesophagitis and the success in its prevention by surgical means. Br J Surg 1972; : 794–798. [DOI] [PubMed] [Google Scholar]
- 6.Vaezi MF. Laryngitis and gastroesophageal reflux disease: increasing prevalence or poor diagnostic tests? Am J Gastroenterol 2004; : 786–788. [DOI] [PubMed] [Google Scholar]
- 7.Sifrim D, Dupont L, Blondeau K et al. . Weakly acidic reflux in patients with chronic unexplained cough during 24 hour pressure, pH, and impedance monitoring. Gut 2005; : 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tutuian R, Mainie I, Agrawal A et al. . Nonacid reflux in patients with chronic cough on acid-suppressive therapy. Chest 2006; : 386–391. [DOI] [PubMed] [Google Scholar]
- 9.Faruqi S, Sedman P, Jackson W et al. . Fundoplication in chronic intractable cough. Cough 2012; : 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zerbib F, Roman S, Bruley Des Varannes S et al. . Normal values of pharyngeal and esophageal 24-hour pH impedance in individuals on and off therapy and interobserver reproducibility. Clin Gastroenterol Hepatol 2013; : 366–372. [DOI] [PubMed] [Google Scholar]
- 11.Smith JA, Houghton LA. The oesophagus and cough: laryngo-pharyngeal reflux, microaspiration and vagal reflexes. Cough 2013; : 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mainie I, Tutuian R, Agrawal A et al. . Combined multichannel intraluminal impedance-pH monitoring to select patients with persistent gastro-oesophageal reflux for laparoscopic Nissen fundoplication. Br J Surg 2006; : 1,483–1,487. [DOI] [PubMed] [Google Scholar]
- 13.Aanen MC, Bredenoord AJ, Samsom M, Smout AJ. Reliability of oesophageal pH recording for the detection of gastro-oesophageal reflux. Scand J Gastroenterol 2008; : 1,442–1,447. [DOI] [PubMed] [Google Scholar]
- 14.Sifrim D, Blondeau K, Mantillla L. Utility of non-endoscopic investigations in the practical management of oesophageal disorders. Best Pract Res Clin Gastroenterol 2009; : 369–386. [DOI] [PubMed] [Google Scholar]
- 15.Janson C, Chinn S, Jarvis D, Burney P. Determinants of cough in young adults participating in the European Community Respiratory Health Survey. Eur Respir J 2001; : 647–654. [DOI] [PubMed] [Google Scholar]
- 16.Kahrilas PJ, Dodds WJ, Hogan WJ et al. . Esophageal peristaltic dysfunction in peptic esophagitis. Gastroenterology 1986; : 897–904. [DOI] [PubMed] [Google Scholar]
- 17.Falk GL, Beattie J, Ing A et al. . Scintigraphy in laryngopharyngeal and gastroesophageal reflux disease: a definitive diagnostic test? World J Gastroenterol 2015; : 3,619–3,627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falk M, Van der Wall H, Falk GL. Differences between scintigraphic reflux studies in gastrointestinal reflux disease and laryngopharyngeal reflux disease and correlation with symptoms. Nucl Med Commun 2015; : 625–630. [DOI] [PubMed] [Google Scholar]
- 19.Ekström T, Johansson KE. Effects of anti-reflux surgery on chronic cough and asthma in patients with gastro-oesophageal reflux disease. Respir Med 2000; : 1,166–1,170. [DOI] [PubMed] [Google Scholar]
- 20.Farrell TM, Richardson WS, Trus TL et al. . Response of atypical symptoms of gastro-oesophageal reflux to antireflux surgery. Br J Surg 2001; : 1,649–1,652. [DOI] [PubMed] [Google Scholar]
- 21.Greason KL, Miller DL, Deschamps C et al. . Effects of antireflux procedures on respiratory symptoms. Ann Thorac Surg 2002; : 381–385. [DOI] [PubMed] [Google Scholar]
- 22.Novitsky YW, Zawacki JK, Irwin RS et al. . Chronic cough due to gastroesophageal reflux disease: efficacy of antireflux surgery. Surg Endosc 2002; : 567–571. [DOI] [PubMed] [Google Scholar]
- 23.Thoman DS, Hui TT, Spyrou M, Phillips EH. Laparoscopic antireflux surgery and its effect on cough in patients with gastroesophageal reflux disease. J Gastrointest Surg 2002; : 17–21. [DOI] [PubMed] [Google Scholar]
- 24.Wright RC, Rhodes KP. Improvement of laryngopharyngeal reflux symptoms after laparoscopic Hill repair. Am J Surg 2003; : 455–461. [DOI] [PubMed] [Google Scholar]
- 25.Brouwer R, Kiroff GK. Improvement of respiratory symptoms following laparoscopic Nissen fundoplication. ANZ J Surg 2003; : 189–193. [DOI] [PubMed] [Google Scholar]
- 26.Duffy JP, Maggard M, Hiyama DT et al. . Laparoscopic Nissen fundoplication improves quality of life in patients with atypical symptoms of gastroesophageal reflux. Am Surg 2003; : 833–838. [PubMed] [Google Scholar]
- 27.Chang AB, Lasserson TJ, Kiljander TO et al. . Systematic review and meta-analysis of randomised controlled trials of gastro-oesophageal reflux interventions for chronic cough associated with gastro-oesophageal reflux. BMJ 2006; : 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rakita S, Villadolid D, Thomas A et al. . Laparoscopic Nissen fundoplication offers high patient satisfaction with relief of extraesophageal symptoms of gastroesophageal reflux disease. Am Surg 2006; : 207–212. [PubMed] [Google Scholar]
- 29.Swoger J, Ponsky J, Hicks DM et al. . Surgical fundoplication in laryngopharyngeal reflux unresponsive to aggressive acid suppression: a controlled study. Clin Gastroenterol Hepatol 2006; : 433–441. [DOI] [PubMed] [Google Scholar]
- 30.Qiu Z, Yu L, Xu S et al. . Cough reflex sensitivity and airway inflammation in patients with chronic cough due to non-acid gastro-oesophageal reflux. Respirology 2011; : 645–652. [DOI] [PubMed] [Google Scholar]
- 31.Patterson RN, Johnston BB, Ardill JE et al. . Increased tachykinin levels in induced sputum from asthmatic and cough patients with acid reflux. Thorax 2007; : 491–495. [DOI] [PMC free article] [PubMed] [Google Scholar]


