Abstract
INTRODUCTION
Linitis plastica (LP) is a particular subtype of diffuse gastric cancer and is thought to have a very poor prognosis. The operative approach in patients with LP has historically been questioned because of the poor outcomes. The aim of this study was to determine the current outcomes in LP patients who undergo radical resection.
METHODS
Patients with a new diagnosis of diffuse gastric adenocarcinoma between 2006 and 2010 were identified from a regional pathology database. LP was diagnosed based on histological, radiological and endoscopic findings. The patients’ health records were analysed retrospectively and mortality data obtained from a regional cancer registry. The primary outcome assessed was overall survival.
RESULTS
Overall, 273 patients with diffuse gastric cancer were identified; 54 of these were diagnosed with LP. In the LP cohort, 17 patients underwent resection compared with 95 of the 219 patients in the non-LP group. The median survival following resection in patients with LP was 16.7 months (95% confidence interval [CI]: 8.3–25.1) while in LP patients who did not have surgery it was 3.6 months (95% CI: 2.2–4.9 months) (p<0.001). There was no significant difference in survival following resection between those with LP and those with non-LP diffuse gastric adenocarcinoma (median: 23.9 months, 95% CI: 15.8–32.1 months) (p=0.331).
CONCLUSIONS
Survival following resection in patients with LP is not significantly different to that in those with non-LP diffuse gastric cancer. A preoperative diagnosis of LP should not be a reason for denying radical treatment and such individuals should be managed in the same way as any other patient with diffuse gastric cancer.
Keywords: Linitis plastica, Surgery, Mortality, Kaplan–Meier estimate, Stomach neoplasm, Prognosis
Gastric adenocarcinoma can be divided into two histological groups: intestinal and diffuse.1 The incidence of the intestinal type of gastric carcinoma is decreasing.2 However, it still remains the most common form of gastric carcinoma. This reducing incidence is thought to be due to a number of features such as decreasing smoking rates and increased Helicobacter pylori eradication. Conversely, the incidence of diffuse gastric cancer has been rising.2 It generally has poorer outcomes than the more common intestinal type.3 Diffuse gastric carcinoma also has further histopathological characteristics that help define this condition, including a poorly cohesive cellular structure due to the loss of cell-to-cell adhesion and the presence of signet ring cells.4 Linitis plastica (LP) is a particular phenotype of diffuse gastric carcinoma, characterised by diffuse submucosal infiltration of the stomach, producing the classic ‘leather bottle’ appearance at endoscopy or on radiological examination (including barium swallow studies and computed tomography [CT]).
There remains considerable debate regarding the best form of treatment for patients with LP as the outcomes have previously been shown to be poor, no matter how they are treated.5,6 The management of gastric cancer has continued to evolve with the more ubiquitous use of staging laparoscopy and peritoneal cytology in the preoperative assessment of patients, and with the development of modern evidence-based chemotherapeutic regimens. It is not yet clear what influence these factors may have on the outcomes of patients with LP as many of the previous studies did not include all of these factors. The aim of this study was to compare the outcomes of LP patients treated operatively versus those who were managed non-operatively and to compare survival of patients with LP versus non-LP diffuse gastric cancer.
Methods
Patients with a new diagnosis of diffuse gastric cancer between January 2006 and December 2010 were eligible for inclusion in the study. Patients were identified retrospectively using a regional pathology database and a regional cancer tracking system. Search terms comprised ‘linitis plastica’ and ‘diffuse gastric cancer’. The additional terms ‘poorly cohesive’, ‘infiltrative’ and ‘signet cell’ were also used when searching pathological records. Patients with this pathological description were included in the study if they also had radiological or endoscopic evidence of diffuse gastric wall thickening in keeping with LP.
A pragmatic approach to the definition of LP was taken, with cases being included if the radiology or endoscopy report used the term ‘linitis plastica’ in the description. In addition, patients were also included if the specific phrase ‘linitis plastica’ was not used but if the endoscopy findings suggested a diffusely abnormal mucosa or poor distension, as were those for whom radiological imaging demonstrated diffuse or circumferential thickening in more than one contiguous area of the stomach. Patients with tumours involving the gastro-oesophageal junction were excluded as these are categorised as oesophageal tumours in the seventh edition of TNM Classification of Malignant Tumours.7
Mortality data were obtained from the Northern Ireland Cancer Registry. The primary outcome assessed was overall survival. All decisions regarding treatment were made through the regional upper gastrointestinal cancer multidisciplinary team meeting. Operability was determined based on the absence of metastatic disease on staging CT and staging laparoscopy combined with negative peritoneal cytology. All patients deemed suitable for radical treatment were also considered for perioperative chemotherapy in the form of three preoperative and three postoperative cycles of epirubicin, cisplatin and capecitabine or fluorouracil. Survival data were analysed in SPSS® version 22 (IBM, New York, US) using the Kaplan–Meier method and compared using the logrank test. A p-value of <0.05 was considered statistically significant. Data were censored at death or 1 June 2014.
Results
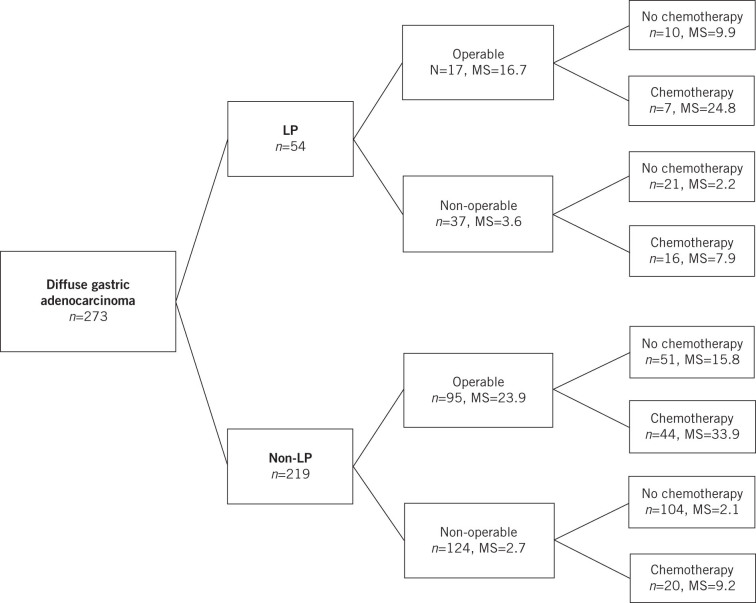
Overall, 273 patients with diffuse gastric cancer were identified and 54 of these had LP using the diagnostic criteria outlined above. In the LP group, 17 patients were operable and underwent surgical resection with curative intent compared with 95 patients in the non-LP cohort. Figure 1 gives a breakdown of the different treatment groups for all patients with the corresponding median survival.
Figure 1.
Treatment groups for patients diagnosed with linitis plastica (LP) and those with non-LP diffuse gastric cancer, with median survival (MS) in months
There was an even distribution of male and female patients in the LP group, with a slight male preponderance in the non-LP group (Table 1). The mean age was 69 years in both cohorts. Just over half (52%) of the patients with LP were found to have metastatic disease at presentation compared with just over a third (37%) in the non-LP group. This resulted in lower rates of surgery for LP patients (31% vs 43%).
Table 1.
Baseline data for LP and non-LP groups
| LP (n=54) | Non-LP (n=219) | |
|---|---|---|
| Male | 27 (50%) | 131 (60%) |
| Female | 27 (50%) | 88 (40%) |
| Mean age in years | 69.6 (SD: 13.6) | 69.5 (SD: 13.4) |
| M1 disease at presentation | 28 (52%) | 82 (37%) |
LP = linitis plastica; SD = standard deviation
Among patients who underwent resection, those with LP tended to have more locally advanced disease (pT3/T4: 82% vs 47%). Nodal involvement was also greater in the LP group: 47% had N2 or N3 disease compared with 37% for non-LP patients. Correspondingly, the rate of complete disease (R0) resections was lower for those with LP (65% vs 82%) (Table 2). Three patients (3%) in the operable non-LP group and one patient (6%) in the operable LP group had a palliative (R2) resection with macroscopic disease left behind.
Table 2.
Pathological staging in patients who underwent resection in the linitis plastica (LP) and non-LP groups
| Stage | LP (n=17) | Non-LP (n=95) |
|---|---|---|
| T1 | 1 (6%) | 16 (17%) |
| T2 | 2 (12%) | 31 (33%) |
| T3 | 9 (53%) | 38 (40%) |
| T4 | 5 (29%) | 7 (7%) |
| Tx | 0 (0%) | 3 (3%) |
| N0 | 4 (24%) | 27 (28%) |
| N1 | 4 (24%) | 29 (31%) |
| N2 | 1 (6%) | 11 (12%) |
| N3 | 7 (41%) | 24 (25%) |
| Nx | 1 (6%) | 4 (4%) |
| M0 | 14 (82%) | 83 (87%) |
| M1 | 2 (12%) | 11 (12%) |
| Mx | 1 (6%) | 1 (1%) |
| R0 | 11 (65%) | 78 (82%) |
| R1 | 5 (29%) | 14 (15%) |
| R2 | 1 (6%) | 3 (3%) |
In the LP cohort, seven patients who underwent resection also had perioperative chemotherapy and ten patients were treated with resection only. Only three of the chemotherapy patients completed the full six cycles. Among the LP patients who were inoperable, 16 had palliative chemotherapy while the remaining 21 patients received best supportive care only.
The median survival following resection in those with LP was 16.7 months (95% confidence interval [CI]: 8.3–25.1 months) compared with 3.6 months (95% CI: 2.2–4.9 months) in LP patients who did not have surgery (p<0.001). There was no significant difference in survival for those with LP who underwent resection and those without LP receiving the same treatment (median: 23.9 months, 95% CI: 15.75–32.11 months) (p=0.331) (Fig 2). The longest survival among LP patients was demonstrated in the group treated with surgery and chemotherapy (median: 24.8 months, 95% CI: 8.0–41.6 months). Patients treated with surgery or chemotherapy alone had a similar median survival of 9.9 months (95% CI: 0–25.0 months) and 7.9 months (95% CI: 7.3–8.6 months) respectively while those who received best supportive care only had a median survival of 2.2 months (95% CI: 1.6–2.9 months).
Figure 2.
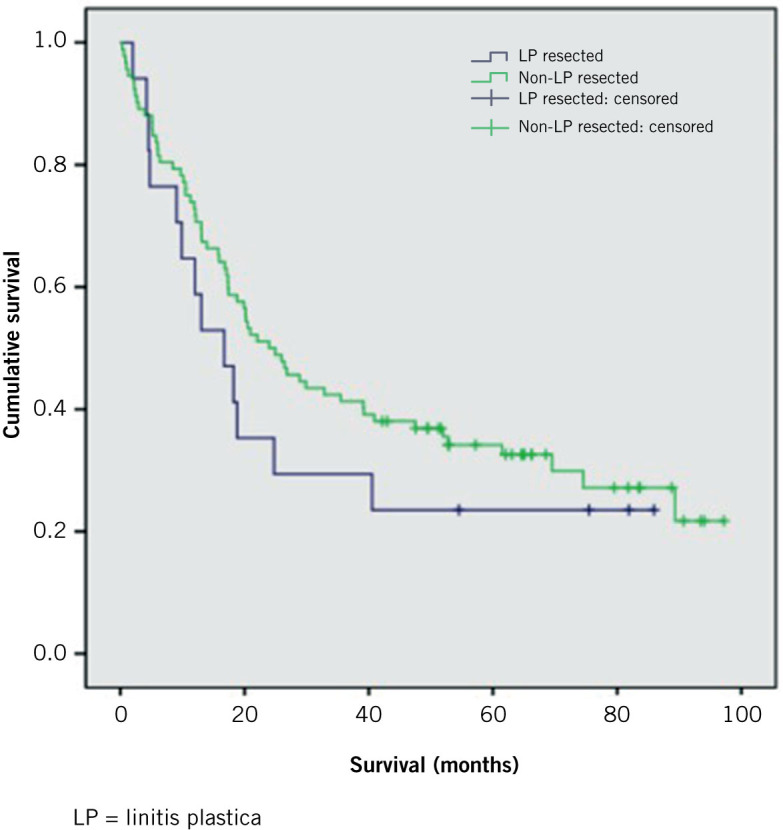
Survival in months after surgery for those with LP and those with non-LP diffuse gastric cancer
Discussion
LP was first reported by Brinton in 1859.8 It can be recognised by the characteristic ‘leather bottle’ appearance of the stomach. The incidence of LP has been reported variably in the literature depending on the definition used but it represents 3–19% of gastric adenocarcinomas.5,9,10
Typical histological findings consistent with LP include signet cell formation, poorly cohesive cells, lack of glandular cell formation and diffuse infiltration.4 There are a small number of papers that have reported survival data for patients with gastric LP. These authors have used variable definitions of LP based on either macroscopic or histological criteria, or a combination of both. In our study, a pragmatic macroscopic definition was used based on findings at CT or endoscopy, in keeping with the original definition of Brinton.8 Our aim was to determine the survival of patients who were considered to have LP preoperatively based on macroscopic appearances in order to challenge the suggestion that patients with LP have poor outcomes irrespective of how they are treated. This has been the conclusion of a number of previous studies.11,12
Diffuse gastric cancer has a rising incidence2 and is known to have a poorer prognosis than the more common intestinal subtype.3 Studies looking specifically at survival following surgery for LP have had differing results. There are a number of reasons why it is difficult to compare the data from all the studies to date, including the heterogeneous criteria used to define LP, the lack of (now standard) staging investigations (eg CT and laparoscopy) in some of the earlier studies and also the lack of chemotherapy in studies prior to the publication of the findings of the MAGIC (Medical Research Council Adjuvant Gastric Infusional Chemotherapy) trial, which demonstrated the survival benefits of perioperative chemotherapy in gastric cancer cases.13 LP has been shown to be a predictor of positive peritoneal cytology and therefore a poorer prognosis,14 emphasising the importance of staging laparoscopy in this group.
Aranha and Georgen reported on 26 patients with a macroscopic definition of LP.11 This was an early study; there was limited use of staging CT (n=14) and no patients had a staging laparoscopy. The median survival for patients who did not undergo surgical resection was 6.6 months, with a modest increase to 7.7 months for those who did have surgery. They also found a modest survival benefit for patients who underwent resection and were given adjuvant chemotherapy, with a mean survival of 11 months. Hamy et alreported a 50% 1-year survival rate and a 7.5% survival rate at 84 months in a cohort of 86 LP patients (defined histologically) who were treated surgically.15
Pedrazzani et al reviewed the outcomes of 102 patients with LP, 92 of whom underwent resection.12 They used a histological definition of LP and did not use staging laparoscopy or perioperative chemotherapy in their cohort. An overall median survival of 5.7 months (95% CI: 3.7–7.5 months) was demonstrated, which increased to 15.8 months (95% CI: 11–20.7 months) for those who had a R0 resection (27.5%).
Kodera et al noted more favourable outcomes in 178 patients with a R0 resection rate of 46%.16 Their definition of LP was more in keeping with ours in that the macroscopic appearance was used. However, as with the study by Pedrazzani et al,12 the patients did not undergo perioperative chemotherapy and staging laparoscopy was not performed as standard. The median survival in patients with a R0 resection was 30.2 months while in those treated with palliative surgery, it was 8.2 months. In those who had no resection (although they did undergo laparotomy), it was 7.2 months. More recently, Schauer et al reported a median survival of 8 months in 120 patients with LP (based on a histological diagnosis) who underwent gastrectomy.17 Their R0 resection rate was 31%, with a median survival of 17 months in this group. Staging laparoscopy was used variably.
Our study compares favourably with these previously reported studies. All patients in our cohort underwent staging with CT and laparoscopy as standard. We did not differentiate between individuals with a suspicion of LP based on preoperative imaging and those with non-LP diffuse gastric cancer. Although this was a retrospective study with inevitable selection bias, it is unlikely that a prospective study with sufficient power to address the aim of this paper would be feasible in practical terms. Perioperative chemotherapy was considered in all patients, regardless of the possibility of LP, in the same way we would manage any patient with gastric cancer (either diffuse or intestinal).
Six patients with LP had perioperative chemotherapy and one patient completed the three preoperative cycles only. The survival in this group of patients was the longest in our LP cohort (median: 24.8 months, 95% CI: 8.0–41.6 months). The median overall survival for all patients with LP who underwent resection was 16.7 months (95% CI: 8.3–25.1 months) compared with 3.6 months (95% CI: 2.2–4.9 months) for LP patients who did not have surgery (p<0.001).
One patient with LP who underwent resection and perioperative chemotherapy had only T1 disease on pathological staging. It is postulated that this may have been due to the downstaging effect of the chemotherapy, underlining the value of multimodal treatment in LP patients. Alternatively, this may have not been a true case of LP, highlighting the difficulty in establishing with certainty what constitutes LP. As discussed previously, various definitions (both macroscopic and microscopic) have been used in the literature. We have tried to use a pragmatic definition based on the radiological and/or endoscopic appearance of the stomach. However, undoubtedly, this relies on a degree of subjective interpretation of the findings.
The R0 resection rate in our study for patients with LP was 65%. This compares favourably with the rates reported in the studies discussed above. In our study, there was a significant survival benefit for LP patients following resection, with the greatest benefit noted in those who had multimodal treatment. In addition, there was no statistically significant difference in survival between patients with LP who underwent resection and those with non-LP diffuse gastric cancer who also had a resection.
Conclusions
This study demonstrates that patients with LP as defined by macroscopic criteria on preoperative investigations have significantly better survival after resection than those managed non-operatively. The greatest survival benefit was derived from multimodal treatment. Survival following resection in patients with LP was not significantly different to that in the group with non-LP diffuse gastric cancer. A preoperative diagnosis of LP based on macroscopic appearance should therefore not be a reason for denying radical treatment and such individuals should be managed in the same way as any other patient with diffuse gastric cancer. We recommend that the use of the subjective term linitis plastica is removed from the lexicon of inoperable gastric cancer. It should be replaced with standard TNM (tumour, lymph nodes, metastasis) classification and there should be defined criteria of operability based on preoperative staging.
References
- 1.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Micobiol Scand 1965; : 31–49. [DOI] [PubMed] [Google Scholar]
- 2.Henson DE, Dittus C, Younes M et al. Differential trends in the interstinal and diffuse types of gastric carcnioma in the United States, 1973–2000. Arch Pathol Lab Med 2004; : 765–770. [DOI] [PubMed] [Google Scholar]
- 3.Hass HG, Smith U, Jäger C et al. Signet ring cell carcinoma of the stomach is significantly associated with poor prognosis and diffuse gastric cancer (Lauren’s): single-center experience of 160 cases. Onkologie 2011; : 682–686. [DOI] [PubMed] [Google Scholar]
- 4.Chiaravalli AM, Klersy C, Tava F et al. Lower- and higher-grade subtypes of diffuse gastric cancer. Hum Pathol 2009; : 1,591–1,599. [DOI] [PubMed] [Google Scholar]
- 5.Moore JR. Gastric carncinoma: 30-year review. Can J Surg 1986; : 25–28. [PubMed] [Google Scholar]
- 6.Visset J, Hamy A, Letessier E et al. Linitis plastica of the stomach. Factors influencing prognosis. Chirurgie 1992; : 236–242. [PubMed] [Google Scholar]
- 7.Sobin L, Gospodarowicz M, Wittekind C. TNM Classification of Malignant Tumours. 7th edn Oxford: Wiley-Blackwell; 2009. [Google Scholar]
- 8.Brinton W. The Diseases of the Stomach. London; Churchill: 1859. [Google Scholar]
- 9.Issam Beyrouti M, Beyrouti R, Ben Amar M et al. Linitis plastica. Presse Med 2007; : 1,782–1,786. [DOI] [PubMed] [Google Scholar]
- 10.Park JC, Lee YC, Kin JH et al. Clinicopathological aspects and prognostic vlaue with respect to age: an analysis of 3,362 consecutive gastric cancer patients. J Surg Oncol 2009; : 395–401. [DOI] [PubMed] [Google Scholar]
- 11.Aranha GV, Georgen R. Gastric linitis plastica is not a surgical disease. Surgery 1989; : 758–762. [PubMed] [Google Scholar]
- 12.Pedrazzani C, Marrelli D, Pacelli F et al. Gastric linitis plastica: which role for surgical resection? Gastric Cancer 2012; : 56–60. [DOI] [PubMed] [Google Scholar]
- 13.Cunningham D, Allum WH, Stenning SP et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; : 11–20. [DOI] [PubMed] [Google Scholar]
- 14.Badgwell B, Cormier JN, Krishnan S et al. Does neoadjuvant treatment for gastric cancer patients with positive peritoneal cytology at staging laparoscopy improve survival? Ann Surg Oncol 2008; : 2,684–2,691. [DOI] [PubMed] [Google Scholar]
- 15.Hamy A, Letessier E, Bizouarn P et al. Study of survival and prognostic factors in patients undergoing resection for gastric linitis plastica: a review of 86 cases. Int Surg 1999; : 337–343. [PubMed] [Google Scholar]
- 16.Kodera Y, Ito S, Mochizuki Y et al. The number of metastatic lymph nodes is a significant risk factor for bone metastasis and poor outcome after surgery for linitis plastica-type gastric carcinoma. World J Surg 2008; : 2,015–2,020. [DOI] [PubMed] [Google Scholar]
- 17.Schauer M, Peiper M, Theisen J, Knoefel W. Prognostic factors in patients with diffuse type gastric cancer (linitis plastica) after operative treatment. Eur J Med Res 2011; : 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]