Abstract
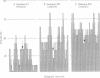
A promising strategy for DNA sequencing exploits transposons to provide mobile sites for the binding of sequencing primers. For such a strategy to be maximally efficient, the location and orientation of the transposon must be readily determined and the insertion sites should be randomly distributed. We demonstrate an efficient probe-based method for the localization and orientation of transposon-borne primer sites, which is adaptable to large-scale sequencing strategies. This approach requires no prior restriction enzyme mapping or knowledge of the cloned sequence and eliminates the inefficiency inherent in totally random sequencing methods. To test the efficiency of probe mapping, 49 insertions of the transposon gamma delta (Tn1000) in a cloned fragment of Drosophila melanogaster DNA were mapped and oriented. In addition, oligonucleotide primers specific for unique subterminal gamma delta segments were used to prime dideoxynucleotide double-stranded sequencing. These data provided an opportunity to rigorously examine gamma delta insertion sites. The insertions were quite randomly distributed, even though the target DNA fragment had both A + T-rich and G + C-rich regions; in G + C-rich DNA, the insertions were found in A + T-rich "valleys." These data demonstrate that gamma delta is an excellent choice for supplying mobile primer binding sites to cloned DNA and that transposon-based probe mapping permits the sequences of large cloned segments to be determined without any subcloning.
Full text
PDF
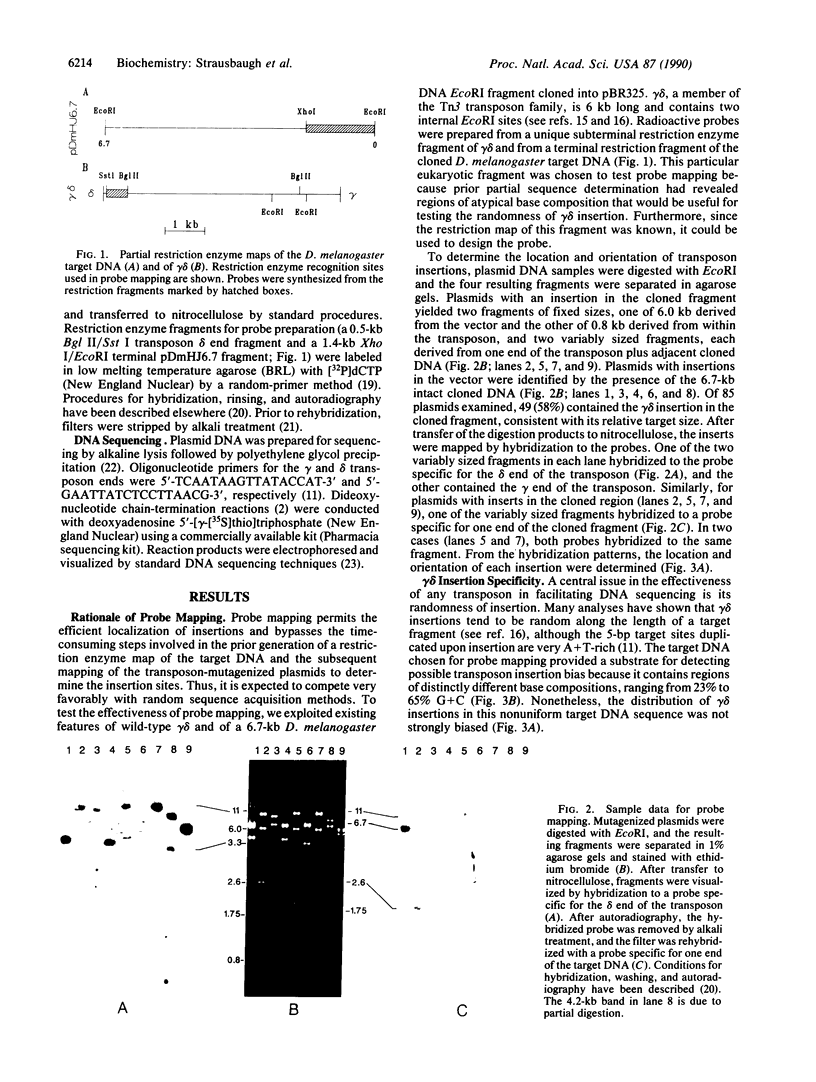
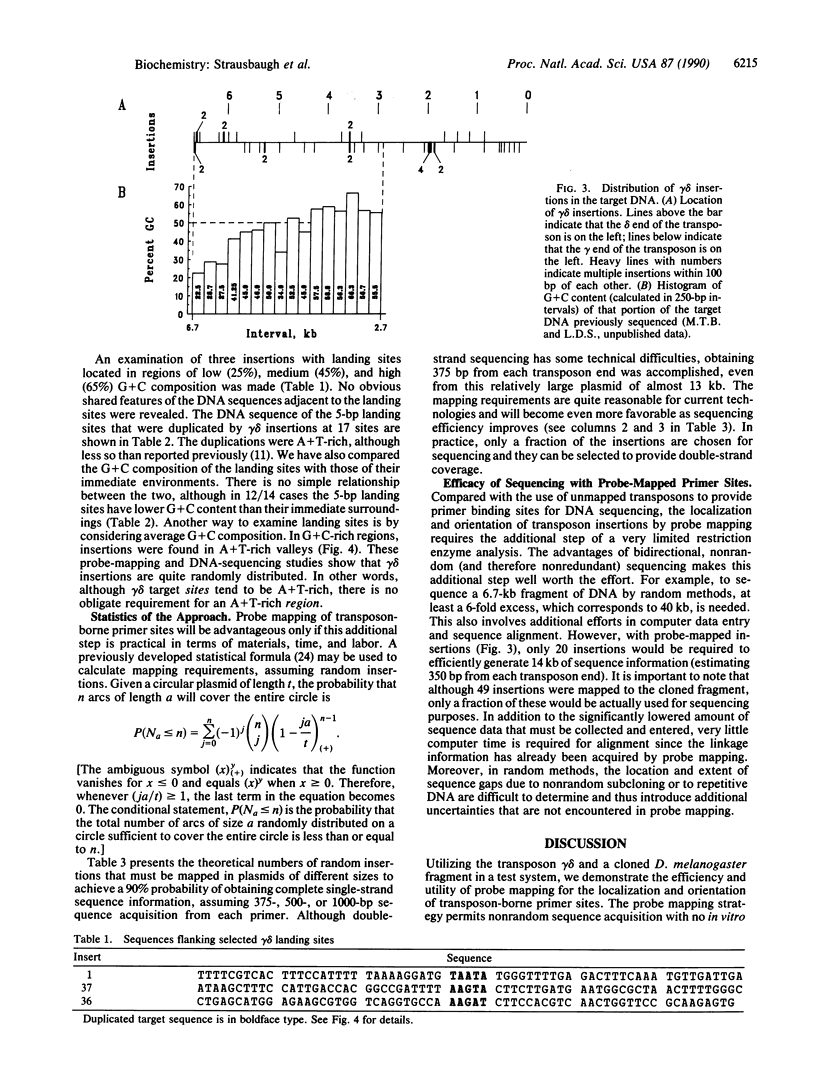
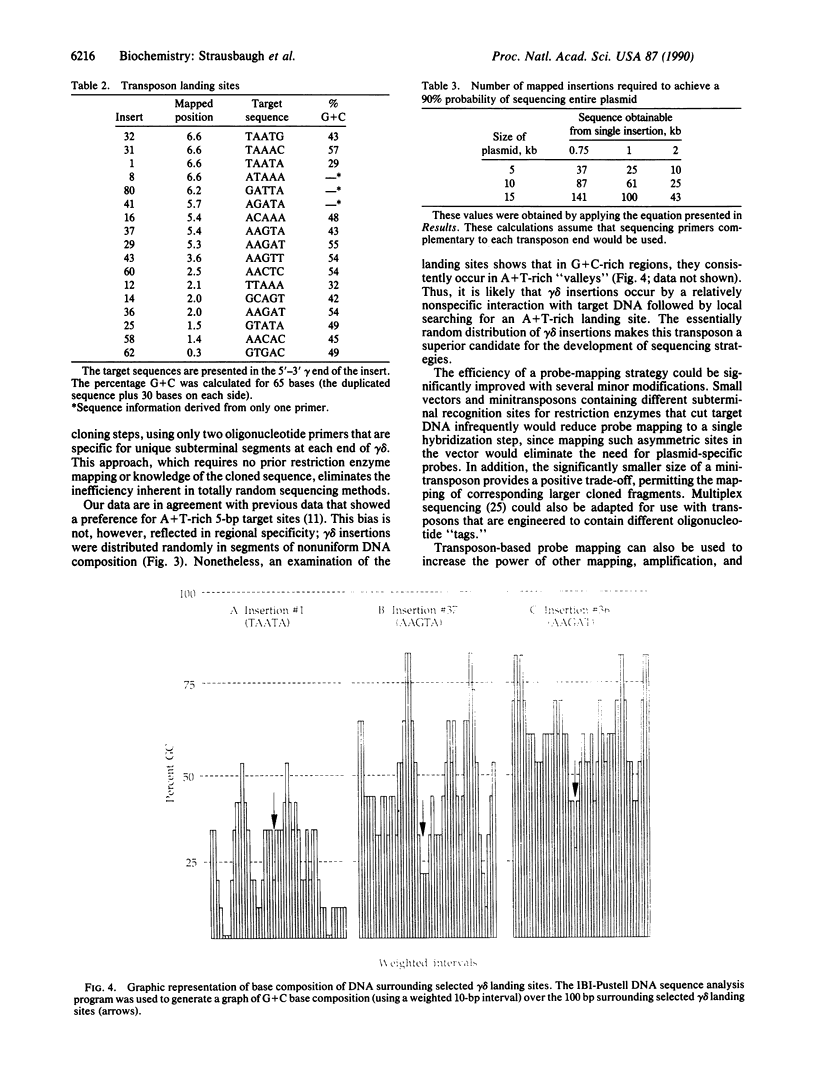

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Mizuuchi M., Robinson E. A., Appella E., O'Dea M. H., Gellert M., Mizuuchi K. DNA sequence of the E. coli gyrB gene: application of a new sequencing strategy. Nucleic Acids Res. 1987 Jan 26;15(2):771–784. doi: 10.1093/nar/15.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed A. Use of transposon-promoted deletions in DNA sequence analysis. Methods Enzymol. 1987;155:177–204. doi: 10.1016/0076-6879(87)55016-9. [DOI] [PubMed] [Google Scholar]
- Bucheton A., Paro R., Sang H. M., Pelisson A., Finnegan D. J. The molecular basis of I-R hybrid dysgenesis in Drosophila melanogaster: identification, cloning, and properties of the I factor. Cell. 1984 Aug;38(1):153–163. doi: 10.1016/0092-8674(84)90536-1. [DOI] [PubMed] [Google Scholar]
- Chow W. Y., Berg D. E. Tn5tac1, a derivative of transposon Tn5 that generates conditional mutations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6468–6472. doi: 10.1073/pnas.85.17.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Kieffer-Higgins S. Multiplex DNA sequencing. Science. 1988 Apr 8;240(4849):185–188. doi: 10.1126/science.3353714. [DOI] [PubMed] [Google Scholar]
- Collins J., Hohn B. Cosmids: a type of plasmid gene-cloning vector that is packageable in vitro in bacteriophage lambda heads. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4242–4246. doi: 10.1073/pnas.75.9.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fitch D. H., Strausbaugh L. D., Barrett V. On the origins of tandemly repeated genes: does histone gene copy number in Drosophila reflect chromosomal location? Chromosoma. 1990 Apr;99(2):118–124. doi: 10.1007/BF01735327. [DOI] [PubMed] [Google Scholar]
- Guyer M. S. Uses of the transposon gamma delta in the analysis of cloned genes. Methods Enzymol. 1983;101:362–369. doi: 10.1016/0076-6879(83)01027-7. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Liu L., Berg C. M. Mutagenesis of dimeric plasmids by the transposon gamma delta (Tn1000). J Bacteriol. 1990 May;172(5):2814–2816. doi: 10.1128/jb.172.5.2814-2816.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Whalen W., Das A., Berg C. M. Rapid sequencing of cloned DNA using a transposon for bidirectional priming: sequence of the Escherichia coli K-12 avtA gene. Nucleic Acids Res. 1987 Nov 25;15(22):9461–9469. doi: 10.1093/nar/15.22.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag D. K., Huang H. V., Berg D. E. Bidirectional chain-termination nucleotide sequencing: transposon Tn5seq1 as a mobile source of primer sites. Gene. 1988 Apr 15;64(1):135–145. doi: 10.1016/0378-1119(88)90487-8. [DOI] [PubMed] [Google Scholar]
- Olson M., Hood L., Cantor C., Botstein D. A common language for physical mapping of the human genome. Science. 1989 Sep 29;245(4925):1434–1435. doi: 10.1126/science.2781285. [DOI] [PubMed] [Google Scholar]
- Phadnis S. H., Huang H. V., Berg D. E. Tn5supF, a 264-base-pair transposon derived from Tn5 for insertion mutagenesis and sequencing DNAs cloned in phage lambda. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5908–5912. doi: 10.1073/pnas.86.15.5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge J. A., Blinderman L. A. ExoMeth sequencing of DNA: eliminating the need for subcloning and oligonucleotide primers. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9208–9212. doi: 10.1073/pnas.86.23.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg N. Bacteriophage P1 cloning system for the isolation, amplification, and recovery of DNA fragments as large as 100 kilobase pairs. Proc Natl Acad Sci U S A. 1990 Jan;87(1):103–107. doi: 10.1073/pnas.87.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. C., Kobori J. A., Siu G., Hood L. E. Specific-primer-directed DNA sequencing. Anal Biochem. 1986 Apr;154(1):353–360. doi: 10.1016/0003-2697(86)90536-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. A strategy for high-volume sequencing of cosmid DNAs: random and directed priming with a library of oligonucleotides. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6917–6921. doi: 10.1073/pnas.86.18.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]