Abstract
Background
Relapse infections resulting from the activation hypnozoites produced by Plasmodium vivax and Plasmodium ovale represent an important obstacle to the successful control of these species. A single licensed drug, primaquine is available to eliminate these liver dormant forms. To date, investigations of vivax relapse infections have been few in number.
Results
Genotyping, based on polymorphic regions of two genes (Pvmsp1F3 and Pvcsp) and four microsatellite markers (MS3.27, MS3.502, MS6 and MS8), of 12 paired admission and relapse samples from P. vivax-infected patients were treated with primaquine, revealed that in eight of the parasite populations in the admission and relapse samples were homologous, and heterologous in the remaining four patients. The patients’ CYP2D6 genotypes did not suggest that any were poor metabolisers of primaquine. Parasitaemia tended to be higher in the heterologous as compared to the homologous relapse episodes as was the IgG3 response. For the twelve pro- and anti-inflammatory cytokine levels measured for all samples, only those of IL-6 and IL-10 tended to be higher in patients with heterologous as compared to homologous relapses in both admission and relapse episodes.
Conclusions
The data from this limited number of patients with confirmed relapse episodes mirror previous observations of a significant proportion of heterologous parasites in relapses of P. vivax infections in Thailand. Failure of the primaquine treatment that the patients received is unlikely to be due to poor drug metabolism, and could indicate the presence of P. vivax populations in Thailand with poor susceptibility to 8-aminoquinolines.
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-017-1877-x) contains supplementary material, which is available to authorized users.
Keywords: Antibodies, Cytokines, Genotyping, Relapse, Plasmodium vivax, Thailand
Background
The origin of renewed parasitaemia following a primary infection by Plasmodium vivax or Plasmodium ovale treated with a schizontocidal drug is either a recrudescence due to erythrocytic parasites that have survived treatment, a re-infection, or a relapse consequent to merozoites released from a dormant liver stage, the hypnozoite, that has activated to resume normal development. Hypnozoites, which are not detectable, significantly extend the duration of the infection and the parasite’s potential to transmit, as a single infected mosquito bite can lead to multiple relapse episodes over many years. Relapse patterns vary between the parasite from temperate and tropical origin, and the risk for multiple relapse is highest in the tropics (50–80%) and lowest in temperate regions (5–10%) [1]. In Southeast Asia, vivax infections are predicted to have a high relapse incidence rate (836 relapses per 100,000 person days) and a relatively rapid mean time from primary episode to first relapse (41 days) [2]. The mechanisms behind the activation of hypnozoites and the determination of their periodicity remain unclear. Hypnozoites can only be eliminated by 8-aminoquinolines of primaquine which is safely administered only to persons without glucose-6-phosphate dehydrogenase deficiency. Thus, relapses are a major concern for efforts to control and eliminate vivax malaria [3].
It was assumed that the parasite populations emerging during relapses would be identical to those present during the primary episode, a notion that received support from early molecular studies [4, 5]. However, later investigations based more detailed genotyping analyses on relapses in diverse geographic regions revealed that the parasites in primary and relapse episodes are often genetically distinct, a phenomenon termed heterologous hypnozoite activation [6–8]. Thus, interpretation of drug efficacy studies against P. vivax in endemic countries cannot be corrected by genotyping analyses, as these will not allow distinguishing between a re-infection, a recrudescence, or a heterologous relapse.
The relapse pattern comprising the latency period and the number of relapse episodes is probably influenced by the level of immunity that the patients may have acquired during the primary attack [1, 9]. A degree of immunity is generally acquired following the primary infection, and experimental vivax infections clearly show that protection is strain-specific [9–11]. However, although the number of investigations on the immune responses in P. vivax is increasing, very few studies have addressed this in the context of relapse episodes [5, 12–16].
In Thailand patients diagnosed with P. vivax receive a standard treatment of chloroquine and primaquine, though approximately 7% relapse within 6 months [17]. Patients who present with P. vivax in Bangkok following a trip to endemic areas cannot be re-infected while they remain there, as malaria transmission does not occur in this city. This afforded the opportunity to recruit a cohort of vivax patients and to identify those with true relapse episodes during extended follow-up. In this manner a set 12 paired admission/relapse blood samples were obtained, and these were used to compare the genotypes of the parasites and a set of immune response markers.
Methods
Samples
The stored packed red blood cells and plasma samples were obtained from Clinical Malaria Research Unit, Faculty of Tropical Medicine at Mahidol University (Bangkok, Thailand). The samples were collected between February 2012 and May 2013 prior to treatment by the Clinical Malaria Research Unit (Faculty of Tropical Medicine) from 12 patients (P1 to P12) on initial admission with P. vivax and when presenting with renewed clinical symptoms during follow-up. These infections were acquired while visiting malaria endemic areas along the Thai-Myanmar border in the west (P1, P2, P3, P4, P5, P6, P7, P9), the Thai–Cambodian border in the east (P10, P11), and the Thai-Lao border in the north (P8, P12). All patients were treated the standard chloroquine/primaquine treatment: once-daily oral chloroquine on day 1 (600 mg), day 2 (600 mg), and day 3 (300 mg), and oral primaquine 15 mg for 14 days from day 2.
The mean age (mean ± SD) of the patients was 29.8 ± 13.3 years. The admission episode was confirmed to be the first following the latest travel to malarial areas through an interview of the patient and an examination of their clinical record. The second episode was confidently classed as a true relapse because: (a) reinfection could be excluded since the relapse episode occurred when the patients were residents in or around Bangkok, a non-malaria area, without any history of travelling to any malaria endemic areas after the initial P. vivax episode; (b) the median time interval to relapse in these patients was 81 days, making it highly unlikely that this was a recrudescence, which usually occurs within 28 days after treatment [18].
Thirty anonymous frozen plasma samples obtained from healthy individuals resident in non-endemic areas were used as controls. These donors had no history of malaria and denied travelling to any endemic area during the 2 years preceding blood collection.
This study was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University (Approval Number: MUTM 2013-034-01).
DNA template preparation
Plasmodium vivax genomic DNA was extracted from 200 µL of frozen-packed red cells using the commercially available DNA Blood kit (MACHEREY–NAGEL, Germany). The final volume of the DNA template was 100 µL, thus each 1 µL corresponds to the DNA present in 2 µL of whole blood. Confirmation of microscopic diagnosis examination was achieved by a multiplex real-time PCR assay using both genus- and species-specific primers [19].
Genotyping of Pvmsp1F3 and Pvcsp genes
Polymorphic fragments of two genes: F3 fragment of the P. vivax merozoite surface protein 1 (Pvmsp1F3) and the central repeat domain of the P. vivax circumsporozoite protein (Pvcsp) were amplified by nested-PCR assay as previously described [20]. The PCR products were purified using the QIAquick Gel Extraction Kit (QIAGEN, Hilden, Germany) and sequenced by AIT Biotech, Singapore. The sequences were aligned and analysed using Sequencher™ software version 5.3 (Gene Codes Corporation, Ann Arbor, USA).
Microsatellite analysis
Four microsatellite (MS) markers (MS3.502, MS3.27, MS6, and MS8) were amplified using semi-nested PCR amplification as previously described [21, 22]. The length variation of labelled PCR products was measured in comparison to internal size standards (GeneScan 500 LIZ; Applied Biosystems) on an ABI Genetic Analyzer (Macogen Inc., South Korea). Allele lengths and peak heights were analysed by using PeakScanner software (Applied Biosystems). Multiple alleles were called when there were multiple peaks per locus and where the height of these minor peaks reached one-third or more (>33%) of the predominant allele peak height.
Detection of anti-Plasmodium vivax IgG and IgG subclasses antibody
The anti-P. vivax IgG, IgG1, IgG2, IgG3, and IgG4 antibodies levels in the plasma samples were determined by the enzyme-linked immunosorbent assay as previously described [23] with some modification using crude P. vivax blood-stage antigens. The optical density (OD) was measured at a wavelength of 450 nm by an ELISA plate Reader (Tecan Sunrise™, Austria) with Magellan™ data analysis software version 7.1 (Tecan, Austria). Plasma from normal healthy individuals was used as controls.
Plasma cytokine quantification
The plasma pro- and anti- inflammatory cytokines were quantified by human cytometric bead array (CBA) kits (BD Biosciences Pharmingen, San Jose, CA, USA) for IL-1β (interleukin-1 beta), IL-6, IL-8, IL-10, IL-12p70 and TNF (tumour necrosis factor), and by CBA Flex Set system (BD Biosciences Pharmingen, San Jose, CA, USA) for IL-2, IL-4, IL-5, IL-13, IL-17A and IFN-γ (interferon gamma). Standard curves for each cytokine were generated by using the reference cytokine concentrations supplied with the kits. All standards and plasma samples were acquired on Accuri™ C6 Plus personal flow cytometer (BD Biosciences). Data analysis was performed using the FCAP Array software version 3.0.1 (BD/Softflow, Pécs, Hungary) and the values were expressed in pg/mL.
CYP2D6 genotyping and phenotyping
The single-nucleotide polymorphisms (SNPs) in the human gene encoding the cytochrome P450 2D6 (CYP2D6) were determined using the Luminex xTAG CYP2D6 Kit v3 (Luminex Corporation). The assay identifies the following alleles: normal function *1, *2, *35; decreased function *9, *10, *17, *29, *41; no enzymatic function *3, *4, *5, *6, *7, *8, *11, *15, and duplications. The CYP2D6 metaboliser phenotypes were assigned according to the rule-base system of Luminex xTAG CYP2D6 Kit v3 [24]. The patients were classified as (1) poor metabolisers (PM) if they harboured two null (non-functional) alleles; (2) intermediate metabolisers (IM) if they harboured two decreased function alleles, or a null allele with a decreased function allele; (3) extensive metabolisers (EM) if they harboured two functional alleles, or a functional allele with either a decreased function allele or a null allele; (4) ultra-rapid metabolizer (UM) if they harboured duplication of functional alleles.
Statistical analyses
Statistical analyses were performed using SPSS 15.0 software (SPSS Inc, Chicago, IL, USA) and Prism v.5 (GraphPad Software Inc, San Diego, CA, USA). Dimension variables were compared using Wilcoxon signed rank test for dependent data and Mann–Whitney U test for independent data. The P value of <0.05 was considered as statistically significant.
Results
Homologous versus heterologous relapses
Genotyping based on the Pvmsp1F3 and Pvcsp was successfully achieved for the parasites in all twelve paired admission and relapse samples (Table 1). Six distinct allelic variants (designated as “a” to “f”) were observed for Pvmsp1F3, and 13 distinct allelic variants were observed for Pvcsp: nine of the VK210 type designated as “a” to “i”, and four genotypes of the VK247 type designated as “j” to “m”. The sequences of the allelic variants are presented in Additional file 1. The parasites in the admission and relapse samples were also successfully genotyped using four microsatellite (MS) markers: MS3.27, MS3.502, MS6 and MS8 (Table 1). The number of distinct allelic variants for these microsatellite markers ranged from 6 to 17. Mixed genotype infections were observed in one or both samples from seven of the patients, with a maximum of two distinct haplotypes detected in a given sample (Table 1).
Table 1.
Genotypes of the P. vivax populations in admission and relapse episodes
| Patient/age (days)a | Date of sample collection (D/M/Y) | Msp1F3 | Csp | Microsatellite | |||
|---|---|---|---|---|---|---|---|
| MS3.27 | MS3.502 | MS 6 | MS 8 | ||||
| P1/47 | AS (12/3/2012) | a | VK247 j | 110.7 | 150.7 | 252.3 | 243.7 |
| (106) | RS (25/6/2012) | a | VK247 j | 110.7 | 150.6 | 252.3 | 243.7 |
| P2/56 | AS (12/3/2012) | a | VK247 j | 110.7 | 150.7 | 252.2 | 243.7 |
| (120) | RS (09/7/2012) | a | VK247 j | 110.7 | 150.6 | 252.3 | 243.7 |
| P3/50 | AS (12/3/2012) | a | VK247 j | 110.7 | 150.7 | 252.3 | 243.7 |
| (106) | RS (25/6/2012) | a | VK247 j | 110.6 | 150.6 | 252.3 | 243.7 |
| P4/18 | AS (23/4/2012) | a | VK247 j | 110.7 | 150.6 | 252.3 | 243.7 |
| (44) | RS (05/6/2012) | a | VK247 j | 110.8 | 150.7 | 252.3 | 243.8 |
| P5/20 | AS (22/5/2012) | a | VK247 k | 95.5 | 150.6 | 252.3 | 311.0 + 314.0 |
| (38) | RS (28/6/2012) | b | VK210 a | 125.9 | 190.9 | 252.2 | 304.8 + 307.8 |
| P6/22 | AS (02/7/2012) | c | VK210 b | 103.1 | 158.8 | 240.4 | 311.1 + 314.1 |
| (120) | RS (29/10/2012) | c | VK210 b | 103.2 | 158.8 | 240.3 | 311.1 + 314.2 |
| P7/22 | AS (16/7/2012) | a | VK210 c | 129.8 | 166.8 | 252.1 | 223.8 |
| (92) | RS (15/10/2012) | a | VK210 c | 129.7 | 166.9 | 252.2 | 223.9 |
| P8/31 | AS (07/2/2013) | a | VK210 d + VK247 l | 118.2 | 158.8 | 246.1 | 249.5 |
| (67) | RS (13/4/2013) | a | VK247 l | 185.9 | 150.7 | 240.3 | 284.3 + 287.3 |
| P9/22 | AS (07/8/2013) | d | VK210 e | 146.3 | 141.7 | 240.4 | 272.7 + 275.5 |
| (68) | RS (13/10/2013) | d | VK210 e | 146.4 | 141.7 | 240.3 | 272.7 + 275.6 |
| P10/22 | AS (18/5/2013) | a + e | VK210 f | 133.8 | 133.1 | 243.2 + 246.3 | 287.1 + 289.9 |
| (153) | AS (17/10/2013) | a | VK210 g | 133.7 | 133.1 | 243.16 | 218.2 |
| P11/21 | AS (11/9/2013) | a | VK210 h + VK247 m | 118.2 | 141.7 | 246.2 | 229.5 |
| (40) | RS (20/10/2013) | a | VK210 i + VK247 m | 110.6 + 118.2 | 141.7 | 240.3 | 229.5 + 246.7 |
| P12/27 | AS (23/10/2013) | f | VK210 b | 150.6 | 190.9 | 261.2 | 278.5 + 281.4 |
| (69) | RS (30/12/2013) | f | VK210 b | 150.6 | 191.0 | 261.2 | 278.4 + 281.4 |
Relapse episodes were classified as heterologous when a new allelic variant was observed in this episode for at least one of the markers
AS admission sample, RS relapse sample. Allelic variants that differ between the paired samples from each patient are presented in bold
aThe number of days between the initial admission and the relapse episode is presented in parenthesis below each patient number
The genotyping data allowed classifying the relapse episodes as homologous or as heterologous, i.e. with genetically similar or distinct parasite populations in the paired samples, respectively. For eight patients (P1, P2, P3, P4, P6, P7, P9 and P12) the relapses were homologous, while for the other four they were clearly heterologous (Table 1).
Median parasitaemia at first presentation was quite similar for the patients who then had a heterologous relapse (median and interquartile range: 349.3, 94.9–536.5 parasites/µL) as compared to those who went on to have a homologous relapse (346.5, 68.5–806.3 parasites/µL). On the other hand, the median parasitaemia at presentation of the relapse episode was higher in those with heterologous relapse (316.3, 52.1–612.3 parasites/µL) as compared to those with a homologous relapse (119.8, 50.4–190.8 parasites/µL) (Fig. 1).
Fig. 1.
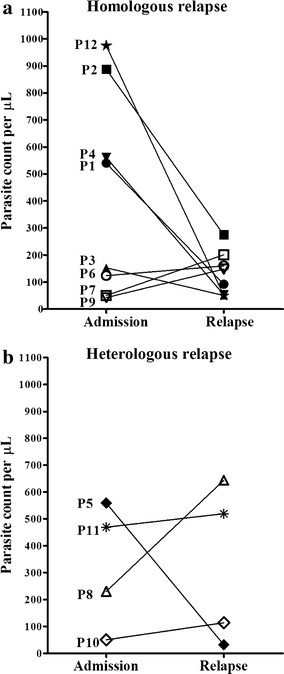
Parasitaemia in admission and relapse episodes of patients with homologous and heterologous relapse. Each line represents data from one patient. Parasite numbers per microliter of blood were determined by counting the number of parasites per 200 white blood cells (WBCs) in thick films and assuming an average of 8000 WBC/µL of blood to calculate the parasitaemia (P/µL of blood)
CYP2D6 genotyping of the patients
The twelve patients recruited in this study had received primaquine in addition to chloroquine as treatment for the initial admission episode. Their relapse episodes could therefore be considered as hypnozoicidal treatment failures. Given that adherence to treatment was observed, failure could be due to reduced susceptibility to 8-aminoquinolines or an inability of the patients to metabolise primaquine to its active form. Recent studies showed that cytochrome P450 2D6 (CYP2D6) is implicated in the metabolism of primaquine [25]. CYP2D6 is a genetically and phenotypically highly polymorphic drug-metabolizing enzyme involved in the biotransformation of numerous drugs [26]. Indeed, two cases where primaquine failure was associated to carriage of a particular CYP2D6 haplotype have been recorded [27, 28].
The CYP2D6 genotypes and the predicted metaboliser phenotypes were obtained for the patients recruited in this study (Table 2). Eight of the patients were classed as extensive metabolisers (EM) while the remaining four were predicted to be intermediate metabolisers (IM), and their proportion in those who had a homologous relapse (3 IM/5 EM) was similar to that in those who experienced a heterologous relapse (Table 2).
Table 2.
CYP2D6 genotypes and predicted phenotypes
| Relapse | Patient | Drug treatment | Genotype | Predicted phenotype |
|---|---|---|---|---|
| Homologous | P1 | C/P | *5/*10 | IM |
| P2 | C/P | *10/*10 | IM | |
| P3 | C/P | *1/*41 | EM | |
| P4 | C/P | *2/*10 | EM | |
| P6 | C/P | *1/*1 | EM | |
| P7 | C/P | *10/*10 | IM | |
| P9 | C/P | *1/*10 | EM | |
| P12 | C/P | *1/*10 | EM | |
| Heterologous | P5 | C/P | *2/*10 | EM |
| P8 | C/P | *1/*10 | EM | |
| P10 | C/P | *10/*41 | IM | |
| P11 | C/P | *1/*2 | EM |
C/P chloroquine/primaquine, IM intermediate metabolisers, EM extensive metabolisers
Analysis of immune responses
Anti-P. vivax antibody levels (IgG, IgG1, IgG2, IgG3 and IgG4) were measured in the plasma samples collected from the patients on for the initial and the relapse episodes (Fig. 2). As expected the median levels (median and interquartile range) of the specific IgG, IgG1, IgG2, IgG3 and IgG4 antibodies in both episodes were significantly higher than those in normal controls (P < 0.05) for all the patients. Significant differences in total IgG, IgG1, IgG2, IgG3 and IgG4 levels in paired admission and relapse episodes were not observed. This was also the case when considering samples from patients with homologous as compared to heterologous relapses, except for IgG3 where the median levels observed in the paired samples tended to be higher in patients who experienced a heterologous relapse (Fig. 2c).
Fig. 2.
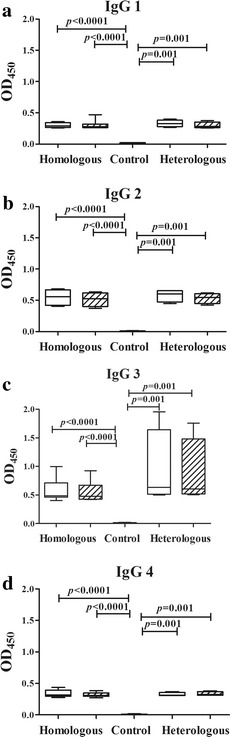
Levels of Plasmodium vivax-specific IgG subclasses in plasma collected at admission and relapse. The admission and relapse episodes are represented by white and crosshatched bars, respectively, while normal controls are indicated by a black bar. The box plots show the median (50th percentile) and 25th and 75th quartiles; error bars show the 10th and 90th percentiles. Mann–Whitney U test was used to establish a significant difference (P < 0.05) between groups pairwise. Wilcoxon signed rank test was used to assess the significant differences in the specific IgG subclass levels between the admission and relapse episodes
At both admission and relapse episodes, the median IgG3 levels in patients with heterologous relapses (admission: 0.63, 0.51–1.64; relapse: 0.61, 0.52–1.48) were relatively higher than those with homologous relapse (admission: 0.49, 0.47–0.72; relapse: 0.48, 0.43–0.67) but these were not statistically significant (P > 0.05) (Fig. 2c).
The cytokine profiles were similarly measured in the plasma samples collected from the patients prior to treatment (Fig. 3). As expected, the median cytokine levels (median and interquartile range) in the admission and relapse episodes were higher levels than those in normal controls. No Differences in median levels were observed for IL-2, IL-4, IL-5, IL-8, IL-12p70, IL-13, IL-17A, TNF or IFN-γ in either patient groups (Fig. 3). IL-6 (Fig. 3a) and IL-10 (Fig. 3b) were higher in the paired samples collected from patients with heterologous relapses as compared with those who had a homologous relapse with a significant difference for IL-6 at admission [heterologous versus homologous: 86.2, 35.4–113.3 pg/mL versus 10.7, 10.1–29.6 pg/mL (P = 0.028)], while significant differences were not seen for IL-6 at relapse [54.7, 23.8–1762.9 pg/mL versus 21.2, 6.1–187.0 pg/mL (P = 0.125)], or for IL-10 for either samples [admission: 130.6, 28.4–666.3 pg/mL versus 18.9, 13.0–58.9 pg/mL (P = 0.283); relapse: 252.5, 62.4-670.2 pg/mL versus 20.9, 6.9–1118.2 pg/mL (P = 0.283)]. IL-1β levels tended to be higher in the relapse as compared to the admission sample for both homologous [relapse versus admission: 6.3, 5.4–7.7 pg/mL versus 5.5, 4.6–5.8 pg/mL (P = 0.078)] and heterologous relapses [6.6, 5.9–7.4 pg/mL versus 6.0, 5.2–6.3 pg/mL (P = 0.375)] (Fig. 3c).
Fig. 3.
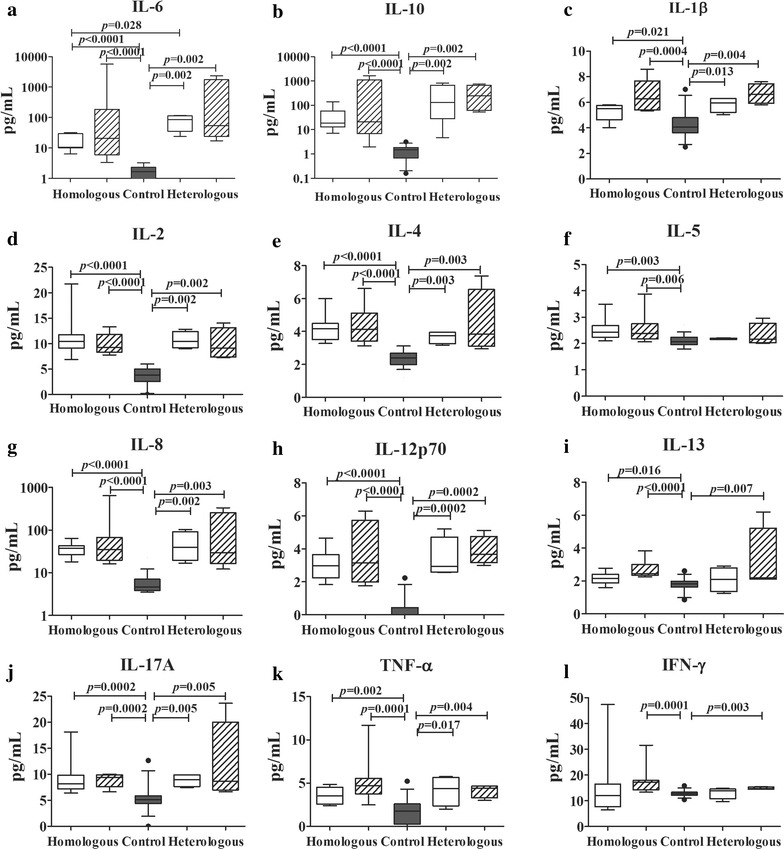
Levels of cytokines in plasma collected at admission and relapse. The admission and relapse episodes were indicated by white and crosshatched bars, respectively, while the normal control was indicated by black bar. The box plots show the median (50th percentile) and 25th and 75th quartiles; error bars show the 10th and 90th percentiles. Mann–Whitney U test was used to establish a significant difference (P < 0.05) between groups pairwise. Wilcoxon signed rank test was used to assess the significant differences in cytokine and chemokine levels between the admission and relapse episodes
Discussion
The biology of hypnozoites remains very poorly investigated; indeed the few hypnozoites consequent to an infectious bite lie dormant in the liver, an organ of a mean weight of 1.5 kg in adults, where they are not amenable to experimental in vivo investigations. Thus, insights into these dormant stages are only practicably inferred indirectly through observations of the relapses they cause. The ability of hypnozoites to survive for months and years and for a subset to activate rather randomly during this time makes this task difficult. In areas where transmission occurs, it is difficult to distinguish a true relapse episode from a recrudescence or a primary episode from a new infection during the follow-up of recruited patients. However, if the patients are treated with an efficacious schizontocidal treatment and observed in areas where transmission does not occur, one can confidently consider any renewed parasitological episodes as true relapses. The first molecular analyses of paired admission/relapse samples were carried out on samples from 6 patients who acquired the infection in various endemic countries who were treated and sampled in Canada [4], and the second from 10 patients recruited in an endemic region of Brazil [5]. In nearly all these cases the parasites in the paired samples were similar, but there were two exceptions (one in each study) where the parasite genotypes differed. The next molecular investigations on relapses that were conducted on a large number of patients, who had acquired their infection in Indonesia [6], Thailand, Myanmar or India [7], clearly demonstrated that the activation of heterologous hypnozoites could actually be quite frequent. Similar subsequent studies from diverse endemic areas confirmed that this phenomenon is indeed common.
Variations in the proportion of heterologous relapses are likely to alter with the levels and the duration of exposure to transmission, as this will influence the number of hypnozoites that accumulate in the liver. Of the 36 patients infected in western Thailand between 1992 and 1998 [7], 28 (78%) had parasite genotypes on relapse that were not observed in the admission sample. In this current study, only four of the twelve (33%) patients recruited between 2012 and 2013 showed a heterologous relapse, a decrease that might reflect the consistent reduction in the transmission intensity of P. vivax in this area (<0.5 infectious bite per person per year). It should be noted that both patients infected at the eastern border and one of the two infected at the north-eastern border showed heterologous relapses, as compared to one of the eight who acquired the infection at the western border of Thailand. Indeed, along the border areas of Thailand, it has been reported that P. vivax genetic diversity and multiplicity of infection remain high despite low malaria transmission [7, 29, 30]. The proximity to the borders of Myanmar, Laos and Cambodia (all areas where malaria endemicity is higher than in Thailand) has probably contributed to increase diversity through the extensive human migration events to and from Thailand. As the genetic diversity in the background population was high, the probability of relapses likely reflects heterologous infections. A larger number of cases would need to be investigated to ascertain whether this represents a significant difference. Nonetheless, the high genetic diversity of the parasites observed in the limited number of samples analysed here, with unequivocal mixed genotype infections in three of the patients, underlines the resilience to standard control efforts that hypnozoites confer to P. vivax.
It is interesting to note that patients P1–P4 had parasites of the same genotype (in both admission and relapse samples). These four patients were admitted within a short period of each other (March/April 2012), making it plausible that were infected by the same strain. The short time to relapse in one of these patients (P4, 44 days) compared to the other three (P1–P3, 106–120 days) might reflect age differences in the level of acquired immunity [31] as P4 was 18 years old whereas P1–P3 were 47–46 years old. Nonetheless, large variations in the time to relapse have been observed in humans inoculated experimentally with P. vivax sporozoites [32, 33]. An earlier relapse might also be due to a febrile illness that was proposed as a potential activator of latent hypnozoites [1]. It should be noted that a clinically discrete or silent relapse would have been missed because the study protocol relied on presentation with clinical symptoms during the follow-up period in order to record a relapse episode.
The patients presented here failed to clear the hypnozoites they were carrying despite the primaquine treatment that they had received. This failure was not related to a reduced ability to metabolise 8-aminoquinolines by cytochrome P450 as a result of CYP2D6 polymorphisms, reflecting previous observations showing similar clearance rates for intermediate and extensive metabolisers [34]. This suggests that some P. vivax lines circulating in Thailand might not be fully susceptible to the standard dose of primaquine (15 mg for 14 days from day 2), which should prompt reconsideration of the suitability of this dosage; all the more urgently should emergent chloroquine-resistant parasites [35] spread further. At present, there is no confirmed genetic marker for primaquine resistance/tolerance, though potential mutations in some parasite genes have been identified in the relapse populations of a single patient [36]. Whether any of these mutations are indeed implicated awaits comparative analysis of P. vivax isolates that have led or not to relapse infections following primaquine administration.
The availability of plasma samples from the patients afforded the possibility to conduct a survey of immune responses that could then be compared between admission and relapse episodes and also between homologous and heterologous episodes. Although the overall number of patients is low, particularly in the two relapse type groups, such studies have been previously rarely carried out and the comparisons could usefully serve to indicate factors that might be worth considering in future studies with larger patient groups. The levels of the twelve pro- and anti-inflammatory cytokines were as expected elevated in all groups as compared to controls, but differences between the groups were only noted for three of these: higher levels of IL-1β in the relapse as compared to the admission episode in patients with either relapse type, and elevated IL-6 and IL-10 in the paired samples from patients with heterologous as compared to homologous relapses. The significance of these observations is as yet unclear. Investigations on the cytokines profiles in P. vivax infections have to our knowledge been confined to admission and convalescent samples (for e.g. [37–40]) with a single study in which recurrences within 6 months, some of which might have been relapses, were included [12]. Given the pleiotropic effects of cytokines, and the diversity of epidemiological settings where the studies were based, conclusions from these studies remain speculative. Overproduction of IL-lβ is known to have deleterious effects on the liver [41]. Elevated levels of this cytokine are associated with the development of hepatic dysfunction in P. vivax malaria patients [40]. In this study, higher IL-1β levels in the relapse than admission episode might affect the development of the hypnozoite reservoir in the liver. Recently, it has been proposed that hypnozoites of different genotypes that have accumulated from previous inoculations could be triggered to activate by a febrile illness, leading to heterologous relapses [1]. Elevated IL-6 levels are associated with high fever in P. vivax malaria patients [39]. The higher IL-6 levels in heterologous as compared to homologous relapses in the current study might indicate the importance of this pyrogenic cytokine in the activation of heterologous hypnozoite. IL-10 has an important regulatory role in controlling the intensity of host immune response against the circulating parasites. Elevated IL-10 levels were shown to be correlated with hyperparasitaemia in P. vivax infected patients [42]. The high production of IL-10 noted for the patients with heterologous relapses suggests that it might be a regulator of immune responses harmful to the host.
Levels of IgG specific to P. vivax were elevated in all groups as compared to controls, which is consistent with previous observations on antibody levels [13, 16], and on the persistence of B cell memory responses to P. vivax blood stage antigens [43]. Previous studies have suggested that B cell memory responses to blood stage antigens could be stably maintained over time in the absence of reinfection even in areas of low malaria transmission [43]. The present investigation provides evidence that IgG antibody response to P. vivax was not greater in relapse compared with admission episodes even in patients with homologous relapses. This observation might be explained in three possibilities. Firstly, homologous relapses might not be associated with an enhanced immune memory-driven antibody response. It is possible that any partially effective immune response to a previous P. vivax strain might have been maximized in terms of driving elevations of antibody response [12]. Secondly, lower parasitaemia in homologous compared with heterologous relapses might be associated with a lower antibody response. Finally, some genotypes present in relapse episode may have been missed or only partially characterized due to insufficient numbers of parasites carrying those genotypes. Probably, in these patients with homologous relapses, the parasites causing relapses might not be identical to those of initial P. vivax infections. P. vivax-specific IgG2 and IgG3 levels were somewhat higher than those of IgG1 and IgG4 in all groups. It is not clear whether the higher median levels of IgG3 observed in both paired samples in heterologous relapses, as compared to those from homologous relapses, are of functional significance, especially as this does not correlate with the somewhat lower parasitaemia observed in homologous as compared to heterologous relapses. However, these observations might be explained by the possibility that the presence of genetically and antigenically distinct strains of the parasites during the course of infection might derive the host to mount diverse immune response against these antigenic variants. Higher parasite density may induce higher antibody responses in patients with malaria infection. In fact, IgG3 has the high binding affinity for the Fcγ receptor on the surface of monocytes, which strongly induces phagocytosis and complement fixing [44]. Recent studies determined that high IgG1 and IgG3 levels were associated with high parasite density [45].
Conclusions
Insights into the biology of hypnozoites, the relapses they cause, and the immunological consequences of these persistent infections are needed to tailor control measures that would effectively reduce and then eliminate vivax malaria. The sampling strategy employed here ensures that true relapse cases are investigated, though only yielding relatively a small number of patients, particularly in areas with declining vivax prevalence. The molecular investigations on the nature of the relapses provides, nonetheless, a means to monitor the efficacy of the recommended first line treatments so as to provide early warning of failing drug dosages. Immunological investigations conducted on carefully selected patients in different endemic setting will be needed to address the specifics of acquired immunity to the P. vivax infections.
Authors’ contributions
SK designed the study; SM prepared DNA extraction, performed the genotyping, ELISA and drafted the manuscript; PG supervised the genotyping techniques; SK provided the samples from P. vivax-infected patients; JT analysed the clinical data; PC performed the cytometric bead arrays; CS performed the Luminex assay; YM gave suggestion; MI provided microsatellite primers and protocols; GS and SK supervised the work and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We wish to thank the staff and the patients at the Clinical Malaria Research Unit, Faculty of Tropical Medicine, Mahidol University, for providing blood samples.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional file 1.
Ethics approval and consent to participate
This study using the stored specimen and data was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University (Approval Number: MUTM 2013-034-01). The blood and plasma of patients were obtained anonymously and recognized only by the study code number from the previous project with written informed consent (Approval Number MUTM 2012-007-02). The forms in which permission to store and use the samples and the data for future related studies are available.
Funding
This publication was made possible through the support from the Royal Golden Jubilee Ph.D. Program (RGJ Program) of the Thailand Research Fund (Grant No. PHD/0095/2554); the Office of Higher Education Commission, Ministry of Education, Thailand, Mahidol University under the National Research Universities Initiative, and the Faculty of Tropical Medicine, Mahidol University.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1. Sequence alignment of Pvmsp1F3 and Pvcsp genes.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12936-017-1877-x) contains supplementary material, which is available to authorized users.
Contributor Information
Sarunya Maneerattanasak, Email: sarunya.man@student.mahidol.ac.th.
Panita Gosi, Email: panitag@afrims.org.
Srivicha Krudsood, Email: srivicha.kru@mahidol.ac.th.
Pattamawan Chimma, Email: pattamawan.chi@mahidol.ac.th.
Jarinee Tongshoob, Email: jarinee.pan@mahidol.ac.th.
Yuvadee Mahakunkijcharoen, Email: yuvadee.mah@mahidol.ac.th.
Chonlaphat Sukasem, Email: chonlaphat.suk@mahidol.ac.th.
Mallika Imwong, Email: mallika.imw@mahidol.ac.th.
Georges Snounou, Email: georges.snounou@upmc.fr.
Srisin Khusmith, Phone: 66-2-3549100-13, Email: srisin.khu@mahidol.ac.th.
References
- 1.White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011;10:297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battle KE, Karhunen MS, Bhatt S, Gething PW, Howes RE, Golding N, et al. Geographical variation in Plasmodium vivax relapse. Malar J. 2014;13:144. doi: 10.1186/1475-2875-13-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price RN, Von Seidlein L, Valecha N, Nosten F, Baird JK, White NJ. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14:982–991. doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig AA, Kain KC. Molecular analysis of strains of Plasmodium vivax from paired primary and relapse infections. J Infect Dis. 1996;174:373–379. doi: 10.1093/infdis/174.2.373. [DOI] [PubMed] [Google Scholar]
- 5.Kirchgatter K, Del Portillo HA. Molecular analysis of Plasmodium vivax relapses using the MSP1 molecule as a genetic marker. J Infect Dis. 1998;177:511–515. doi: 10.1086/517389. [DOI] [PubMed] [Google Scholar]
- 6.Chen N, Auliff A, Rieckmann KH, Gatton ML, Cheng Q. Relapses of Plasmodium vivax infection result from clonal hypnozoites activated at predetermined intervals. J Infect Dis. 2007;195:934–941. doi: 10.1086/512242. [DOI] [PubMed] [Google Scholar]
- 7.Imwong M, Snounou G, Pukrittayakamee S, Tanomsing N, Kim JR, Nandy A, et al. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J Infect Dis. 2007;195:927–933. doi: 10.1086/512241. [DOI] [PubMed] [Google Scholar]
- 8.Restrepo E, Imwong M, Rojas W, Carmona-Fonseca J, Maestre A. High genetic polymorphism of relapsing P. vivax isolates in northwest Colombia. Acta Trop. 2011;119:23–29. doi: 10.1016/j.actatropica.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKenzie FE, Smith DL, O’Meara WP, Riley EM. Strain theory of malaria: the first 50 years. Adv Parasitol. 2008;66:1–46. doi: 10.1016/S0065-308X(08)00201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins WE, Jeffery GM, Roberts JM. A retrospective examination of reinfection of humans with Plasmodium vivax. Am J Trop Med Hyg. 2004;70:642–644. [PubMed] [Google Scholar]
- 11.Snounou G, Pérignon J-L. Malariotherapy—insanity at the service of malariology. Adv Parasitol. 2013;81:223–255. doi: 10.1016/B978-0-12-407826-0.00006-0. [DOI] [PubMed] [Google Scholar]
- 12.Chaves YO, Da Costa AG, Pereira MLM, De Lacerda MVG, Coelho-Dos-Reis JG, Martins-Filho OA, et al. Immune response pattern in recurrent Plasmodium vivax malaria. Malar J. 2016;15:445. doi: 10.1186/s12936-016-1501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuquiyauri R, Molina DM, Moss EL, Wang R, Gardner MJ, Brouwer KC, et al. Genome-scale protein microarray comparison of human antibody responses in Plasmodium vivax relapse and reinfection. Am J Trop Med Hyg. 2015;93:801–809. doi: 10.4269/ajtmh.15-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins WE, Lunde MN, Skinner JC. Development of antibodies to Plasmodium vivax as measured by two different serologic techniques. Am J Trop Med Hyg. 1975;24:412–416. doi: 10.4269/ajtmh.1975.24.412. [DOI] [PubMed] [Google Scholar]
- 15.Khusmith S, Tharavanij S, Bunnag D. Antigenic disparity of Plasmodium vivax causing initial symptoms and causing relapse. Southeast Asian J Trop Med Public Health. 1998;29:519–524. [PubMed] [Google Scholar]
- 16.Tobie JE, Abele DC, Wolff SM, Contacos PG, Evans CB. Serum immunoglobulin levels in human malaria and their relationship to antibody production. J Immunol. 1966;97:498–505. [PubMed] [Google Scholar]
- 17.Llanos-Cuentas A, Lacerda MV, Rueangweerayut R, Krudsood S, Gupta SK, Kochar SK, et al. Tafenoquine plus chloroquine for the treatment and relapse prevention of Plasmodium vivax malaria (DETECTIVE): a multicentre, double-blind, randomised, phase 2b dose-selection study. Lancet. 2014;383:1049–1058. doi: 10.1016/S0140-6736(13)62568-4. [DOI] [PubMed] [Google Scholar]
- 18.Baird JK. Chloroquine resistance in Plasmodium vivax. Antimicrob Agents Chemother. 2004;48:4075–4083. doi: 10.1128/AAC.48.11.4075-4083.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamau E, Tolbert LS, Kortepeter L, Pratt M, Nyakoe N, Muringo L, et al. Development of a highly sensitive genus-specific quantitative reverse transcriptase real-time PCR assay for detection and quantitation of Plasmodium by amplifying RNA and DNA of the 18S rRNA genes. J Clin Microbiol. 2011;49:2946–2953. doi: 10.1128/JCM.00276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maneerattanasak S, Gosi P, Krudsood S, Tongshoob J, Lanteri CA, Snounou G, et al. Genetic diversity among Plasmodium vivax isolates along the Thai–Myanmar border of Thailand. Malar J. 2016;15:75. doi: 10.1186/s12936-016-1136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imwong M, Boel ME, Pagornrat W, Pimanpanarak M, McGready R, Day NPJ, et al. The first Plasmodium vivax relapses of life are usually genetically homologous. J Infect Dis. 2012;205:680–683. doi: 10.1093/infdis/jir806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imwong M, Nair S, Pukrittayakamee S, Sudimack D, Williams JT, Mayxay M, et al. Contrasting genetic structure in Plasmodium vivax populations from Asia and South America. Int J Parasitol. 2007;37:1013–1022. doi: 10.1016/j.ijpara.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Tangteerawatana P, Perlmann HK, Hayano M, Kalambaheti T, Troye-Blomberg M, Khusmith S. IL4 gene polymorphism and previous malaria experiences manipulate anti-Plasmodium falciparum antibody isotype profiles in complicated and uncomplicated malaria. Malar J. 2009;8:286. doi: 10.1186/1475-2875-8-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanwong N, Ngamsamut N, Hongkaew Y, Nuntamool N, Puangpetch A, Chamnanphon M, et al. Detection of CYP2D6 polymorphism using Luminex xTAG technology in autism spectrum disorder: CYP2D6 activity score and its association with risperidone levels. Drug Metab Pharmacokinet. 2016;31:156–162. doi: 10.1016/j.dmpk.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Pybus BS, Marcsisin SR, Jin X, Deye GA, Sousa JC, Li Q, et al. The metabolism of primaquine to its active metabolite is dependent on CYP 2D6. Malar J. 2013;12:212. doi: 10.1186/1475-2875-12-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou S-F, Liu J-P, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41:89–295. doi: 10.1080/03602530902843483. [DOI] [PubMed] [Google Scholar]
- 27.Bennett JW, Pybus BS, Yadava A, Tosh D, Sousa JC, McCarthy WF, et al. Primaquine failure and cytochrome P-450 2D6 in Plasmodium vivax malaria. N Engl J Med. 2013;369:1381–1382. doi: 10.1056/NEJMc1301936. [DOI] [PubMed] [Google Scholar]
- 28.Ingram RJH, Crenna-Darusallam C, Soebianto S, Noviyanti R, Baird JK. The clinical and public health problem of relapse despite primaquine therapy: case review of repeated relapses of Plasmodium vivax acquired in Papua New Guinea. Malar J. 2014;13:488. doi: 10.1186/1475-2875-13-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosaisavee V, Hastings I, Craig A, Lek-Uthai U. The genetic polymorphism of Plasmodium vivax genes in endemic regions of Thailand. Asian Pac J Trop Med. 2011;4:931–936. doi: 10.1016/S1995-7645(11)60221-6. [DOI] [PubMed] [Google Scholar]
- 30.Lin JT, Patel JC, Kharabora O, Sattabongkot J, Muth S, Ubalee R. Plasmodium vivax isolates from Cambodia and Thailand show high genetic complexity and distinct patterns of P. vivax multidrug resistance gene 1 (pvmdr1) polymorphisms. Am J Trop Med Hyg. 2013;88:1116–1123. doi: 10.4269/ajtmh.12-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luxemburger C, Thwai KL, White NJ, Webster HK, Kyle DE, Maelankirri L, et al. The epidemiology of malaria in a Karen population on the western border of Thailand. Trans R Soc Trop Med Hyg. 1996;90:105–111. doi: 10.1016/S0035-9203(96)90102-9. [DOI] [PubMed] [Google Scholar]
- 32.Coatney GR, Cooper WC, Young MD. Studies in human malaria. XXX. A summary of 204 sporozoite-induced infections with the Chesson strain of Plasmodium vivax. J Natl Malar Soc. 1950;9:381–396. [PubMed] [Google Scholar]
- 33.Jeffery GM. Relapses with Chesson strain Plasmodium vivax following treatment with chloroquine. Am J Trop Med Hyg. 1956;5:1–13. doi: 10.4269/ajtmh.1956.5.1. [DOI] [PubMed] [Google Scholar]
- 34.St Jean PL, Xue Z, Carter N, Koh GCKW, Duparc S, Taylor M, et al. Tafenoquine treatment of Plasmodium vivax malaria: suggestive evidence that CYP2D6 reduced metabolism is not associated with relapse in the Phase 2b DETECTIVE trial. Malar J. 2016;15:97. doi: 10.1186/s12936-016-1145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rijken MJ, Boel ME, Russell B, Imwong M, Leimanis ML, Phyo Aung Pyae, et al. Chloroquine resistant vivax malaria in a pregnant woman on the western border of Thailand. Malar J. 2011;10:113. doi: 10.1186/1475-2875-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bright AT, Thamer A, Sandra S, Joel T, Giacomo MP, Nicholas JW, et al. Genetic analysis of primaquine tolerance in a patient with relapsing vivax malaria. Emerg Infect Dis. 2013;19:802. doi: 10.3201/eid1905.121852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park JW, Park SH, Yeom JS, Huh AJ, Cho YK, Ahn JY, et al. Serum cytokine profiles in patients with Plasmodium vivax malaria: a comparison between those who presented with and without thrombocytopenia. Ann Trop Med Parasitol. 2003;97:339–344. doi: 10.1179/000349803235002416. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues-Da-Silva RN, Lima-Junior JDC, Antas PR, Baldez A, Storer FL, Santos F, Banic DM, Oliveira-Ferreira JD, et al. Alterations in cytokines and haematological parameters during the acute and convalescent phases of Plasmodium falciparum and Plasmodium vivax infections. Mem Inst Oswaldo Cruz. 2014;109:154–162. doi: 10.1590/0074-0276140275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seoh JY, Khan M, Park SH, Park HK, Shin MH, Ha EH, et al. Serum cytokine profiles in patients with Plasmodium vivax malaria: a comparison between those who presented with and without hyperpyrexia. Am J Trop Med Hyg. 2003;68:102–106. [PubMed] [Google Scholar]
- 40.Yeom J-S, Park S-H, Ryu S-H, Park H-K, Woo S-Y, Ha E-H, et al. Serum cytokine profiles in patients with Plasmodium vivax malaria: a comparison between those who presented with and without hepatic dysfunction. Trans R Soc Trop Med Hyg. 2003;97:687–691. doi: 10.1016/S0035-9203(03)80104-9. [DOI] [PubMed] [Google Scholar]
- 41.Liu TZ, Lee KT, Chern CL, Cheng JT, Stern A, Tsai LY. Free radical-triggered hepatic injury of experimental obstructive jaundice of rats involves overproduction of proinflammatory cytokines and enhanced activation of nuclear factor kappaB. Ann Clin Lab Sci. 2001;31:383–390. [PubMed] [Google Scholar]
- 42.da Costa AG, Antonelli LR, Costa PA, Pimentel JP, Garcia NP, Tarrago AM, et al. The robust and modulated biomarker network elicited by the Plasmodium vivax infection is mainly mediated by the IL-6/IL-10 axis and is associated with the parasite load. J Immunol Res. 2014:318250. [DOI] [PMC free article] [PubMed]
- 43.Wipasa J, Suphavilai C, Okell LC, Cook J, Corran PH, Thaikla K, et al. Long-lived antibody and B Cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLoS Pathog. 2010;6:e1000770. doi: 10.1371/journal.ppat.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barnadas C, Ratsimbasoa A, Tichit M, Bouchier C, Jahevitra M, Picot S. Plasmodium vivax resistance to chloroquine in Madagascar: clinical efficacy and polymorphisms in pvmdr1 and pvcrt-o genes. Antimicrob Agents Chemother. 2008;52:4233–4240. doi: 10.1128/AAC.00578-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Q, Zhao Z, Zhang X, Li X, Zhu M, Li P, et al. Naturally acquired antibody responses to Plasmodium vivax and Plasmodium falciparum merozoite surface protein 1 (MSP1) C-terminal 19 kDa domains in an area of unstable malaria transmission in Southeast Asia. PLoS ONE. 2016;11:e0151900. doi: 10.1371/journal.pone.0151900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Additional file 1.


