Abstract
Hypoxic tumors exhibit increased resistance to radiation, chemical, and immune therapies. 18F-fluoromisonidazole (18F-FMISO) PET is a noninvasive, quantitative imaging technique used to evaluate the magnitude and spatial distribution of tumor hypoxia. In this study, pharmacokinetic analysis (PKA) of 18F-FMISO dynamic PET extended to 3 h after injection is reported for the first time, to our knowledge, in stage III–IV non–small cell lung cancer (NSCLC) patients. Methods: Sixteen patients diagnosed with NSCLC underwent 2 PET/CT scans (1–3 d apart) before radiation therapy: a 3-min static 18F-FDG and a dynamic 18F-FMISO scan lasting 168 ± 15 min. The latter data were acquired in 3 serial PET/CT dynamic imaging sessions, registered with each other and analyzed using pharmacokinetic modeling software. PKA was performed using a 2-tissue, 3-compartment irreversible model, and kinetic parameters were estimated for the volumes of interest determined using coregistered 18F-FDG images for both the volume of interest–averaged and the voxelwise time–activity curves for each patient’s lesions, normal lung, and muscle. Results: We derived average values of 18F-FMISO kinetic parameters for NSCLC lesions as well as for normal lung and muscle. We also investigated the correlation between the trapping rate (k3) and delivery rate (K1), influx rate (Ki) constants, and tissue-to-blood activity concentration ratios (TBRs) for all tissues. Lesions had trapping rates 1.6 times larger, on average, than those of normal lung and 4.4 times larger than those in muscle. Additionally, for almost all cases, k3 and Ki had a significant strong correlation for all tissue types. The TBR–k3 correlation was less straightforward, showing a moderate to strong correlation for only 41% of lesions. Finally, K1–k3 voxelwise correlations for tumors were varied, but negative for 76% of lesions, globally exhibiting a weak inverse relationship (average R = −0.23 ± 0.39). However, both normal tissue types exhibited significant positive correlations for more than 60% of patients, with 41% having moderate to strong correlations (R > 0.5). Conclusion: All lesions showed distinct 18F-FMISO uptake. Variable 18F-FMISO delivery was observed across lesions, as indicated by the variable values of the kinetic rate constant K1. Except for 3 cases, some degree of hypoxia was apparent in all lesions based on their nonzero k3 values.
Keywords: non-small cell lung cancer, hypoxia, 18F-fluoromisonidazole, compartmental analysis, pharmacokinetic analysis
Lung cancer is the malignancy with the highest mortality for both men and women. Approximately 70% of these patients are diagnosed with nonsquamous non–small cell lung cancer (NSCLC). The 5-y survival of patients treated with surgery alone is 73%, 58%, and 13% for stages IA, IB, and IIIA NSCLC, respectively. Treatment failures are usually associated with distant metastatic recurrences. Even with multimodality treatment (including surgery, chemotherapy, and radiotherapy) in nonresponders to neoadjuvant chemotherapy, the 2-y local control and survival rates are less than 60% (1). Tumor hypoxia has been shown to be an independent prognostic marker in several malignancies, including NSCLC (2). Hypoxic tumors generally express a more aggressive phenotype and are radioresistant and thus have an increased likelihood of locoregional recurrence, distant metastasis, and poor overall outcome (3,4). Nordsmark et al. showed, in fact, that the most predictive factor of survival in head and neck cancer was the proportion of the measurements in each lesion with a pO2 < 2.5 mm Hg; a steep decline in survival was observed when more than 20% of a lesion’s measurements had a pO2 < 2.5 mm Hg (5). In NSCLC, 38% of patients had lesions, with >20% of the measurements having a pO2 < 2.5 mm Hg (6). Potential treatment strategies for overcoming tumor hypoxia and improving local control rates include the use of radiosensitizing drugs and biologic image-guided dose escalation to hypoxic tumor subregions (7–11). 18F-fluoromisonidazole (18F-FMISO) PET has been widely investigated as a noninvasive method for detecting tumor hypoxia in several solid tumors, including NSCLC (2,3). Rajendran et al., building on previous work in animals by Koh et al., used 18F-FMISO uptake at 120 min after injection to quantify tissue hypoxia; they defined a tumor fractional hypoxic volume (FHV) by including voxels in the tumor image with tissue-to-blood activity concentration ratios (TBRs) > 1.4 (12,13). However, the application of 18F-FMISO to assess the FHV in NSCLC has yet to be investigated. Different TBRs and tumor-to-muscle and tumor-to-mediastinum activity concentration ratios (TMRs and TMeRs, respectively) have been proposed as semiquantitative criteria for delineating hypoxic tumor volumes (3,12–14); for example, Eschmann et al. showed that a TMeR > 2 was a predictive factor for local recurrence in lung cancer. However, Thorwarth et al. demonstrated that no single ratio threshold in the late 18F-FMISO images may be sufficient to accurately define the spatial distribution of tumor hypoxia (3,15). Instead, they suggested that pharmacokinetic analysis (PKA) of dynamic 18F-FMISO PET images should be used to extract local hypoxia and perfusion characteristics of tumor tissue (15). In a separate study, Wang et al. argued that the physiologic clearance of 18F-FMISO from highly perfused normal tissue may result in TBRs comparable to those in a tumor at the time of patient imaging (16). Eschmann identified 3 types of time–activity curves, representing rapid washout, intermediate or delayed washout, or continuing accumulation, respectively, which correlated with treatment outcome after radiotherapy (3). In particular, an accumulation-type curve, a high 18FMISO SUV, and a high TMeR at 4 h after injection were highly suggestive of incomplete response to treatment (3).
In this study, we used compartmental modeling to investigate the presence and extent of tumor hypoxia, as measured by 18F-FMISO PET, in a cohort of NSCLC patients, before treatment. In addition, tumor parametric maps of hypoxia surrogate (k3, Ki, and TBR) and delivery (K1) metrics were derived and correlations among these parameters evaluated.
MATERIALS AND METHODS
Patients Cohort
Sixteen NSCLC (10 men and 6 women; mean age ± SD, 66 ± 12 y) scheduled for definitive radiation therapy (RT) were included in this study. The institutional review board approved this study, and all subjects signed a written informed consent form. Patient characteristics are summarized in Table 1. The mean tumor volume of the 16 patients included in this study was approximately 18.8 cm3 (range, 1.7–147.0 cm3). All patients underwent a pretherapy 18F-FDG PET/CT scan for RT planning. A baseline 18F-FMISO PET/CT scan was then obtained up to 3 d after the 18F-FDG study.
TABLE 1.
Patient Characteristics
| Injected dose (MBq) |
||||
| Patient no. | 18F-FMISO | 18F-FDG | Sex | Age (y) |
| 1 | 358 | 435 | F | 79 |
| 2 | 336 | 419 | F | 75 |
| 3 | 338 | 411 | F | 66 |
| 4 | 323 | 426 | F | 66 |
| 5 | 367 | 395 | M | 33 |
| 6 | 361 | 419 | M | 65 |
| 7 | 371 | 429 | M | 59 |
| 8 | 336 | 462 | F | 65 |
| 9 | 365 | 441 | M | 78 |
| 10 | 242 | 441 | M | 66 |
| 11 | 349 | 430 | M | 71 |
| 12 | 376 | 429 | M | 64 |
| 13 | 359 | 437 | F | 57 |
| 14 | 382 | 415 | M | 60 |
| 15 | 326 | 450 | F | 82 |
| 16 | 352 | 432 | M | 73 |
| μ ± σ | 346 ± 33 | 429 ± 16 | 66 ± 11 | |
18F-FDG PET/CT
Patients were required to fast for at least 6 h before intravenous injection with 429 ± 16 MBq of 18F-FDG. Patients underwent 18F-FDG PET/CT at 60 ± 10 min after injection while immobilized in an RT simulation cradle. An additional 3-min free-breathing 18F-FDG scan was acquired over 1 PET field of view centered over the primary lesion, followed by a low-dose 4-dimensional CT (120 kVp; 10 mA; tube rotation, 0.5 s). A time-averaged CT (CTavg) was generated retrospectively and used for attenuation correction of free-breathing PET images. PET/CT images were acquired on a Discovery ST PET/CT scanner in 3-dimensional mode (GE Healthcare). PET emission data were corrected for attenuation, scatter, and randoms and iteratively reconstructed using standard clinical parameters (16 subsets, 2 iterations, transaxial postprocessing filter gaussian filter 6.0 mm in full width at half maximum, Heavy 3-point smoothing axially).
18F-FMISO PET/CT
Patients were injected intravenously with an average of 346 ± 33 MBq (range, 242–382 MBq) of 18F-FMISO simultaneously with the start of data acquisition (bolus duration, ∼5 s). No fasting was required before the 18F-FMISO injection. The 18F-FMISO was synthesized in our facility using a commercially available cassette (GE Healthcare), with radiochemical purity of at least 95% by high-performance liquid chromatography. The patients were scanned while supine on a flat-top couch insert on a Discovery 690 PET/CT scanner with time of flight (GE Healthcare) and immobilized in the RT simulation cradle. Dynamic 18F-FMISO PET (FMISO-1) acquisition over 1 bed position (centered over the primary lesion) was performed and consisted of 12 × 10, 8 × 60, and 7 × 300 s frames (total duration, 45 min). This was followed by 2 additional 10-min static image acquisitions: FMISO-2 at 95 ± 12 min (range, 79–98 min) and FMISO-3 at 168 ± 15 (range, 146–181 min) after injection. A low-dose 4-dimensional CT (same acquisition parameters as for the 18F-FDG PET/CT study) scan preceded each of the 3 18FMISO PET sessions; the corresponding CTavg was used for attenuation correction and image registration purposes. All 18F-FMISO PET images were reconstructed using 20 subsets, 2 iterations, transaxial gaussian postprocessing filter 6.4 mm in full width at half maximum, and Heavy 3-point smoothing axially.
Image Postprocessing and Preliminary Measurements
The PET tumor volumes from 18F-FDG, FMISO-2, and FMISO-3 were coregistered to that of FMISO-1 by means of their corresponding CTavg images, using the Advantage Workstation rigid registration software tool (version 4.7; GE Healthcare). For patients exhibiting multiple lesions, the registration process and the following processing were performed separately for each lesion. The FMISO-2 and FMISO-3 image sets were decay corrected to the start time of the FMISO-1 acquisition, and then these 3 datasets were merged using PMOD (version 3.609; PMOD Technologies, Inc.) into 1 dynamic image set (18F-FMISO dynamic PET).
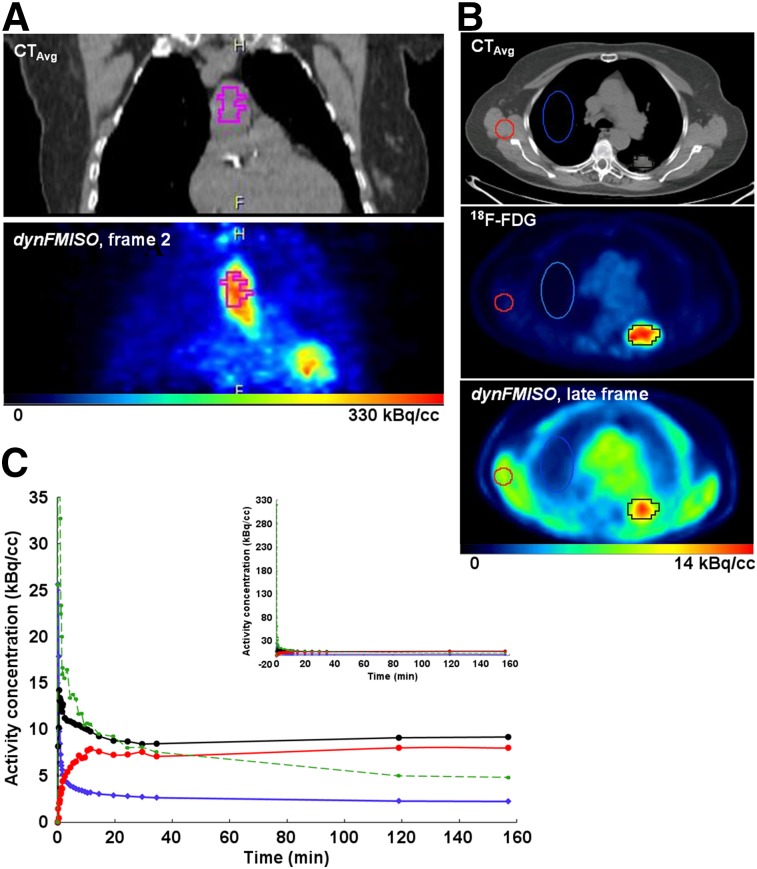
Because the heart was within the imaged field of view, image-derived input functions (IFs) were used for this study. Recent work by Nguyen-Kim has shown that, in NSCLC, the bronchial contribution to pulmonary circulation is higher than the pulmonary (17). Thus, a volume of interest (VOI) was drawn over the aortic arch on the CTavg image, well within the edges of the structure to avoid spillover effects (Fig. 1A, left), then superimposed on the frames of the 18F-FMISO dynamic PET image set (Fig. 1A, right). The time–activity curves of the 25 VOI voxels with highest activity concentration during the second frame were averaged to obtain the IF (Fig. 1C, green).
FIGURE 1.
Examples of aortic arch VOI (A) and VOITumor (black), VOINormal (blue), and VOIMuscle (red) (B) time–activity curves (C) corresponding to IF (green), time–activity curveAvg (black), time–activity curveNormal (blue), and time–activity curveMuscle (red).
Tumor volumes were delineated on the registered 18F-FDG (Fig. 1B, middle) image and then segmented using a 50%-of-maximum threshold. The background in the 18F-FDG images was at least 10 times smaller than the uptake in the lesions. Thus, it was not necessary to account for it as a caveat for adjusting the threshold. A fused PET/CT image was used to ascertain that the VOI was within the tumor on CTavg. The VOI thus derived, VOITumor, was then superimposed on the frames of the 18F-FMISO dynamic PET image set (Fig. 1B, right). Voxelwise time–activity curves were obtained for all voxels within the VOITumor and then averaged to provide a mean VOITumor time–activity curve (time–activity curveAvg). Finally, 2 additional VOIs were drawn for each patient: VOINormal, an ellipsoid drawn over the contralateral normal lung, and VOIMuscle, drawn over muscle in the latissimus dorsi region (Fig. 1B, blue/red, respectively). PKA was performed on the mean time–activity curve of each region (time–activity curveNormal and time–activity curveMuscle, Fig. 1C).
Voxelwise TBRs were calculated for the last frame time, tLate, for each VOI:
| Eq. 1 |
where ACLate and IFLate are the last-frame activity concentrations for a given voxel time–activity curve and IF, respectively. The maximum TBR (TBRmax) was then determined. On the basis of previous work, the tumor hypoxic volume (HV) was defined as the volume containing voxels with TBRs > 1.2 (18). The FHV was calculated as:
| Eq. 2 |
In addition, the time–activity curves of all voxels within the HV were averaged to obtain time–activity curveFHV for each lesion and PKA performed of each. For each VOI, the last-frame SUVbw was also calculated:
| Eq. 3 |
where bw is the body weight (kg). Finally, TMR for each lesion is calculated as .
PKA
The time–activity curve from dynamic PET data, CPET(t), represents the averaged activity concentration in a volume (voxel or VOI) at acquisition time t after injection and is modeled by
| Eq. 4 |
Every volume, whether a voxel or VOI, is heterogeneous, comprising various tissues and vascular components. The fraction of the vascular space in a volume, vB, accounts for the activity arising from the blood within the volume and (1 − vB) represents the fraction of activity in tissue, which is extravascular. The contributions to the measured CPET(t) in a given volume are those from tracer, which is circulating within the plasma (p), (Cp(t)); extracted from plasma into tissue and remaining unbound/free (C1(t)); and irreversibly bound, (C2(t)) (19,20). Bruehlmeier et al. have shown that the percentage of metabolic degradation of 18F-FMISO is negligible, so metabolite correction is unnecessary. The rate of change of the activity in a given compartment, as a function of time, is a linear combination of the fluxes, scaled by rate constants, into and out of it. This relationship may be described via coupled differential equations:
| Eq. 5 |
| Eq. 6 |
The pharmacokinetic rate constants K1 and k2 represent the rate of transport from plasma into and the efflux rate out from the unbound compartment, respectively, whereas k3 is the rate at which 18F-FMISO is trapped. Therefore, k3 is often considered a possible surrogate hypoxia metric (21).
Modeling was performed using PMOD with a 2-tissue, 3-compartment (plasma, free, bound) model with irreversible binding (k4 = 0). Fitting was performed with Levenberg–Marquardt weighted least-squares optimization to obtain the parameters K1, k2, k3, and vB (22,23). The weights were obtained by (16):
| Eq. 7 |
where c (0.064 as we previously calculated (24)) is the scaling factor, Δti the frame duration, CPET (ti) the decay-corrected activity concentration at time ti, and the decay constant.
The PKA results were compared for tumor, normal lung, and muscle VOIs voxelwise and averaged over the whole target volume (i.e., time–activity curveAvg). The influx rate was also calculated, because it has been suggested as a possible surrogate measure of hypoxia (16,21,25).
RESULTS
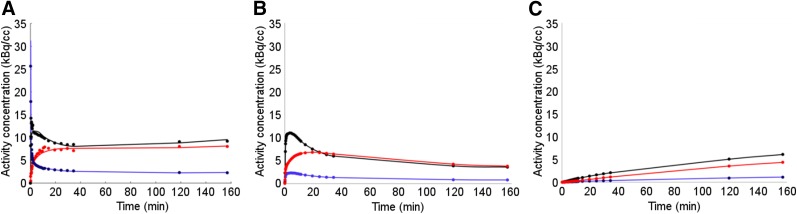
Figure 2A shows representative model curves, CModel(t), and the corresponding unbound and trapping compartment curves, C1(t) and C2(t) (Figs. 2B and 2C, respectively), for VOITumor (black), VOINormal (blue), and VOIMuscle (red) average time–activity curves, respectively.
FIGURE 2.
Total and compartment time–activity curves for patient 1 VOIs determined by PKA: Cmodel(t) (A), C1(t) (B), and C2(t) (C) for time–activity curveAvg (black), time–activity curveNormal (blue), and time–activity curveMuscle (red).
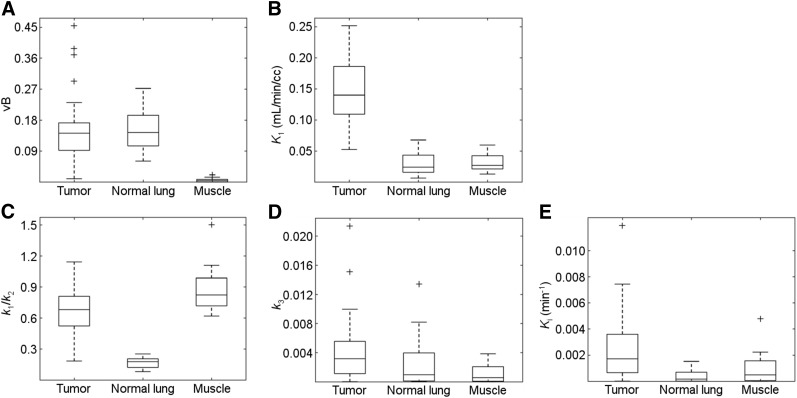
PKA results for the tumors’ time–activity curveAvg for the 16 patients (34 lesions) and values of TBR, lesion volume, and maximum SUVbw are tabulated in Tables 2 and 3 and Supplemental Tables 1 and 2 (supplemental materials are available at http://jnm.snmjournals.org). The average values (μ) and corresponding SD and ranges for each parameter were vB = 0.15 ± 0.10 (range, 0.01–0.45); K1 = 0.15 ± 0.06 (range, 0.05–0.25) mL/min/cm3; K1/k2 = 0.69 ± 0.23 (range, 0.18–1.14); and k3 = 0.0041 ± 0.0045 (range, 0.00–0.021) min−1. The average TBR across lesions was 1.80 ± 1.45 (range, 0.54–7.97). The results for normal lung and muscle are summarized in Tables 4 and 5 (also Supplemental Tables 3 and 4). Results for all tissues are summarized and compared in Figure 3.
TABLE 2.
Statistics of PKA Results for Average Lesion Time–Activity Curves for Each Patient
| Data | vB | %SE vB | K1 (mL/min/cm3) | %SE K1 | K1/k2 | %SE K1/k2 | k3 (min−1) | %SE k3 | Ki (mL/min/cm3) | %SE Ki |
| Average | 0.15 | 20 | 0.15 | 11 | 0.69 | 3.4 | 4.1E−03 | 25 | 4.1E-03 | 31 |
| σ | 0.10 | 13 | 0.06 | 5.0 | 0.23 | 2.1 | 4.5E−03 | 27 | 4.5E-03 | 26 |
| Minimum | 0.01 | 8.7 | 0.05 | 4.2 | 0.18 | 1.0 | 0.0E+00 | 5.4 | 0.0E+00 | 8.3 |
| Maximum | 0.45 | 80 | 0.25 | 25 | 1.10 | 12 | 2.1E-02 | 100 | 2.1E-02 | 110 |
Supplemental Table 1 provides patient/lesionwise results.
TABLE 3.
Statistics of Quantitative Lesion Parameters for Each Tumor VOI and Patient
| Data | SUVmax | TBR | Volume (cm3) | FHV |
| Average | 3.0 | 1.8 | 19 | 39 |
| σ | 2.2 | 1.5 | 27 | 43 |
| Minimum | 1.1 | 0.54 | 1.7 | 0.0 |
| Maximum | 13 | 8.0 | 150 | 100 |
Supplemental Table 2 provides patient/lesionwise results.
TABLE 4.
PKA Results for Average Normal Lung Time–Activity Curves
| Data | vB | %SE vB | K1 (mL/min/cm3) | %SE K1 | K1/k2 | %SE K1/k2 | k3 (min−1) | %SE k3 | SUVmax | TBR |
| Average | 0.16 | 14 | 0.029 | 26 | 0.17 | 12 | 1.8E−03 | 23 | 1.1 | 0.7 |
| σ | 0.063 | 9.7 | 0.019 | 14 | 0.052 | 6.8 | 2.4E−03 | 9.3 | 0.5 | 0.2 |
| Minimum | 0.064 | 6.2 | 0.0061 | 15 | 0.079 | 5.9 | 0.0E+00 | 12 | 0.4 | 0.3 |
| Maximum | 0.27 | 41 | 0.068 | 71 | 0.25 | 32 | 8.2E-03 | 33 | 2.2 | 1.1 |
Supplemental Table 3 provides patient/lesionwise results.
TABLE 5.
PKA Results for Average Muscle Time–Activity Curves
| Data | vB | %SE vB | K1 (mL/min/cm3) | %SE K1 | K1/k2 | %SE K1/k2 | k3 (min−1) | %SE k3 | SUVmax | TBR |
| Average | 5.9E−03 | 45 | 0.031 | 4.2 | 0.87 | 6.7 | 1.1E−03 | 35 | 1.9 | 1.3 |
| σ | 7.1E−03 | 57 | 0.013 | 1.5 | 0.22 | 3.5 | 1.3E−03 | 14 | 0.5 | 0.5 |
| Minimum | 0.0E+00 | 13 | 0.013 | 2.6 | 0.62 | 2.6 | 0.0E+00 | 15 | 1.3 | 0.9 |
| Maximum | 2.1E−02 | 200 | 0.059 | 7.5 | 1.5 | 18 | 3.8E−03 | 53 | 2.9 | 2.8 |
Supplemental Table 4 provides patient/lesionwise results.
FIGURE 3.
Comparison between kinetic parameters determined for the average time–activity curves of the 3 VOIs investigated (VOITumor, VOINormal, and VOIMuscle): (A) vB, (B) K1, (C) K1/k2, and (E) Ki. Points are labeled as outliers (+) if they are farther than from the median (midline), where Q1 and Q3 are the 25th and 75th percentiles of the sample data, respectively.
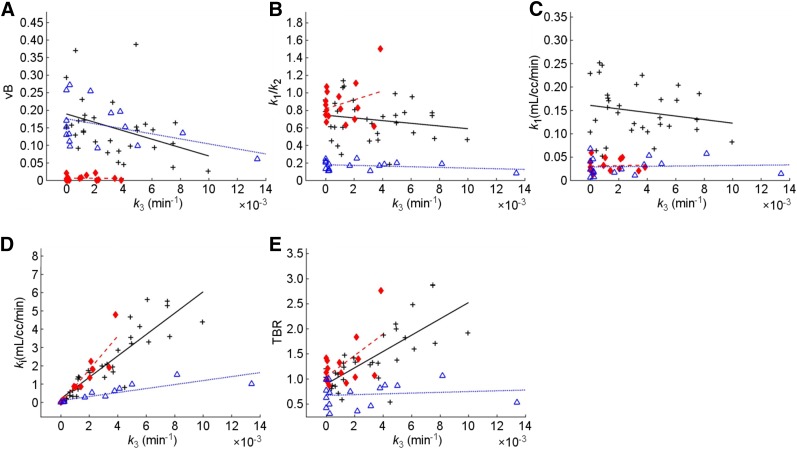
To elucidate the general relationships between k3 and other parameters across patients, scatterplots were constructed for k3 of time–activity curveAvg (tumor) versus the remaining PKA parameters as well as versus Ki and TBR, for each tissue VOI analyzed (Fig. 4); each data point represents 1 lesion (there is only 1 VOI per patient for normal lung or muscle). The corresponding Pearson correlation coefficients (R) are given in Table 6. A strong correlation was defined as R > 0.75, a weak one as R < 0.5, and all others as moderate.
FIGURE 4.
Scatterplots of k3 versus vB (A), K1/k2 (B), K1 (C), Ki (D), and TBR (E), for average time–activity curves: time–activity curveAvg (black), time–activity curveNormal (blue), and time–activity curveMuscle (red). Each point represents 1 lesion (some patients had multiple lesions).
TABLE 6.
Correlation Results Between k3 for Average Time–Activity Curves and Relevant Parameters Across Patients/Lesions
| Tumor |
Normal lung |
Muscle |
||||
| Data | R | P | R | P | R | P |
| K1 vs. k3 | −0.17 | 0.32 | 0.58 | 0.19 | 0.28 | 0.32 |
| K1/k2 vs. k3 | −0.22 | 0.33 | 0.13 | 0.65 | −0.25 | 0.33 |
| TBR vs. k3 | 0.84 | <0.001 | 0.41 | 0.13 | −0.05 | <0.001 |
| Ki vs. k3 | 0.91 | <0.001 | 0.97 | <0.001 | 0.95 | <0.001 |
| vB vs. k3 | −0.38 | 0.036 | −0.19 | 0.5 | −0.02 | 0.036 |
For tumors’ time–activity curveAvg, K1 versus k3 results show a weak negative correlation (R = −0.17), in contrast to what is observed for normal lung (R = 0.58) and muscle (R = 0.28). Additionally, Table 6 also shows R for k3 versus Ki and TBR, which are of interest because these parameters have all been proposed as possible hypoxia indices. This is supported by the degree of concordance between these parameters in tumor (R = 0.84 for TBR–k3 and R = 0.91 for Ki–k3); these correlations are highly significant (P < 0.001). Interestingly, R is even larger (0.97 and 0.95, P < 0.001) in the case of the normal tissues studied.
Table 7 summarizes the results of voxelwise correlations calculated between k3 and the foregoing parameters for each VOI (also Supplemental Table 5). Variable voxelwise correlations between K1 and k3 were observed for lesion VOIs (−0.97–0.72 and R < 0 in 24 out 34 lesions, 20 of which were significant, with P < 0.001), which averaged across lesions depicts a weak inverse relationship (average R = −0.23 ± 0.39). For both normal tissues, R for K1–k3 was positive for 81% of normal lung (75% with P < 0.05) and 75% of muscle (50% with P < 0.05) patients; 13% (lung) and 44% (muscle) of those exhibited moderate to high correlations (R > 0.5).
TABLE 7.
Results of Voxelwise Correlation Analysis
| Tumor R |
Normal lung R |
Muscle R |
||||||||||
| Data | K1–k3 | K1/k2–k3 | TBR–k3 | Ki–k3 | K1–k3 | K1/k2–k3 | TBR–k3 | Ki–k3 | K1–k3 | K1/k2–k3 | TBR–k3 | Ki–k3 |
| Average | −0.23 | −0.42 | 0.26 | 0.70 | 0.29 | −0.22 | 0.23 | 0.85 | 0.29 | −0.81 | 0.20 | 0.95 |
| σ | 0.39 | 0.39 | 0.44 | 0.29 | 0.25 | 0.26 | 0.26 | 0.08 | 0.43 | 0.22 | 0.35 | 0.09 |
| Minimum | −0.97 | −0.97 | −0.52 | −0.02 | −0.17 | −0.64 | −0.17 | 0.70 | −0.51 | −0.96 | −0.36 | 0.64 |
| Maximum | 0.72 | 0.62 | 0.85 | 0.99 | 0.76 | 0.40 | 0.88 | 0.96 | 0.90 | −0.03 | 0.66 | 1.00 |
Statistics shown are determined for multiple patients and lesions. Supplemental Table 5 provides patient/lesionwise results.
The influx rate constant, Ki, which has been proposed as a surrogate measure for hypoxia, showed a positive correlation with k3 in 33 of 34 tumors, 31 of which had an R > 0.5 (P < 0.001). Likewise, 100% of the patients had a Ki–k3 R > 0.5 for normal lung and muscle. As k2 > >k3, it is possible to deduce that The highly positive correlation between Ki and k3 in all 3 tissue types is therefore as expected. For 71% of lesions, TBR–k3 had an R > 0 and 53% showed moderate to strong correlations (P < 0.001), likewise for both normal lung and muscle tissues. Finally, 67% of lesions had nonzero FHVs. Of these, 38% had an FHV > 0.5 (half the tumor volume).
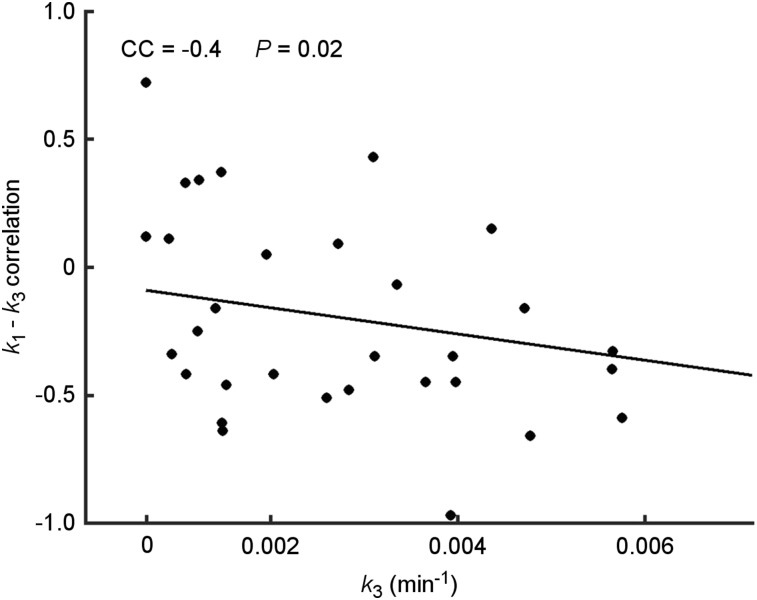
Finally, plotting the K1–k3 voxelwise R versus the lesion’s respective average k3 shows that as the lesion becomes less hypoxic (as indicated by k3), the correlation between delivery and trapping becomes more positive (Fig. 5).
FIGURE 5.
Scatterplot of K1–k3 voxelwise correlation versus average k3 in VOITumor. Each point represents 1 lesion.
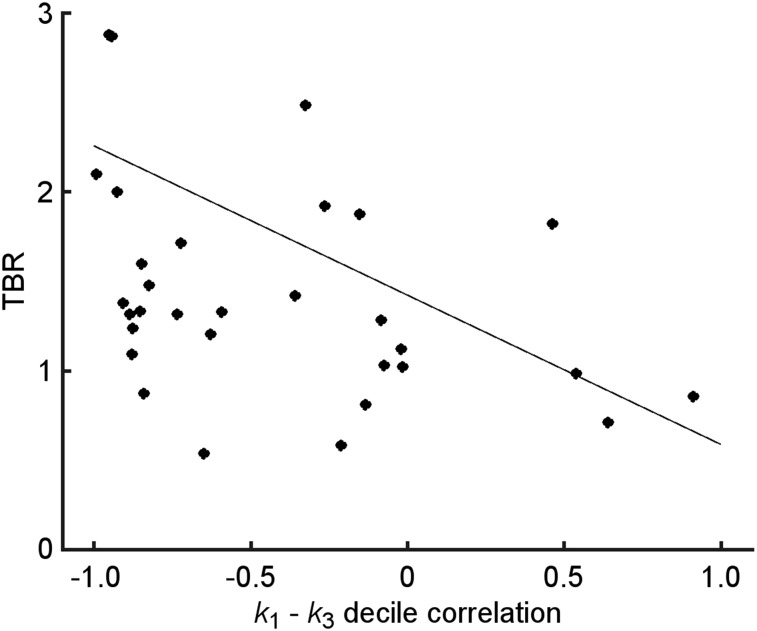
To increase the statistical power of the correlations, a similar analysis was carried by first grouping voxels into k3-deciles using the voxelwise parametric maps. Supplemental Figures 1A and 1B show bar graphs of R for K1–k3 for the respective results from normal lung and muscle; each bar corresponds to 1 patient (as does the color for comparison to tumor results because several patients had multiple lesions). Supplemental Figure 1D shows a similar plot for the decilewise analysis; in this case, each bar represents a lesion (each color a patient as in A and B). Eighty-eight percent of the lesions had an R < 0 for K1–k3, 59% of which had moderate to strong anticorrelations. Supplemental Figure 1C shows the same figure for the voxelwise results discussed in the previous section, for comparison. Figure 6 shows that as the TBR decreases, the R for K1–k3 changes from R < 0 to R > 0. Similar results were found for TMR (Supplemental Fig. 2).
FIGURE 6.
Scatterplot of TBR for VOITumor versus K1–k3 decile correlation coefficient. Each point represents 1 lesion.
Finally, PKA of the time–activity curveFHV showed that for only 8 of 23 lesions k3 was significantly different (P < 0.05) from that of time–activity curveAvg. Of those 8, only 5 had a k3 higher than that of time–activity curveAvg. Additionally, in every case, the maximum TBR in HV was larger than the TBR for the voxel with the maximum k3. Moreover, the maximum k3 voxel was inside the HV in 15 of 23 lesions.
DISCUSSION
18F-FMISO was first introduced as a promising hypoxia tracer because it is preferentially retained in cells with low oxygen pressure (26). 18F-FMISO is lipophilic, diffusing passively through the cell membrane and taken up by both normoxic and hypoxic tissues. Once inside the cell, it may undergo a first reduction into R-NO2, which is reversible reaction under normoxic conditions. However, at low (hypoxic) pO2s, R-NO2 is further reduced into R-NHOH, which binds to intracellular proteins, becoming irreversibly bound. Hence, there is a relationship between 18F-FMISO trapping in cells and the degree of hypoxia. The most widely used surrogate metric of tumor hypoxia in 18F-FMISO PET is its TBR derived from a single late-time-point image. Investigators have reported times from 2–4 h after 18F-FMISO administration (12,27,28). An image voxel is considered to be hypoxic if TBR exceeds a predetermined value, usually 1.2–1.4 (18,27). This approach, although simple to implement, may misidentify hypoxic voxels, a consequence of the slow 18F-FMISO clearance from regions of high tumor perfusion. This variable uptake and slow clearance means that it is not always possible to unambiguously differentiate the impact on uptake of hypoxia and perfusion by static PET imaging alone. The acquisition of dynamic PET images combined with PKA provides a potential methodology to distinguish delivery from trapping. This approach is more complex and acquisitions more time-consuming. Nevertheless, a few studies (15,18,21,29,30), mainly for head and neck cancers, have demonstrated its feasibility and benefit.
In this study, we performed PKA of 18F-FMISO dynamic PET images of patients with NSCLC. All lesions investigated demonstrated uptake of 18F-FMISO, and 31 of 34 studied showed some degree of irreversible trapping, that is, k3 > 0 within the limits of the associated SE. The generic shape of the tumor and normal lung time–activity curves during early frames exhibits a sharp peak, reflecting the transit of the initial injection bolus through the tissue of interest, followed by the diffusion of radiotracers from the vascular compartment into the perivascular space.
We performed PKA of lung tumors, normal lung, and muscle. The average lesion K1, associated radiotracer delivery, resulted in the most marked difference between these tissue types among the parameters studied. Its value was larger in tumors than in either normal lung or muscle tissue by at least a factor of 1.7 in every case (range, 2–33, μ ± SD = 7 ± 7; and range, 1.7–12, μ ± SD = 6 ± 3, respectively), with a lower degree of overlap with the normal tissues than for any of the other parameters (Fig. 3B). The significantly (P < 0.001) larger K1 values measured for lesions may reflect the abnormal tumor vasculature and easier diffusion than that of normal tissues. However, it is important to consider that the data for normal lung has not been corrected for its lower density relative to tumor and muscle. Normal lung tissue has significant components of air and blood. As a result, activity concentration is underestimated for voxels in the parenchyma. Because the air and blood fractions in the voxels are highly variable throughout the lung, corrections cannot be simply implemented via scaling. Holman et al., extending the work of Lambrou, have proposed methods for voxelwise corrections using CTAvg, and showed that K1, Ki, and SUVbw were all found to be affected (31,32). Nevertheless, the method has not been validated and, thus, not applied in this study. Thus, it is expected that the values of K1 and Ki reported here for normal lung would be lower had corrections been applied.
For the 2-compartment model, the rate constant k3, representing irreversible 18F-FMISO trapping, is considered a surrogate hypoxia metric. The expectation would be that such trapping would be more significant in tumors than in normal tissues. In fact, the results in this study show that whereas the average lesion k3 is generally larger in tumors, there is some overlap across tissues; namely, 5 lesions had an average k3 value, which was smaller than in their corresponding normal lung tissue. Three of these 5 lesions had small values of k3 (<0.001 min−1), and their oxygen status might, in accordance with their trapping rate constant, be considered closer to normoxia, as expected for normal lung tissue. However, 2 of 5 patients had values of k3 in normal lung, indicating unexpectedly high rates of 18F-FMISO trapping. The cause of this uptake in the normal lung tissue is unclear and a possible consequence of lung fibrosis, inflammation, or some other nonspecific retention in these unhealthy patients.
Single average parameter (derived from time–activity curveavg) comparisons may not draw clear distinctions between tissue types and lesion subvolumes. However, it is possible to determine relationships among parameters that are indicative of the hypoxic status of tumors. The average of voxelwise parameter correlations, Figure 5, shows that k3 increases with decreased delivery both within the tumor and across patients (30). This pattern is indicative of possible structural or functional differences between diseased and normal tissues, leading to a fundamentally different relationship between forward transport into the metabolic compartment and 18F-FMISO trapping, as might be expected. This is further reinforced by the fact that the concordance between K1 and k3 becomes positive as k3 becomes smaller. This result may imply that the magnitude of R might be indicative of weaker trapping within the tissue due to more normoxic conditions within the tumor.
Despite the theoretic advantages of modeling dynamic PET images, there are practical difficulties that may lead to inaccuracies in the estimated parameters, beyond those associated with errors due to statistical fluctuations. The data were acquired in 3 parts to minimize patient discomfort associated with scans longer than 45 min. In turn, it is necessary to coregister the 2 late image sets to the first to create 1 complete dynamic image series. Thus, the time–activity curve data may contain inaccuracies due to registration errors. This effect would have a larger impact on analyses of voxelwise time–activity curves than of average VOI time–activity curves. Coregistration of the 18F-FDG scan to FMISO-1 for tumor delineation and segmentation may cause further inaccuracies. Additionally, it is inevitable that there will be patient motion during the scan, especially for FMISO-1, which we seek to minimize by use of the RT mold. However, especially in lung, breathing also introduces an additional complication. In this study, no correction for breathing motion was applied as all the lesions studied were assumed to be minimally affected by motion effects because they were either large or located in regions (e.g., mediastinum or upper lung lobe) in which motion is minimal. Additionally, we observed that most patients quickly fell asleep or were relaxed within a few minutes, leading to shallow breathing. Nevertheless, breathing motion corrections could improve the accuracy of results.
Although the current results provide evidence for the presence of hypoxic subvolumes within each lesion, there is currently no known method for translating a value of k3 into an amount of hypoxia. Several authors have proposed TBR thresholds of 1.2–1.4 (30) as an empiric criterion for hypoxia. Twenty-three of 34 lesions studied here contained subvolumes with a TBR > 1.2, with a significant (P < 0.001) correlation identified between TBR and the average lesion k3. When hypoxic regions are defined solely on the basis of TBR > 1.2, more than 30% of lesions would appear to lack macroscopic areas of hypoxia. However, 81% of those are 18F-FMISO–avid (k3 > 0, P < 0.05), showing that TBR and k3 are not always in agreement as to determining region hypoxia. The average and maximum k3 for the latter are significantly (P < 0.05) lower than for those lesions with HVs. Finally, voxelwise only 52% of lesions have a significant (P < 0.05) R > 0.5.
Finally, the results presented in this article provide further evidence that there is not always concordance between the parametric descriptors of hypoxia such as k3 and TBR. It is possible that such discrepancies are a consequence of underlying different tumor tissue pathologies such as chronic hypoxia and ribbonlike hypoxia that is observed by immunohistochemical staining (33) but not resolved by PET imaging.
CONCLUSION
We have successfully performed PKA of baseline dynamic 18F-FMISO PET images in a cohort of NSCLC patients. Lesions for all patients showed evidence of hypoxia as depicted by relatively increased 18F-FMISO concentrations, which was corroborated by the increased k3 values relative to those in normal lung and muscle. Because this was a prospective study, the prognostic value of our measurements could not be assessed. This investigation demonstrates the feasibility of dynamic 18F-FMISO PET imaging in thoracic disease. In particular, future directions of this work include investigating the prognostic value of the rate constants and macroparameters derived from them.
Finally, although in this particular study we included only lesions minimally affected by motion, we are developing tools with which to implement corrections for breathing motion of dynamic PET. This will allow us to assess the impact of breathing motion on kinetic rate constants when studying lesions in thoracic regions with significant breathing motion effects.
DISCLOSURE
This study was supported by a grant from NIH/NCI U01 CA157442-3 (principal investigator Sadek A. Nehmeh) and the cancer center grant P30 CA008748 (principal investigator Craig B. Thompson). No other potential conflict of interest relevant to this article was reported.
Supplementary Material
REFERENCES
- 1.Rosenzweig KE, Amols H, Ling CC. New radiotherapy technologies. Semin Surg Oncol. 2003;21:190–195. [DOI] [PubMed] [Google Scholar]
- 2.Sura S, Gupta V, Yorke E, Jackson A, Amols H, Rosenzweig KE. Intensity-modulated radiation therapy (IMRT) for inoperable non-small cell lung cancer: the Memorial Sloan-Kettering Cancer Center (MSKCC) experience. Radiother Oncol. 2008;87:17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eschmann SM, Paulsen F, Reimold M, et al. Prognostic impact of hypoxia imaging with 18F-misonidazole PET in non-small cell lung cancer and head and neck cancer before radiotherapy. J Nucl Med. 2005;46:253–260. [PubMed] [Google Scholar]
- 4.Le QT, Chen E, Salim A, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non-small cell lung cancers. Clin Cancer Res. 2006;12:1507–1514. [DOI] [PubMed] [Google Scholar]
- 5.Nordsmark M, Bentzen SM, Rudat V, et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy: an international multi-center study. Radiother Oncol. 2005;77:18–24. [DOI] [PubMed] [Google Scholar]
- 6.Graves EE, Vilalta M, Cecic IK, et al. Hypoxia in models of lung cancer: implications for targeted therapeutics. Clin Cancer Res. 2010;16:4843–4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee NY, Mechalakos JG, Nehmeh S, et al. Fluorine-18-labeled fluoromisonidazole positron emission and computed tomography-guided intensity-modulated radiotherapy for head and neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys. 2008;70:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das SK, Miften MM, Zhou S, et al. Feasibility of optimizing the dose distribution in lung tumors using fluorine-18-fluorodeoxyglucose positron emission tomography and single photon emission computed tomography guided dose prescriptions. Med Phys. 2004;31:1452–1461. [DOI] [PubMed] [Google Scholar]
- 9.van Lin EN, Futterer JJ, Heijmink SW, et al. IMRT boost dose planning on dominant intraprostatic lesions: gold marker-based three-dimensional fusion of CT with dynamic contrast-enhanced and 1H-spectroscopic MRI. Int J Radiat Oncol Biol Phys. 2006;65:291–303. [DOI] [PubMed] [Google Scholar]
- 10.Madani I, Duthoy W, Derie C, et al. Positron emission tomography-guided, focal-dose escalation using intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2007;68:126–135. [DOI] [PubMed] [Google Scholar]
- 11.Vanderstraeten B, Duthoy W, De Gersem W, De Neve W, Thierens H. [18F]fluoro-deoxy-glucose positron emission tomography ([18F]FDG-PET) voxel intensity-based intensity-modulated radiation therapy (IMRT) for head and neck cancer. Radiother Oncol. 2006;79:249–258. [DOI] [PubMed] [Google Scholar]
- 12.Koh WJ, Bergman KS, Rasey JS, et al. Evaluation of oxygenation status during fractionated radiotherapy in human nonsmall cell lung cancers using [F-18]fluoromisonidazole positron emission tomography. Int J Radiat Oncol Biol Phys. 1995;33:391–398. [DOI] [PubMed] [Google Scholar]
- 13.Rajendran JG, Wilson DC, Conrad EU, et al. [18F]FMISO and [18F]FDG PET imaging in soft tissue sarcomas: correlation of hypoxia, metabolism and VEGF expression. Eur J Nucl Med Mol Imaging. 2003;30:695–704. [DOI] [PubMed] [Google Scholar]
- 14.Rasey JS, Koh WJ, Evans ML, et al. Quantifying regional hypoxia in human tumors with positron emission tomography of [18F]fluoromisonidazole: a pretherapy study of 37 patients. Int J Radiat Oncol Biol Phys. 1996;36:417–428. [DOI] [PubMed] [Google Scholar]
- 15.Thorwarth D, Eschmann SM, Paulsen F, Alber M. A kinetic model for dynamic [18F]-Fmiso PET data to analyse tumour hypoxia. Phys Med Biol. 2005;50:2209–2224. [DOI] [PubMed] [Google Scholar]
- 16.Wang W, Georgi JC, Nehmeh SA, et al. Evaluation of a compartmental model for estimating tumor hypoxia via FMISO dynamic PET imaging. Phys Med Biol. 2009;54:3083–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milne EN. Circulation of primary and metastatic pulmonary neoplasms. A postmortem microarteriographic study. Am J Roentgenol Radium Ther Nucl Med. 1967;100:603–619. [DOI] [PubMed] [Google Scholar]
- 18.Rajendran JG, Schwartz DL, O’Sullivan J, et al. Tumor hypoxia imaging with [F-18] fluoromisonidazole positron emission tomography in head and neck cancer. Clin Cancer Res. 2006;12:5435–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grunbaum Z, Freauff SJ, Krohn KA, Wilbur DS, Magee S, Rasey JS. Synthesis and characterization of congeners of misonidazole for imaging hypoxia. J Nucl Med. 1987;28:68–75. [PubMed] [Google Scholar]
- 20.Chapman JD, Franko AJ, Sharplin J. A marker for hypoxic cells in tumours with potential clinical applicability. Br J Cancer. 1981;43:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Lee NY, Georgi JC, et al. Pharmacokinetic analysis of hypoxia 18F-fluoromisonidazole dynamic PET in head and neck cancer. J Nucl Med. 2010;51:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta N, Gill H, Graeber G, Bishop H, Hurst J, Stephens T. Dynamic positron emission tomography with F-18 fluorodeoxyglucose imaging in differentiation of benign from malignant lung/mediastinal lesions. Chest. 1998;114:1105–1111. [DOI] [PubMed] [Google Scholar]
- 23.Hoekstra CJ, Hoekstra OS, Lammertsma AA. On the use of image-derived input functions in oncological fluorine-18 fluorodeoxyglucose positron emission tomography studies. Eur J Nucl Med. 1999;26:1489–1492. [DOI] [PubMed] [Google Scholar]
- 24.Grkovski M, Schwartz J, Gonen M, et al. Feasibility of 18F-fluoromisonidazole kinetic modeling in head and neck cancer using shortened acquisition times. J Nucl Med. 2016;57:334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li F, Joergensen JT, Hansen AE, Kjaer A. Kinetic modeling in PET imaging of hypoxia. Am J Nucl Med Mol Imaging. 2014;4:490–506. [PMC free article] [PubMed] [Google Scholar]
- 26.Rasey JS, Grunbaum Z, Magee S, et al. Characterization of radiolabeled fluoromisonidazole as a probe for hypoxic cells. Radiat Res. 1987;111:292–304. [PubMed] [Google Scholar]
- 27.Fleming IN, Manavaki R, Blower PJ, et al. Imaging tumour hypoxia with positron emission tomography. Br J Cancer. 2015;112:238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muzi M, Krohn KA. Imaging Hypoxia with 18F-fluoromisonidazole: challenges in moving to a more complicated analysis. J Nucl Med. 2016;57:497–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casciari JJ, Graham MM, Rasey JS. A modeling approach for quantifying tumor hypoxia with [F-18]fluoromisonidazole PET time-activity data. Med Phys. 1995;22:1127–1139. [DOI] [PubMed] [Google Scholar]
- 30.Bruehlmeier M, Roelcke U, Schubiger PA, Ametamey SM. Assessment of hypoxia and perfusion in human brain tumors using PET with 18F-fluoromisonidazole and 15O-H2O. J Nucl Med. 2004;45:1851–1859. [PubMed] [Google Scholar]
- 31.Holman BF, Cuplov V, Millner L, et al. Improved correction for the tissue fraction effect in lung PET/CT imaging. Phys Med Biol. 2015;60:7387–7402. [DOI] [PubMed] [Google Scholar]
- 32.Lambrou T, Groves AM, Erlandsson K, et al. The importance of correction for tissue fraction effects in lung PET: preliminary findings. Eur J Nucl Med Mol Imaging. 2011;38:2238–2246. [DOI] [PubMed] [Google Scholar]
- 33.van Laarhoven HW, Kaanders JH, Lok J, et al. Hypoxia in relation to vasculature and proliferation in liver metastases in patients with colorectal cancer. Int J Radiat Oncol Biol Phys. 2006;64:473–482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.