Abstract
18F-DCFPyL is a small-molecule inhibitor of the prostate-specific membrane antigen that has shown promise for evaluation of primary and metastatic prostate cancer using PET. Measuring the variability in normal-organ uptake of 18F-DCFPyL is necessary to understand its biodistribution, aid image interpretation, judge the reliability of scan quantification, and provide a basis for therapeutic monitoring. Methods: Sixty-five consecutive 18F-DCFPyL PET/CT scans from 64 patients with a history of prostate cancer were analyzed. Volumes of interest were defined for the lacrimal glands, major salivary glands, liver, spleen, and both kidneys. The mean SUV normalized to body mass or to lean body mass (SUL) was calculated for each volume of interest. The average SUV across all scans, the SD, and the coefficient of variation (COV) for each organ were calculated. The same parameters were also derived for a 3-cm sphere drawn in the center of the right lobe of the liver. Results: The average SUVmean for all selected organs measured was 6.6 ± 1.8 for the right lacrimal gland, 6.4 ± 1.8 for the left lacrimal gland, 9.1 ± 2.0 for the right parotid gland, 9.0 ± 2.1 for the left parotid gland, 9.6 ± 2.3 for the right submandibular gland, 9.4 ± 2.2 for the left submandibular gland, 5.0 ± 0.7 for the whole liver, 5.1 ± 0.7 for a 3-cm sphere in the liver, 4.0 ± 1.5 for the spleen, 20.1 ± 4.6 for the right kidney, and 19.4 ± 4.5 for the left kidney. SULmean was lower overall, although demonstrating similar trends. The COV of SUVmean and SULmean was lower in the liver (13.8% and 14.5%, respectively) than in any other organ and was less than the comparable COV for 18F-FDG PET. The COV of SUVmean and SULmean in the 3-cm sphere in the liver was also low and similar to the variability in the whole liver (14.2% and 14.7%, respectively). Conclusion: 18F-DCFPyL uptake in normal liver demonstrates less variability than in other 18F-DCFPyL–avid organs, and its variability is less than the reported variability of 18F-FDG in liver. Variability was slightly less for SUVmean than for SULmean, suggesting that SUVmean may be the preferable parameter for quantification of images obtained with 18F-DCFPyL.
Keywords: prostate-specific membrane antigen, positron emission tomography, molecular imaging, SUV, prostate cancer
Prostate-specific membrane antigen (PSMA) is a human transmembrane protein that is highly expressed in prostate cancer, and the degree of expression correlates positively with tumor aggression, metastatic disease, and recurrence (1–3). Several reports have suggested that PSMA-targeted PET imaging has utility in prostate cancer (4–11). Many of these have been retrospective studies that have used 68Ga-labeled PSMA ligands such as 68Ga-PSMA-HBED-CC (4–7). We have focused on 18F-labeled radiotracers for PSMA-targeted imaging (8–12) given the improved prospects for centralized radiotracer production and the potential for better image quality (13). We developed 2-(3-(1carboxy-5-(6-[18F]fluoro-pyridine-3-carbonyl)amino]-pentyl)-ureido)-pentanedioic acid (18F-DCFPyL) as a second-generation fluorinated PSMA-targeted PET radiotracer to improve the tissue distribution of our first-generation agent (12,14).
PET/CT imaging of cancer is important in assessing tumor response or progression during or after therapy. 18F-FDG PET/CT, in particular, has emerged as a useful approach to the assessment of metabolic response in a variety of tumors, partly on the basis of its ability to quantify radiotracer uptake within tumors and quantitatively determine response to therapy (15–18). These characteristics of PET offer significant advantages over anatomic imaging, in which size and morphologic changes must be used to judge the presence or absence of disease and response to therapy.
18F-DCFPyL is a new clinical radiotracer that has initially shown promise in identifying lesions caused by prostate cancer and may be an alternative approach for therapeutic monitoring (10,11,19). However, before embarking on studies to assess the capacity for such monitoring, the intrinsic variability of 18F-DCFPyL uptake in normal organs must be understood. Any change in tumor uptake on serial quantitative studies can be assessed only in the context of the known intrinsic variability of the imaging test (20).
The primary aim of this study was to initially characterize the between-patient variability in normal-organ uptake in patients with prostate cancer undergoing18FDCFPyL PET/CT. Characterization of variability with 18F-DCFPyL may increase our understanding of other PSMA-targeted radiotracers such as those radiolabeled with 68Ga or other radioisotopes.
A secondary aim of this work was to investigate whether, for 18F-DCFPyL, it would be preferable to use SUV corrected for body mass or for lean body mass (i.e., SUL). Previous work has demonstrated that SUV showed a positive correlation with body mass in 18F-FDG PET scans (21,22). Many centers use SUL for 18F-FDG PET so that uptake is independent of patient mass (23). The question of whether to use SUV or SUL has not been specifically addressed for PSMA-targeted agents such as 18F-DCFPyL.
MATERIALS AND METHODS
Patients
We retrospectively reviewed 65 consecutive 18F-DCFPyL PET/CT scans acquired between May 2014 and November 2015 from 64 patients with a history of prostate cancer. The mean age of the patients was 63.8 y (range, 45–88 y). Fifty-four patients (84.4%) were white, 8 (12.5%) were black, and 2 (3.1%) were of Asian ancestry. All patients were imaged with protocols approved by the local Institutional Review Board, and all gave written informed consent before undergoing imaging. The patients were enrolled in imaging trials for preoperative staging (12, 18.8%), to delineate sites of disease in the context of recurrence after radical retropubic prostatectomy (44, 68.8%), or to evaluate metastatic disease (9, 14.0%). One patient had a past history of splenectomy, but there were no other major abdominal organ resections in this patient cohort.
18F-DCFPyL PET/CT Imaging
18F-DCFPyL was produced using a dual-run 18F-FDG synthesis module according to a modification of a synthetic route previously described by Chen et al. (12). Fifty-nine of the PET scans were performed on a Discovery RX PET/CT scanner (GE Healthcare), and the remaining 6 scans were acquired on a Biograph mCT scanner (Siemens Healthcare). Both devices were operated in 3-dimensional emission acquisition mode and used CT for attenuation correction.
The patients fasted for 4–6 h before injection of 18F-DCFPyL. Approximately 1 h after intravenous injection of 18F-DCFPyL (≤333 MBq [≤9 mCi]), PET images of the supine patients were acquired over 6–8 bed positions (depending on the scanner used and the patient height) from the mid thighs to the skull vertex. PET and CT acquisition parameters were similar between the two scanners, although some differences are noted in Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org). Routine quality assurance phantoms confirmed that PET images from the two scanners were quantitatively comparable, and quality evaluations of clinical 18F-FDG studies indicated no statistically significant difference (Student t test, P = 0.39) between the mean liver SULs obtained from the two systems.
Image Analysis
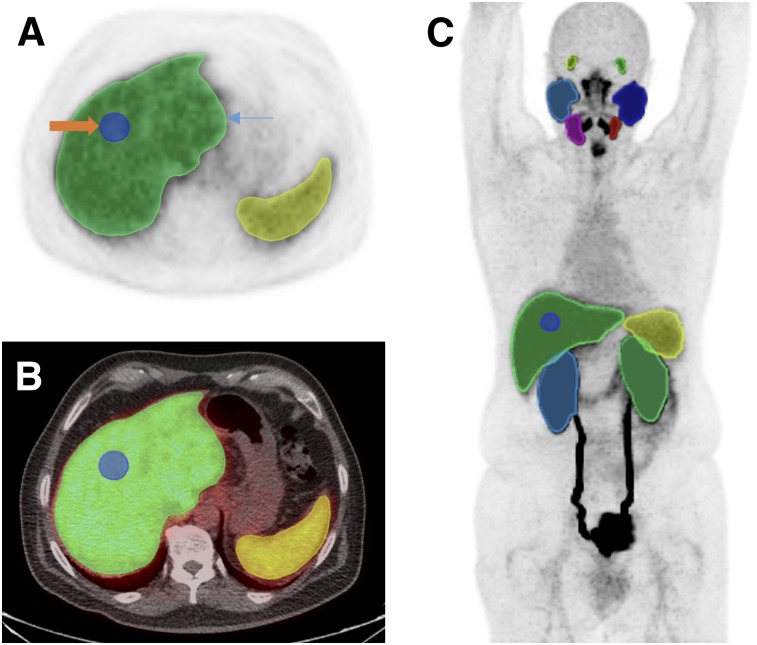
PET images were analyzed using XD3 Software (Mirada Medical), which allowed review of PET, CT, and fused-image data. Volumes of interest (VOIs) were manually drawn over the entire organ volume using the best visual approximation of the organ edge as has previously been described (20). Given the biodistribution of 18F-DCFPyL, these VOIs included both lacrimal glands, all 4 major salivary glands, the liver, the spleen, and both kidneys for all patients. Representative VOIs displayed on a maximum-intensity-projection image are shown in Figure 1. The CT images were available only for localization and were not used to guide delineation of the VOIs.
FIGURE 1.
(A) axial 18F-DCFPyL PET image through upper abdomen including liver and spleen showing whole-organ VOI (blue arrow) and 3-cm-sphere VOI in right lobe of liver (orange arrow). (B) Corresponding axial fused 18F-DCFPyL PET/CT images. (C) Representative organ VOIs displayed on maximum-intensity-projection image.
Between-patient variability was assessed for each organ and SUV definition (SUV or SUL) by taking the average, SD, and coefficient of variation (COV) across all patients. The same parameters were also derived for a 3-cm sphere drawn in the center of the nondiseased right hepatic lobe of the liver, a potentially more convenient VOI for clinical application.
Statistical Analysis
Data are presented as mean ± SD and the COV for each organ. The independent-samples t test was used to compare the difference in uptake values between the 3-cm-sphere VOI and the whole-liver VOI. The relationship of SUVmean and SULmean to patient body weight was assessed by the Pearson correlation coefficient using SPSS software (version 17.0).
RESULTS
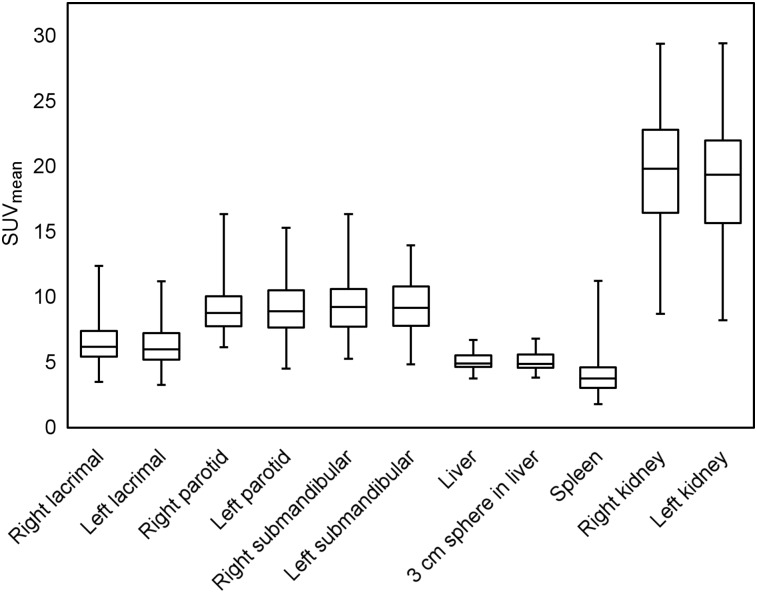
Sixty-three of 64 patients (98.4%) had visually normal biodistribution findings. One patient’s biodistribution differed only in lacking a spleen, consistent with a history of prior splenectomy. The calculated averages and SDs for SUVmean and SULmean for each organ are displayed in Table 1. As expected from visual assessment, SUVmean and SULmean were higher in the kidneys than in any other examined organ (SUVmean, 20.1 ± 4.6 on the right and 19.4 ± 4.5 on the left; SULmean, 14.8 ± 3.4 on the right and 14.3 ± 3.3 on the left). The major salivary glands had, on average, higher uptake values than any other organ except for the kidneys, with SUVmean ranging from 9.0 ± 2.1 to 9.6 ± 2.3 and SULmean ranging from 6.7 ± 1.7 to 7.1 ± 1.6. The lacrimal glands also demonstrated high average uptake, with an SUVmean of 6.6 ± 1.8 on the right and 6.4 ± 1.8 on the left and an SULmean of 4.9 ± 1.3 on the right and 4.8 ± 1.3 on the left. The highly similar values obtained between the paired organs suggests that the images are spatially reliable and that there were no significant errors in the manner in which the VOIs were manually rendered.
TABLE 1.
SUVmean and SULmean for Each Organ Across All Patients
| Organ | SUVmean | SULmean |
| Right lacrimal gland | 6.6 ± 1.8 | 4.9 ± 1.3 |
| Left lacrimal gland | 6.4 ± 1.8 | 4.8 ± 1.3 |
| Right parotid gland | 9.1 ± 2.0 | 6.8 ± 1.6 |
| Left parotid gland | 9.0 ± 2.1 | 6.7 ± 1.7 |
| Right submandibular gland | 9.6 ± 2.3 | 7.1 ± 1.6 |
| Left submandibular gland | 9.4 ± 2.2 | 6.9 ± 1.6 |
| Liver | 5.0 ± 0.7 | 3.8 ± 0.5 |
| Spleen | 4.0 ± 1.5 | 2.9 ± 1.1 |
| Right kidney | 20.1 ± 4.6 | 14.8 ± 3.4 |
| Left kidney | 19.4 ± 4.5 | 14.3 ± 3.3 |
Data are average and SD of uptake parameters.
Regarding the 2 unpaired organs in this study, the liver demonstrated overall higher uptake than the spleen. SUVmean for the liver was 5.0 ± 0.7, and SULmean was 3.8 ± 0.5. SUVmean for the spleen was 4.0 ± 1.5, and SULmean was 2.9 ± 1.1 (Fig. 2).
FIGURE 2.
Box-and-whisker chart shows uptake of 18F-DCFPyL in different organs. SUVmean in kidneys was highest of any examined organs, followed by salivary and lacrimal glands. Lowest uptake was in spleen, that in liver being slightly higher.
The calculated COVs across all patients for SUVmean and SULmean are included in Table 2. Variabilities between paired organs are similar regardless of the use of SUV or SUL (e.g., 26.9% for the right lacrimal gland and 27.6% for the left lacrimal gland when using SUVmean and 26.3% for the right lacrimal gland and 26.5% for the left lacrimal gland when using SULmean). The lowest variability of any of the studied organs was in the liver, where the COV was 13.8% for SUVmean and 14.5% for SULmean, whereas the highest variability was in the spleen (38.7% with SUVmean and 38.8% for SULmean). For reference, the COV of the liver with 18F-FDG PET has been reported to be 21.0%–23.1% using SULmean (24). Only 1 of the 64 patients included in this study was imaged at more than a single time point, so no data are available on the relative roles of intrapatient (test–retest) versus interpatient variability.
TABLE 2.
COVs for SUVmean and SULmean for Each Organ Across All Patients
| Organ | SUVmean | SULmean |
| Right lacrimal gland | 26.9% | 26.3% |
| Left lacrimal gland | 27.6% | 26.5% |
| Right parotid gland | 22.0% | 23.5% |
| Left parotid gland | 23.3% | 24.5% |
| Right submandibular gland | 24.3% | 23.3% |
| Left submandibular gland | 23.5% | 23.7% |
| Liver | 13.8% | 14.5% |
| Spleen | 38.7% | 38.8% |
| Right kidney | 23.1% | 22.6% |
| Left kidney | 23.2% | 22.7% |
Although the whole-organ VOIs would likely be the most reliable means of determining average uptake in normal organs, drawing such VOIs is time-consuming and unlikely to be undertaken in the context of a busy clinical workflow. We investigated the ability of a simple 3-cm-diameter sphere placed within visually normal liver parenchyma (Fig. 1) to represent the activity in the whole-organ liver VOI. SUVmean, SULmean, and COV for the 3-cm-sphere VOIs were found to be similar to the whole-organ VOI uptake values (Table 3). The average SUVmean of the 3-cm spheres in the liver was 5.1 ± 0.7 (compared with 5.0 ± 0.7), whereas the average SULmean in the 3-cm spheres was 3.8 ± 0.6 (compared with 3.8 ± 0.5). We used independent-samples t testing and found no significant difference between 3-cm-sphere and whole-liver VOIs (SULmean, t = −0.37, P = 0.71; SUVmean, t = −0.40, P = 0.69). The COVs in the 3-cm-sphere VOIs (14.2% using SUVmean and 14.7% using SULmean) were similar to those of the whole-liver VOIs (13.8% using SUVmean and 14.5% using SULmean).
TABLE 3.
SUVmean, SULmean, and COV for 3-Centimeter-Sphere and Whole-Liver VOIs
| VOI |
||
| Parameter | 3-cm sphere | Whole liver |
| SUVmean | 5.1 ± 0.7 | 5.0 ± 0.7 |
| SULmean | 3.8 ± 0.6 | 3.8 ± 0.5 |
| SUVmean COV | 14.2% | 13.8% |
| SULmean COV | 14.7% | 14.5% |
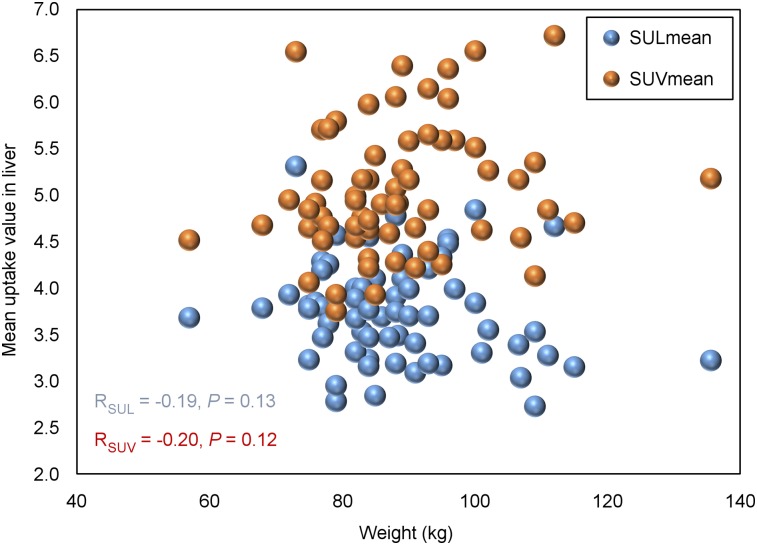
The average body mass of the 64 male patients was 88.5 ± 12.8 kg (range, 57.0–136.0 kg). That relatively wide range of patient masses allowed us to investigate for the presence of any correlation between body mass and uptake parameters. Because the variability in the liver across all scans was less than that in any other included organ, we plotted SUVmean in the liver against body mass and found that there was no significant correlation between them (Pearson analysis: r = 0.195, P = 0.119; Fig. 3). SULmean in the liver was also tested for a correlation with body mass, and again, no significant correlation was found (r = −0.190, P = 0.130; Fig. 3).
FIGURE 3.
Scatterplots of SUVmean and SULmean in liver against body mass. SUVmean in liver had no significant correlation with mass based on Pearson analysis (r = 0.195, P = 0.119). There was also no significant correlation between SULmean in liver and body mass (r = −0.19, P = 0.13).
DISCUSSION
As PSMA-targeted radiotracers continue to be used in the detection of prostate cancer lesions in a wide variety of clinical settings, it will become increasingly important to understand the quantitative and semiquantitative aspects of these new agents. When PSMA-guided focal therapies such as stereotactic body radiation gain further clinical acceptance, and as PSMA-based endoradiotherapy continues to be used in more locations for widely metastatic disease (25,26), the need to quantitatively evaluate tumors and their response to therapy will increase.
PSMA is also consistently and abundantly expressed on the neovascular endothelium in a wide variety of human solid tumors (27–30), suggesting that agents such as 18F-DCFPyL may be used as general radiotracers for oncologic imaging. This has recently been demonstrated for metastatic clear cell renal cell carcinoma (11,12,31). For these reasons, we addressed the semiquantitative aspects of imaging with 18F-DCFPyL as they relate to normal-organ variability.
The levels of variation in normal organs on 18F-DCFPyL PET scans must be known so that changes in uptake in malignant lesions can be confidently attributed to a change in disease or therapeutic response as opposed to the intrinsic variability of the scan. In 18F-FDG PET studies, the quantitative framework of PERCIST 1.0 required a 30% decline in SUV for a tumor to be considered to have a true response (16). Liver was chosen as the basis of quantitative reliability in PET images given its moderate level of 18F-FDG uptake in normal parenchyma and the low variability in uptake relative to other organs (16). In a metaanalysis on the repeatability of 18F-FDG uptake measurements in tumors, a minimal relative change of 20% in combination with a 1.2-unit change in SUVmean was presumed to represent a biologic change (32).
Here, we assessed the variability of 18F-DCFPyL uptake in a variety of solid organs (salivary glands, lacrimal glands, liver, spleen, and kidneys). Analogous to 18F-FDG, uptake in the normal liver was moderate and appeared visually homogeneous. Uptake in the liver had the lowest variability of any of the organs included in this study (COV of 13.8% when using SUVmean and 14.5% when using SULmean, both of which are lower than the 21%–23% COVs previously reported for 18F-FDG PET in liver (24)). These data imply that 18F-DCFPyL PET images, and perhaps PSMA-targeted PET images in general, can be quantified reliably and possibly with higher fidelity than the corresponding 18F-FDG images.
A spheric 3-cm-diameter VOI in the normal-liver parenchyma accurately represented uptake within the entire liver volume, with no significant difference in average uptake when either SUVmean or SULmean was used (P > 0.05). This is an important consideration in the clinical translation of the principles of this study given the impracticality of delineating whole-organ VOIs during clinical workflow.
Uptake seen on PET scans is commonly normalized to body mass (SUV) or lean body mass (SUL). SUL is preferred by many centers over SUV for quantification in 18F-FDG studies because there is a strong positive correlation between the blood SUV and body mass (r = 0.705, P < 0.001), but SUL has shown no correlation with body mass (r = −0.010, P = 0.904) (23,33). In the course of our evaluation of normal-organ uptake variability, we investigated both SUV and SUL for 18F-DCFPyL. We calculated the correlation between both SUVmean and SULmean and body mass for the liver, and the scatterplots showed no significant correlation (r = 0.195, P = 0.119, for SUVmean; r = −0.190, P = 0.130, for SULmean). We concluded from these findings that both SUVmean and SULmean can be used in the assessment of 18F-DCFPyL uptake, although the lower normal-organ variability calculated with SUVmean (COV 13.76% vs. COV 14.47% with SULmean) would argue that SUV should be used.
A prospective test–retest study that scans patients twice with 18F-DCFPyL within a short period and without intervening therapy will ultimately be needed to assess the ground-state variability of 18F-DCFPyL in normal organs and tumors and to continue to lay the groundwork for threshold cutoffs for determining response to therapy. Given the similarities in biodistribution between 18F-DCFPyL and other PSMA-based radiotracers such as the 68Ga-labeled agents (4–7), it is likely that quantitative and semiquantitative principles learned with one such radiotracer will apply broadly to other agents in this class. Even without dedicated test–retest studies, the data in this study suggest that semiquantitative analysis is both feasible and promising.
Potential limitations of this study include the possibility that partial-volume effects at the periphery of organs will degrade the reliability of average uptake measurements and increase variability, although we believe such effects are likely minimal given the similarity in both absolute uptake and variability between whole-liver VOIs and spheric VOIs in the middle of the liver parenchyma. Any partial-volume effects at the edge would likely be greatest for smaller organs such as the lacrimal glands. With regard to nearby organs, such as the liver and right kidney or the spleen and left kidney, these could cause some overlap in uptake that would be difficult to avoid when drawing whole-organ VOIs.
Furthermore, we included patients undergoing imaging for multiple indications and with a widely ranging amount of radiotracer-avid disease, potentially increasing the measured variability in normal-organ uptake through redistribution of radiotracer to sites of disease. Including such a breadth of patients, however, increases the generalizability of the findings, as the variability results described herein should be applicable to many different patients undergoing PSMA-targeted imaging.
We should also note that we have previously advocated imaging with 18F-DCFPyL at 2 h after injection because of the possibility of identifying subtle lesions at a later time point as well as improving tumor-to-background ratios (34); however, for reasons of clinical expediency, we have generally imaged patients at 1 h after injection. Even greater stability in normal-organ uptake variation might be achievable at a later time point, but we have not specifically investigated this possibility.
CONCLUSION
Variability in normal-liver uptake was less for 18F-DCFPyL than has been shown for 18F-FDG. This finding implies that 18F-DCFPyL PET images, and perhaps PSMA-targeted PET images in general, can be reliably quantified, laying the groundwork for future studies involving therapeutic monitoring. Neither SUVmean nor SULmean correlated significantly with body mass; however, the variability in liver uptake calculated from SUVmean was marginally less than that calculated from SULmean, favoring adoption of SUV for 18F-DCFPyL PSMA-targeted PET scans.
DISCLOSURE
This work was supported by the National Institutes of Health (grants CA134675 and CA183031) and by philanthropic funds provided to the James Buchanan Brady Urological Institute and Department of Urology. Martin G. Pomper is a coinventor on a U.S. patent covering 18F-DCFPyL and as such is entitled to a portion of any licensing fees and royalties generated by this technology. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict-of-interest policies. No other potential conflict of interest relevant to this article was reported.
Supplementary Material
REFERENCES
- 1.Perner S, Hofer MD, Kim R, et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38:696–701. [DOI] [PubMed] [Google Scholar]
- 2.Haberkorn U, Eder M, Kopka K, et al. New strategies in prostate cancer: prostate-specific membrane antigen (PSMA) ligands for diagnosis and therapy. Clin Cancer Res. 2016;22:9–15. [DOI] [PubMed] [Google Scholar]
- 3.Silver DA, Pellicer I, Fair WR, et al. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 4.Afshar-Oromieh A, Malcher A, Eder M, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–495. [DOI] [PubMed] [Google Scholar]
- 5.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. [DOI] [PubMed] [Google Scholar]
- 6.Afshar-Oromieh A, Avtzi E, Giesel FL, et al. The diagnostic value of PET/CT imaging with the 68Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eder M, Neels O, Müller M, et al. Novel preclinical and radiopharmaceutical aspects of [68Ga]Ga-PSMA-HBED-CC: a new PET tracer for imaging of prostate cancer. Pharmaceuticals (Basel). 2014;7:779–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho SY, Gage KL, Mease RC, et al. Biodistribution, tumor detection, and radiation dosimetry of 18F-DCFBC, a low-molecular-weight inhibitor of prostate-specific membrane antigen, in patients with metastatic prostate cancer. J Nucl Med. 2012;53:1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rowe SP, Gage KL, Faraj SF, et al. 18F-DCFBC PET/CT for PSMA-based detection and characterization of primary prostate cancer. J Nucl Med. 2015;56:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowe SP, Macura KJ, Ciarallo A, et al. Comparison of prostate-specific membrane antigen-based 18F-DCFBC PET/CT to conventional imaging modalities for detection of hormone-naïve and castration-resistant metastatic prostate cancer. J Nucl Med. 2016;57:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of [18F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Pullambhatla M, Foss CA, et al. 2-(3-{1-carboxy-5-[(6-[18F]fluoropyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011;17:7645–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez-Crespo A. Comparison of gallium-68 and fluorine-18 imaging characteristics in positron emission tomography. Appl Radiat Isot. 2013;76:55–62. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Foss CA, Byun Y, et al. Radiohalogenated prostate-specific membrane antigen (PSMA)-based ureas as imaging agents for prostate cancer. J Med Chem. 2008;51:7933–7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O JH, Lodge MA, Wahl RL. Practical PERCIST: a simplified guide to PET response criteria in solid tumors 1.0. Radiology. 2016;280:576–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahl RL, Jacene H, Kasamon Y, et al. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avril S, Muzic RF, Jr, Plecha D, et al. 18F-FDG PET/CT for monitoring of treatment response in breast cancer. J Nucl Med. 2016;57(suppl 1):34S–39S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheikhbahaei S, Wray R, Young B, et al. 18F-FDG-PET/CT therapy assessment of locally advanced pancreatic adenocarcinoma: impact on management and utilization of quantitative parameters for patient survival prediction. Nucl Med Commun. 2016;37:231–238. [DOI] [PubMed] [Google Scholar]
- 19.Rowe SP, Mana-Ay M, Javadi MS. PSMA-based detection of prostate cancer bone lesions with 18F-DCFPyL PET/CT: a sensitive alternative to 99mTc-MDP bone scan and Na18F PET/CT? Clin Genitourin Cancer. 2016;14:e115–e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowe SP, Vicente E, Anizan N, et al. Repeatability of radiotracer uptake in normal abdominal organs with 111In-pentetreotide quantitative SPECT/CT. J Nucl Med. 2015;56:985–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batallés SM, Villavicencio RL, Quaranta A, et al. Variations of the hepatic SUV in relation to the body mass index in whole body PET-CT studies. Rev Esp Med Nucl Imagen Mol. 2013;32:26–32. [DOI] [PubMed] [Google Scholar]
- 22.Kuhnert G, Boellaard R, Sterzer S, et al. Impact of PET/CT image reconstruction methods and liver uptake normalization strategies on quantitative image analysis. Eur J Nucl Med Mol Imaging. 2016;43:249–258. [DOI] [PubMed] [Google Scholar]
- 23.Sugawara Y, Zasadny KR, Neuhoff AW, Wahl RL. Reevaluation of the standardized uptake value for FDG: variations with body weight and methods for correction. Radiology. 1999;213:521–525. [DOI] [PubMed] [Google Scholar]
- 24.Viner M, Mercier G, Hao F, et al. Liver SULmean at FDG PET/CT: interreader agreement and impact of placement of volume of interest. Radiology. 2013;267:596–601. [DOI] [PubMed] [Google Scholar]
- 25.Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016;57:1170–1176. [DOI] [PubMed] [Google Scholar]
- 26.Zechmann CM, Afshar-Oromieh A, Armor T, et al. Radiation dosimetry and first therapy results with a 124I/131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41:1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang SS, Reuter VE, Heston WD, et al. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor associated neovasculature. Cancer Res. 1999;59:3192–3198. [PubMed] [Google Scholar]
- 28.Chang SS, O’Keefe DS, Bacich DJ, et al. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin Cancer Res. 1999;5:2674–2681. [PubMed] [Google Scholar]
- 29.Schülke N, Varlamova OA, Donovan GP, et al. The homodimer of prostate specific membrane antigen is a functional target for cancer therapy. Proc Natl Acad Sci USA. 2003;100:12590–12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haffner MC, Kronberger IE, Ross JS, et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum Pathol. 2009;40:1754–1761. [DOI] [PubMed] [Google Scholar]
- 31.Rowe SP, Gorin MA, Hammers HJ, et al. Imaging of metastatic clear cell renal cell carcinoma with PSMA-targeted 18F-DCFPyL PET/CT. Ann Nucl Med. 2015;29:877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Langen AJ, Vincent A, Velasquez LM, et al. Repeatability of 18F-FDG uptake measurements in tumors: a meta-analysis. J Nucl Med. 2012;53:701–708. [DOI] [PubMed] [Google Scholar]
- 33.Zasadny KR, Wahl RL. Standardized uptake values of normal tissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: variations with body weight and a method for correction. Radiology. 1993;189:847–850. [DOI] [PubMed] [Google Scholar]
- 34.Rowe SP, Macura KJ, Mena E, et al. PSMA-based [18F]DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol Imaging Biol. 2016;18:411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.