Abstract
Myocardial blood flow (MBF) is the critical determinant of cardiac function. However, its response to increases in partial pressure of arterial CO2 (PaCO2), particularly with respect to adenosine, is not well characterized because of challenges in blood gas control and limited availability of validated approaches to ascertain MBF in vivo. Methods: By prospectively and independently controlling PaCO2 and combining it with 13N-ammonia PET measurements, we investigated whether a physiologically tolerable hypercapnic stimulus (∼25 mm Hg increase in PaCO2) can increase MBF to that observed with adenosine in 3 groups of canines: without coronary stenosis, subjected to non–flow-limiting coronary stenosis, and after preadministration of caffeine. The extent of effect on MBF due to hypercapnia was compared with adenosine. Results: In the absence of stenosis, mean MBF under hypercapnia was 2.1 ± 0.9 mL/min/g and adenosine was 2.2 ± 1.1 mL/min/g; these were significantly higher than at rest (0.9 ± 0.5 mL/min/g, P < 0.05) and were not different from each other (P = 0.30). Under left-anterior descending coronary stenosis, MBF increased in response to hypercapnia and adenosine (P < 0.05, all territories), but the effect was significantly lower than in the left-anterior descending coronary territory (with hypercapnia and adenosine; both P < 0.05). Mean perfusion defect volumes measured with adenosine and hypercapnia were significantly correlated (R = 0.85) and were not different (P = 0.12). After preadministration of caffeine, a known inhibitor of adenosine, resting MBF decreased; and hypercapnia increased MBF but not adenosine (P < 0.05). Conclusion: Arterial blood CO2 tension when increased by 25 mm Hg can induce MBF to the same level as a standard dose of adenosine. Prospectively targeted arterial CO2 has the capability to evolve as an alternative to current pharmacologic vasodilators used for cardiac stress testing.
Keywords: hypercapnia, myocardial perfusion, coronary artery disease, cardiac stress testing
More than 5 million cardiac stress tests performed annually in the United States use intravenously administered pharmacologic stress agents, such as adenosine or one of its analogs. However, these drugs have well-known side effects (1). Moreover, consumption of caffeine confounds cardiac stress tests, as it is a potent nonselective inhibitor of receptors in the coronary arteries that counteracts the downstream effects of commonly used pharmacologic vasodilators, such as adenosine. Hence, alternative pharmacologic vasodilators for cardiac stress testing are highly desirable.
One such potential coronary vasodilator is arterial carbon dioxide (CO2), because changes in arterial blood CO2 tension (PaCO2) are known to modulate smooth muscle relaxation (2). To date, a large number of studies have investigated the effect of PaCO2 on myocardial blood flow (MBF) (3–10), but their conclusions on the sensitivity of CO2 to invoke an increase in MBF have been variable. Specifically, studies have reported that an increase in PaCO2 has marked (3,4), minimal (6,7), or no (8,11) effect on MBF. These findings may be explained by one or more of the commonly shared aspects of these studies: an indistinct stimulus due to the inability to independently, precisely, and rapidly control PaCO2; confounding physiologic effects resulting from the use of isolated heart-lung or in situ preparations; and uncertain measures of the outcome variable due to limitations of the surrogate metrics of MBF.
The objectives of this study were 2-fold: the first aim was to investigate the effects of PaCO2 on MBF while minimizing contributions from factors that can unintentionally reduce or inaccurately report on sensitivity of PaCO2 on MBF; and the second aim was to assess whether an independent, precise, and rapid establishment of a physiologically tolerable level of hypercapnia could produce hyperemia equivalent to that caused by adenosine, a commonly used pharmacologic stimulus for cardiac stress testing, with and without preadministration of caffeine.
MATERIALS AND METHODS
To address the aims of this study, we used a clinically relevant animal model along with validated strategies for precisely and rapidly establishing desired levels of PaCO2, while holding PaO2 constant; quantifying MBF in vivo; and analyzing images to derive MBF values across the different coronary supply territories. We compared our findings to the effects of a standard dose of adenosine in the same animal models with and without coronary stenosis to quantify MBF and flow deficit regions under peak tolerable PaCO2. To determine whether MBF response to PaCO2 overlapped the same mechanistic path as adenosine, we quantified MBF under hypercapnia and adenosine after caffeine administration.
Animal Model and Preparation
Study Groups
Three groups of animals were studied: intact animals (group intact, n = 10), animals were fasted for 18 h, sedated and anesthetized with propofol (2.0–5.0 mg/kg, intravenously), and intubated; animals with coronary stenosis (group stenosis, n = 10), in addition to the above preparation, each animal was implanted with an occluder to implement stenosis of the left-anterior descending coronary artery (LAD), as previously described (12); and preadministration of intact animals with caffeine (group caffeine, n = 5), where animals were prepared as described for group intact but imaging was performed before and 10 min after caffeine administration (5 mg/kg, intravenously).
Anesthesia and Ventilation
Animals were transferred to the scanner table and were initially mechanically ventilated. A secondary sequential gas delivery circuit, functionally similar to that used for spontaneous ventilation, was interposed between the animal and the ventilator to enable end-tidal prospective gas control as previously described (12). Electrocardiogram, peripheral blood oxygenation (SpO2), heart rate (3-lead electrocardiogram), and blood pressure (diastolic, systolic, and mean based on pressure cuffs) were monitored. During the imaging studies, anesthesia was maintained with a continuous infusion of low-dose propofol (0.03–0.1 mg/kg/min, intravenously).
Targeting of PaCO2 and PaO2
Prospective targeting of PaCO2 and PaO2 was accomplished using an algorithm-driven computerized gas blender administering gases to a sequential gas delivery breathing circuit (RespirAct; Thornhill Research Inc.) as previously described (12,13). At rest and during adenosine infusion, end-tidal PCO2 (PETCO2) was targeted at approximately 35 mm Hg, and during hypercapnia end-tidal PO2 (PETO2) was targeted at about 60 mm Hg, all while targeting isoxia at PETO2 at about 125 mm Hg.
Stenosis Studies
In group stenosis, imaging was performed within 2 min of establishing non–flow-limiting stenosis at rest by ensuring that occlusion did not lead to ST elevation at rest. In addition, first-pass perfusion MRI (14) at rest before release of occlusion to determine flow abnormalities, cine MRI to ascertain any wall motion abnormalities or edema (15), and late-gadolinium enhancement MRI to rule out myocardial infarction were performed (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). Three of the 10 animals from group stenosis showed evidence of rest perfusion defects and mild subendocardial evidence of late-gadolinium enhancement in the anterior lateral wall and were excluded from further analysis. Thus, a total of 8 animals from group stenosis were included in the analysis.
Noninvasive Assessment of MBF with 13N PET
PET images were acquired using a clinical 3-T PET/MR system (Biograph mMR; Siemens Healthcare) in 3-dimensional list-mode using 13N-ammonia (200 MBq, intravenous bolus [3–5 s]) as the blood flow tracer. Before each PET scan, MR images were acquired to correct for photon attenuation. Attenuation correction was performed using 2-point Dixon MR images as previously described (16,17). Data acquisition spanned over 10 min and began a few seconds before the 13N-ammonia injection. In group intact, images were acquired during hypercapnia and adenosine infusion at rest. Specifically, under adenosine, PET acquisitions were prescribed after 2 min of adenosine infusion; and under hypercapnia, 1 min after the desired PETCO2 value was reached. A time delay was introduced between the sequential PET acquisitions at each physiologic condition to ensure sufficient decay of each 13N-ammonia dose (5 half-lives, ∼50 min). In group stenosis, images were acquired at rest and under hypercapnia and adenosine before and after infliction of LAD coronary stenosis. In group caffeine, rest scans were acquired before and after caffeine administration. This was followed by scans under hypercapnia and adenosine after caffeine administration. Other aspects of the imaging protocols implemented in groups stenosis and caffeine were similar to that implemented in group intact. The order of adenosine and hypercapnia stimulations was randomized, and the animals were allowed to recover to baseline PETCO2 between the protocols. A schematic representation of the sequential execution of the study protocols in the 3 different groups of animals is provided in Supplemental Figure 2.
PET Reconstruction and Quantifications of MBF and Myocardial Perfusion Reserve (MPR)
Sixteen dynamic PET frames were reconstructed (12 × 10 s, 2 × 30 s, 1 × 1 min, and 1 × 6 min frames, for a total of 10 min). Standard reconstruction (2-dimensional attenuation-weighted ordered-subsets expectation maximization) was used with 3 iterations and 14 subsets and 3-dimensional postfiltering with a 5-mm gaussian kernel (18). Transverse data were reformatted to a 168 × 168 × 47 matrix with 2-mm pixels for each dynamic frame. Late perfusion images were reconstructed using 7 min of the acquisition after a 2-min delay to allow for blood-pool clearance. The reconstruction parameters were identical to the dynamic reconstruction.
Global and regional MBF and MPR values were derived automatically from the PET data using the automated QPET software (Cedars-Sinai Medical Center, Los Angeles), as described previously (18,19). MPR was computed as a ratio, by dividing each stress polar map sample by the corresponding rest sample at each point, and was corrected by adjusting the resting flow by the resting rate–pressure product in QPET. The global MBF at stress and rest was computed within the whole LV region bounded by the LV plane. The regional MPR was then obtained by dividing the polar map into 3 regions (LAD, left circumflex coronary artery [LCx], and right coronary artery [RCA]) using the 17-segment model of the American Heart Association. Regional and global MPR values were extracted automatically for further analysis.
Quantification of Total Reduction in Perfusion Volume and Visual Scoring
The myocardial perfusion defect territory of group stenosis was measured using the stress–rest change analysis in QPET software as previously described (18,20). Total reduction in myocardial perfusion volume was derived as a fraction of the total LV myocardial volume (TRP, %LV). The extent of perfusion defect was also measured by consensus of 2 observers following guidelines of the American Society of Nuclear Cardiology. Images were scored using the 17-segment model and 5-point scoring (0, normal perfusion; 1, mild count reduction; 2, moderate count reduction; 3, severe count reduction; 4, absent uptake). Accuracy, sensitivity, and specificity for perfusion defect detection with hypercapnia were evaluated against adenosine using visual scores of 3 or greater as the cutoff for disease.
Statistical Analysis
All statistical analyses were performed using SPSS (version 21.0; IBM). Statistical significance was established for a P value of less than 0.05. All results are reported as mean ± SD. Two-way repeated-measurement ANOVA tests were used to examine the regional MBF and MPR in response to hypercapnia and adenosine. One-way repeated-measurement ANOVA tests were used to examine the differences of global MBF (at rest, hypercapnia, and adenosine) and hemodynamic indices. For both tests, post hoc comparison with Bonferroni adjustment was used if the null hypothesis was rejected. Visual scores of perfusion defect determined with hypercapnia and adenosine were compared using a paired-sampled Wilcoxon signed-rank test. Global MPR from all studies and TRP with hypercapnia and adenosine were compared using a paired t test. An equivalence test was performed with a confidence interval of 90% to determine whether the global MBF and MPR were different under adenosine and hypercapnia in group intact. Linear correlation analyses were performed between hypercapnia and adenosine to compare regional MBF and MPR and TRP across relevant groups. Bland–Altman analysis was performed to determine the limits of agreement and bias in MBF, MPR, and TRP between hypercapnia and adenosine.
RESULTS
Prospectively Targeted Hypercapnia as Potent Stimulator of MBF
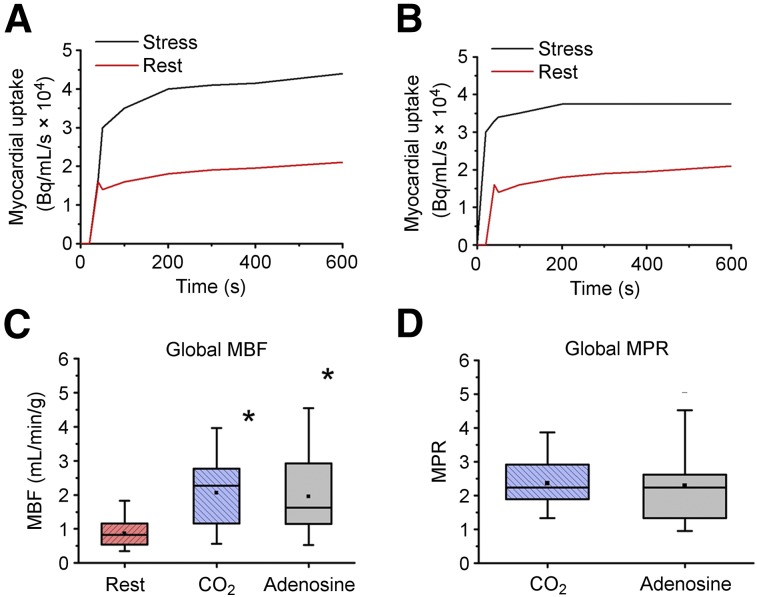
Table 1 summarizes the mean arterial CO2, O2, and hemodynamic variables of interest in group intact. Figure 1 shows the mean global MBF and MPR response to hypercapnia in relation to adenosine (numeric values are tabulated in Supplemental Table 1). Global MBF values under adenosine and hypercapnia were both higher than at rest (P < 0.05, for both) and were not different from one another (P = 0.33). Global MBF increases under adenosine and hypercapnia were equivalent, with a margin of equivalence of 0.26 mL/min/g (1/3 of the SD of the difference in MBF, 0.8 mL/min/g) at α = 0.05. Global MBF values normalized by rate–pressure product under adenosine (1.16 × 10−5 ± 0.80 × 10−5) and hypercapnia (1.26 × 10−5 ± 0.56 × 10−5) were significantly different from rest (0.67 × 10−5 ± 0.33 × 10−5, P < 0.05 for both) but were not different from one another (P = 1.00). Mean global MPRs with adenosine and hypercapnia were significantly greater than 2 and were equivalent with a margin of equivalence of 0.50 (1/3 of the SD of the difference in MPR, 1.54) at α = 0.05. The mean global MPRs did not differ under hypercapnia and adenosine (P = 1.00). Similar observations were evident for regional MBF and MPR with hypercapnia and adenosine (Supplemental Fig. 3). The observed range of MBF at rest (0.44–1.93 mL/min/g) and adenosine (0.47–5.10 mL/min/g) and range of MPR under adenosine (1.20–4.57) across the animals are consistent with previous reports (21). Notably, these results indicate that an increase in MBF is not different from that are observed with adenosine and is not attributable to changes in myocardial oxygen consumption (work) indexed by rate–pressure product.
TABLE 1.
Targeted Arterial Blood Gas Estimates and Hemodynamic Parameters
| Group | SBP (mm Hg) | HR (per min) | RPP (mm Hg/min × 103) | ||
| Intact (n = 10) | |||||
| PETCO2 (mm Hg) | PETO2 (mm Hg) | ||||
| Adenosine | 37.5 ± 2.9 | 119.9 ± 7.0 | 128.6 ± 16.8 | 99.6 ± 34.7* | 12.3 ± 4.9* |
| Hypercapnia | 60.6 ± 1.4* | 121.3 ± 5.8 | 130.2 ± 14.3 | 81.8 ± 16.8 | 10.9 ± 2.4 |
| Rest | 36.9 ± 1.6 | 118.3 ± 5.2 | 121.2 ± 12.1 | 65.4 ± 15.2 | 8.2 ± 2.3 |
| Stenosis (n = 7) | |||||
| PaCO2 (mm Hg) | PaO2 (mm Hg) | ||||
| Adenosine | 37.3 ± 2.2 | 128.0 ± 4.9 | 111.6 ± 32.2 | 119.8 ± 26.9* | 14.9 ± 6.1* |
| Hypercapnia | 61.6 ± 1.1* | 124.7 ± 5.6 | 118.1 ± 25.2 | 100.2 ± 22.8 | 12.5 ± 5.7* |
| Rest | 35.9 ± 3.4 | 126.1 ± 4.4 | 118.0 ± 30.5 | 75.4 ± 17.0 | 8.9 ± 2.9 |
| Caffeine (n = 5) | |||||
| PaCO2 (mm Hg) | PaO2 (mm Hg) | ||||
| Adenosine | 35.1 ± 0.6 | 123.7 ± 3.0 | 125.8 ± 8.0 | 65.4 ± 29.9 | 6.9 ± 1.7 |
| Hypercapnia | 59.9 ± 2.3* | 128.9 ± 6.8 | 128.3 ± 11.6 | 69.8 ± 10.8 | 9.3 ± 3.6 |
| Rest | 34.5 ± 1.2 | 125 ± 3.6 | 124.0 ± 11.2 | 56.6 ± 23.2 | 7.1 ± 4.0 |
P < 0.05 in comparison to rest values.
SBP = systolic arterial blood pressure; HR = heart rate; RPP = rate–pressure product (mean arterial pressure × HR).
FIGURE 1.
Global and regional MBF response to hypercapnia and adenosine in intact canines. (A and B) Corresponding dynamic radiotracer uptake curves, which show increased myocardial uptake responses to hypercapnia and adenosine stresses relative to rest. (C and D) Global mean MBF and corresponding MPR at rest and under hypercapnia and adenosine. *P < 0.05.
Prospectively Targeted Hypercapnia for Identifying Regional Impairments in MBF and MPR
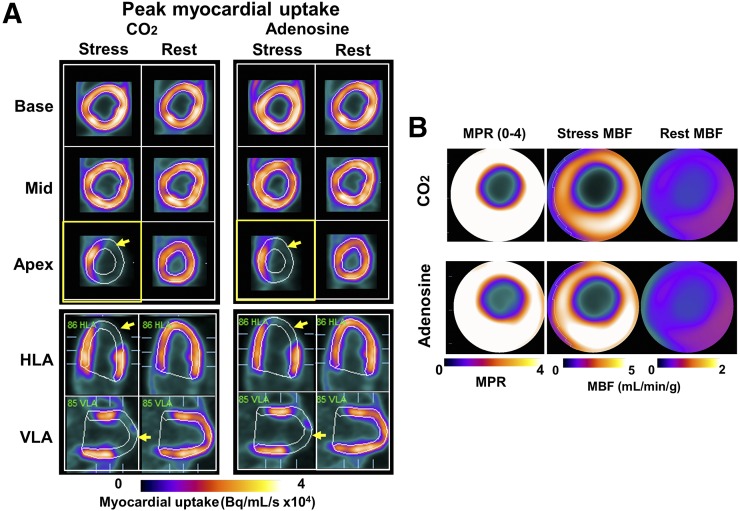
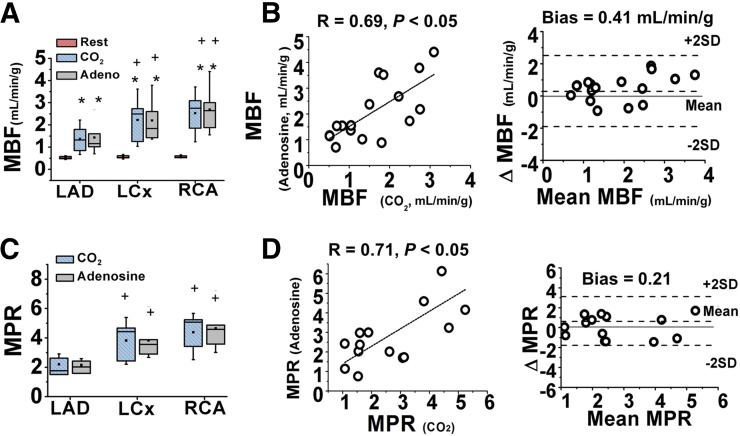
Table 1 summarizes the estimates of mean arterial CO2, O2, and hemodynamic variables of interest in group stenosis. Figure 2 shows the mean global and regional MBF and MPR response to hypercapnia in relation to adenosine (numeric values are tabulated in Supplemental Table 2). Figure 3 shows the mean regional MBF in LAD, LCx, and RCA supply territories at rest, hypercapnia, and adenosine. MBF values were not different at rest among the different supply territories (P > 0.4, for all). MBF increased under hypercapnia and adenosine (P < 0.05, for all territories), albeit the increase in the LAD territory was significantly lower than in the LCx and RCA territories (with hypercapnia and adenosine; both P < 0.05). MBF under hypercapnia was not different between the LCx and RCA territories (hypercapnia, P = 0.21); the same was true under adenosine (P = 0.50). For each myocardial supply territory, MBF under hypercapnia and adenosine was not different (P = 1.00, for all). Collective comparisons of regional MBF between hypercapnia and adenosine showed significant correlation (R = 0.69, P < 0.05) and good agreement (bias = 0.41 mL/min/g). MPR values were not higher than 2.0 in LAD territories (hypercapnia, P = 0.48, and adenosine, P = 0.52) but were higher than 2.0 in the LCx and RCA (P < 0.05 for hypercapnia and adenosine) territories. MPR under hypercapnia was not different between the LCx and RCA territories (hypercapnia, P = 0.59); the same was true under adenosine (P = 0.34). For each myocardial supply territory, MPRs under hypercapnia and adenosine were not different (P > 0.5 for all). Collective comparisons of regional MPR between hypercapnia and adenosine showed significant correlation (R = 0.71, P < 0.05) and good agreement (bias = 2.11%).
FIGURE 2.
Regional MBF response to hypercapnia and adenosine in presence of coronary stenosis. (A) Representative short- and long-axis PET images of peak myocardial uptake of 13N-ammonia during hypercapnia of PaCO2 ∼60 mm Hg (CO2), standard clinical dose of adenosine (Adenosine) and at rest with PaCO2 ∼35 mm Hg (Rest) in a canine with LAD stenosis. Note lower uptake of radiotracer in anterior lateral wall (lower signal in distal LAD segments, yellow arrows) under hypercapnia and adenosine. For case in A, rest and stress MBF (under hypercapnia and adenosine) and corresponding MPR are shown as polar maps in B. These images show marked reduction in MBF and MPR in LAD territory, which are visually evident and spatially concordant under hypercapnia and adenosine. HLA = horizontal long axis; VLA = vertical long axis.
FIGURE 3.
Quantitative measurements of regional MBF response to hypercapnia and adenosine in presence of coronary stenosis. (A and B) Mean regional MBF at rest, hypercapnia, and adenosine. Regional MBF under hypercapnia and adenosine showed good correlation and agreement. (C and D) Corresponding MPR under hypercapnia and adenosine with similar results. *P < 0.05 compared with conditions of rest. +P < 0.05 compared with LAD under stress.
Perfusion Defect Volumes and Visual Scoring Under Hypercapnia Versus Adenosine
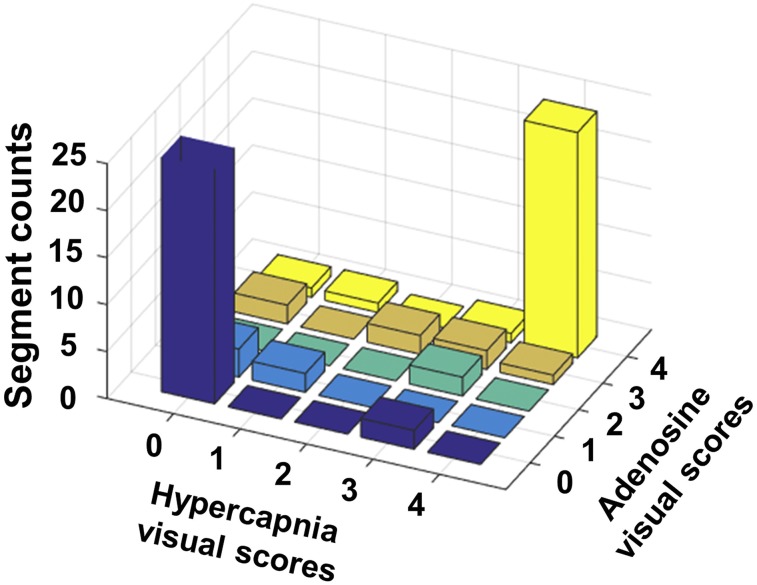
The total reduction in perfusion volume (TRP, %LV) between stress and rest states is shown in Figure 4. TRP obtained under hypercapnia and adenosine was not different: 25% ± 19% (hypercapnia) versus 27% ± 15% (adenosine) (P = 0.12). Direct comparisons of TRP within the same subjects under hypercapnia and adenosine were highly correlated (R = 0.85, P < 0.05) and were in good agreement (bias = 2%). Similar trends were observed from visual scoring analysis. No difference between the scores from hypercapnia and adenosine were observed from a paired-sample Wilcoxon signed-rank test (P = 0.26). Visual scores between hypercapnia and adenosine were concordant, with most segments falling onto the diagonal of the scoring matrix (Fig. 5). Hypercapnia also showed high accuracy (0.95), sensitivity (0.89), and specificity (0.98) for detecting affected segments compared with adenosine.
FIGURE 4.
Total myocardial perfusion defect due to coronary stenosis under hypercapnia and adenosine. (A) Perfusion defects detected from change analysis estimated from time-averaged myocardial uptake images at rest and stress (hypercapnia and adenosine), and polar images highlighting total perfusion defects (right). Note near identical correspondence in perfusion defect territories identified in slices and whole heart under hypercapnia and adenosine. (B) Mean TRP (%LV) under hypercapnia and adenosine. No significant difference in TRP (%LV) was observed under hypercapnia and adenosine. (C and D) Results from linear regression and Bland–Altman analyses.
FIGURE 5.
Visual scoring of perfusion defects under hypercapnia and adenosine in presence of LAD coronary stenosis. Visual scoring (counts) from segments in stenosis studies are presented in 3-dimensional bar plot. Excellent correspondence in visual scoring between hypercapnia and adenosine is observable (high counting rates along diagonal).
Effect of Preadministration of Caffeine on MBF Under Hypercapnia Versus Adenosine
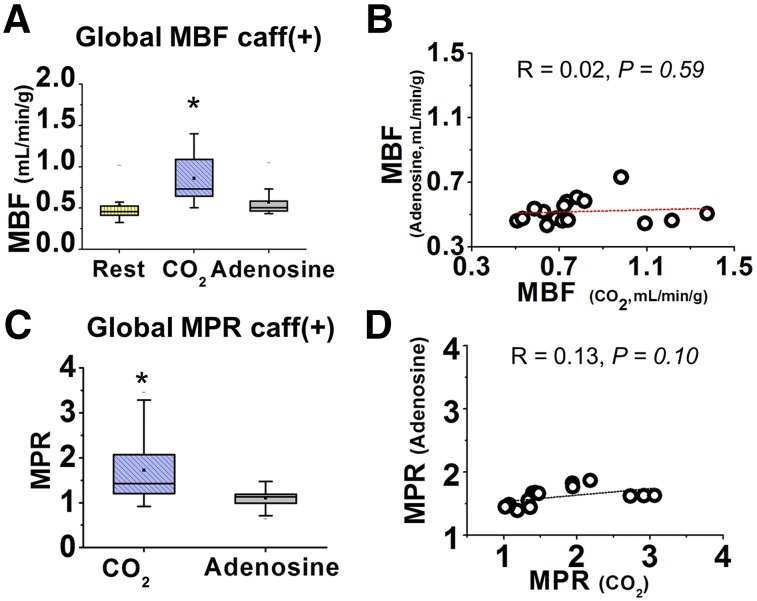
Table 1 summarizes the estimates of mean arterial CO2, O2, and hemodynamic variables of interest in group caffeine. Mean global MBF and MPR after pre- and postcaffeine administration are shown in Figures 6 and 7 (numeric values are tabulated in Supplemental Table 3). Results showed that although there is no change in MBF between rest and adenosine, hypercapnia was able to induce myocardial hyperemia. There was a trend toward higher resting MBF before caffeine administration, but this was not statistically significant (P = 0.09). However, the resting MBF normalized by rate–pressure product was significantly higher before caffeine (1.5 × 10−5 [preadministration] vs. 1.0 × 10−5 [postadministration], P = 0.03). These observations are consistent with reports in humans (22) and are likely related to the influence of caffeine on calcium cycling at rest, which is known to promote vascular smooth muscle contraction (23,24). Mean global MBF at rest (postcaffeine) and under adenosine was not different (P = 1.00) and was significantly lower than hypercapnia (P < 0.05, for both). Under caffeine, there was no correlation between MBF under adenosine and hypercapnia (R = 0.02, P = 0.59). Under caffeine, the global MPR under adenosine was lower than under hypercapnia (P < 0.05). Regional MPR regressed against adenosine, and hypercapnia showed a weak and nonsignificant correlation (R = 0.13, P = 0.10). These findings of differential MBF response to hypercapnia and adenosine after the preadministration of caffeine suggest that the mechanism of action mediating myocardial hyperemia by these stimuli are at least partly different.
FIGURE 6.
Global and regional MBF response to hypercapnia and adenosine after caffeine administration. (A) Representative short- and long-axis PET images acquired during peak myocardial uptake of 13N-ammonia under hypercapnia of PaCO2 ∼60 mm Hg (CO2), standard adenosine dose (Adenosine) and at baseline conditions with PaCO2 ∼35 mm Hg (Rest) after caffeine administration. These visual results show that increase in myocardial uptake of radiotracer relative to rest to occur only under hypercapnia, but not under adenosine. For the case in A, rest and stress MBF (under hypercapnia and adenosine) and corresponding MPR are shown as polar maps in B. (C) MBF at rest before (Caff(−)) and after (Caff(+)) caffeine administration. HLA = horizontal long axis; VLA = vertical long axis.
FIGURE 7.
MBF and MPR response under hypercapnia and adenosine after caffeine administration. (A) Global and regional mean MBF at rest and under hypercapnia and adenosine after caffeine infusion (Caff+). (B) Results from linear regression analysis between regional MBF under adenosine and hypercapnia. (C and D) Corresponding MPR response. *P < 0.05.
DISCUSSION
Although the arterial pressure of CO2 is known to regulate MBF, the extent of its effect on MBF, especially in relation to adenosine, has not been quantified in the presence or absence of coronary stenosis. In particular, previous investigations have not been able to isolate the effects of PaCO2 on MBF primarily because it has not been possible to independently and rapidly control PaCO2. This limitation, coupled with limited access to noninvasive quantification of MBF changes with PET, made it difficult to determine whether hypercapnia could be as potent a coronary vasodilator as adenosine on the heart. We used precise, independent, and rapid control of PaCO2, 13N-ammonia PET, a validated image analysis approach, and a clinically relevant animal model to rigorously study whether a physiologically viable hypercapnic stimulus is a potent mediator of MBF. We found that when the PETCO2 is altered from rest (∼35 mm Hg) to about 60 mm Hg under isoxic conditions, MBF increases to levels observed with the clinical dose of adenosine. Specifically, these changes in MBF and MPR were both globally and regionally not different from those observed with adenosine in the absence and presence of coronary stenosis. Preadministration of caffeine abolished myocardial hyperemia to adenosine, as expected, but not to hypercapnia.
Translational Importance of Arterial CO2 Tension as Potent Modulator of MBF
Canines have been the preclinical model of choice in the literature for examining the MBF response to various pharmacologic agents (2) that are now routinely used as part of cardiac stress testing, including dipyridamole, adenosine, and regadenoson (25). Hence our finding of marked increase in MBF under isoxic hypercapnia has significant translational value especially within the framework of cardiac stress testing. Because elevated levels of PaCO2 can introduce myocardial hyperemia to the same extent as adenosine, PaCO2 has the potential to be an alternative to these pharmacologic agents. A desirable feature of hypercapnia-based vasodilation compared with pharmacologic stress agents is that the effects of PaCO2 could be rapidly reversed within 2–3 breaths with prospective gas control methods as used in the study (13). In comparison, the effects of pharmacologic agents, in particular long-acting vasodilators such as regadenoson, are only reversed with metabolic breakdown of the administered agent or after rapid delivery of antidote, such as aminophylline (26). On the other hand, hypercapnia of 25 mm Hg can induce respiratory acidosis (pH drop ∼0.1); however, previous studies have shown that such an increase in PaCO2 is safe and tolerable in humans (27,28). The major side effect of hypercapnia is the psychic feeling of dyspnea. Physiologically, the change in PaCO2 we administered is within the range of everyday experience. A previous study on more than 400 consecutive hypercapnic tests for brain vascular reactivity (29) in patients ranging in age from 9 to 88 y with many non–life-threatening comorbidities reported transient symptoms, such as shortness of breath, headache, and dizziness, to be evident in 11.1% of subjects. Since then, the group has performed more than 1,200 such studies on patients with cerebrovascular disease and accompanying comorbidities (Joseph A. Fisher, oral communication, December 2016). Notably, the presence of coronary artery disease is not known to affect tolerability to hypercapnia (30,31). This has been documented in several clinical publications, which have studied patients subjected to PaCO2 levels, well in excess of the proposed 25 mm Hg increase (30–32). This suggests that translating hypercapnic stimulus of 25 mm Hg in patients with coronary artery disease for the purpose of cardiac stress testing is likely feasible.
There are several advantages hypercapnia would provide over adenosine: it is noninvasive, it is inexpensive, and its onset is rapid (within 1 breath). As opposed to adenosine, its blood concentration is known continuously from the end-exhaled concentration; its level is controlled within 2 mm Hg continuously throughout the test; the termination of a CO2 stimulus occurs within 10–15 s, as does its side effects; and there is no sudden severe headache, hypotension, tachycardia, diarrhea, allergy, or interaction with other drugs. As a further margin of safety, in the absence of hypoxia, there is no lethal level of PCO2. Whereas hypercapnia in the PCO2 range of greater than 90 mm Hg may cause obtundation and even loss of consciousness, it is immediately reversible by ventilation (∼5–10 breaths). Our target range of approximately 60 mm Hg is within what is commonly experienced in daily living. Another key advantage of hypercapnia as a coronary vasodilator is that it appears to have the capacity to impart significant increase in MBF even when adenosine cannot. Although this remains to be proven in humans, if translated, it may have significant impact on cardiac stress testing, because caffeine ingestion is a common confounder in clinical cardiac stress testing. However, CO2 retainers—and people who chronically ventilate their PCO2 into the low 30s mm Hg at altitude, for that matter—do not have a chronically altered myocardial blood, renal, or cerebral blood flow. This is because there is metabolic compensation after about 8 h of a PaCO2 change, and the pH of the CO2 retainers, and baseline organ blood flows, returns to baseline. Notably, chronic obstructive pulmonary disease and CO2 retention would be a contraindication for a hypercapnic stimulus because they would not be able to mobilize the CO2 at the end of the test. In these patients, an alternate stimulus such as adenosine would be needed.
As noted earlier, a large number of studies have investigated the effects of PaCO2 on MBF. However, the reported sensitivity to invoke myocardial hyperemia, especially within the physiologically tolerable range of PaCO2, from these studies has not indicated that PaCO2 is a potent coronary vasodilator. Eberlein et al. (3) using closed-chest canines showed that when PaCO2 was altered from 45 to 82 mm Hg, the mean increase in MBF was 2.8%/mm Hg increase in PaCO2. Using 133Xe clearance, Ledingham et al. (4) showed, also in closed-chest canines, that when PaCO2 was changed from 40 to 100 mm Hg, mean MBF increase was 0.50%/mm Hg increase in PaCO2. Using 131I aminopyrin, Scheuer et al. (5) showed that when PaCO2 was increased from 38 to 91 mm Hg in canines, along with large bicarbonate infusion, the mean increase in MBF was 3.4%/mm Hg increase in PaCO2. In comparison, our closed-chest canine studies using 13N-ammonia PET showed that when PaCO2 was changed from 35 to 60 mm Hg, the increase in MBF was a 5.6%/mm Hg increase in PaCO2—nearly a 2-fold increase in coronary sensitivity to hypercapnia than the highest reported values in the literature.
The higher sensitivity of PaCO2 toward MBF we observed may be explained by the methodologic differences between our study and those of others. Previous studies were relatively slow to establish, maintain, or modulate steady-state levels of hypercapnia before making the MBF measurements (typically 10s of minutes). These time-consuming manipulations may have contributed to the reduced MBF response to hypercapnia because it has been shown that the onset and extent of MBF response to hypercapnia is fast and degrades with exposure time (4). In comparison, we established the target PaCO2 within a few seconds (2–3 breaths), initiating the MBF measurements within 1–2 min and terminating the exposure to hypercapnia within 5–6 min. Next, most of the previous studies did not tightly control PaCO2 or PaO2. This is particularly important for the studies that were performed under hyperoxia, because hyperoxia is known to cause coronary vasoconstriction, which can counteract the vasodilatory response from hypercapnia (2). In this study, we maintained isoxia and independently altered PaCO2 with a precision of 1 mm Hg. Moreover, our measurements, particularly those in intact animals, were made in the most noninvasive manner possible. These results remain to be extended into humans.
A limitation of this study is that we used the 17-segment model of the American Heart Association to define the perfusion territories, despite its inaccuracies in humans (33) and unintended use for canines. Although it is known that the coronary anatomy between canines and humans is different, the overall mapping of the coronary arteries to perfusion territories is similar to humans but not strictly the same (34). Notwithstanding this, we observed a significant MBF difference between the assigned LAD and other supply territories in group stenosis. Further, there was no statistical difference between the regional territories in groups intact and caffeine. Hence, the American Heart Association segmentation appears to be a useful model to assign MBF values. Finally, we examined only the effect of preadministration of caffeine on MBF in the absence of coronary stenosis. Further studies are needed to characterize redistribution of MBF in the presence of coronary stenosis after the preadministration of caffeine.
CONCLUSION
When the arterial partial pressure of blood CO2 is increased by 25 mm Hg, it induces MBF to the same level as a standard dose of adenosine, suggesting that prospectively targeted arterial CO2 has the capacity to evolve as a potent vasodilator for clinical cardiac stress testing.
DISCLOSURE
This work was supported in part by a grant from NIH/NHLBI (HL091989). Joseph A. Fisher, Michael Klein, and Olivia Sobczyk are part-time employees of Thornhill Research Inc., and Xiaoming Bi is an employee of Siemens Medical Solutions. No other potential conflict of interest relevant to this article was reported.
Supplementary Material
REFERENCES
- 1.FDA warns of rare but serious risk of heart attack and death with cardiac nuclear stress test drugs Lexiscan (regadenoson) and Adenoscan (adenosine). U.S. Food and Drug Administration website. https://www.fda.gov/Drugs/DrugSafety/ucm375654.htm. Updated January 16, 2016. Accessed March 8, 2017.
- 2.Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205. [DOI] [PubMed] [Google Scholar]
- 3.Eberlein HJ. Coronary blood supply and oxygen supply of the heart under various CO2 tensions and anesthetics [in German]. Arch Kreislaufforsch. 1966;50:18–87. [PubMed] [Google Scholar]
- 4.Ledingham IM, McBride TI, Parratt JR, Vance JP. The effect of hypercapnia on myocardial blood flow and metabolism. J Physiol (Lond). 1970;210:87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheuer J. The effects of respiratory and metabolic alkalosis on coronary flow, hemodynamics and myocardial carbohydrate metabolism. Cardiologia. 1968;52:275–286. [DOI] [PubMed] [Google Scholar]
- 6.van den Bos GC, Drake AJ, Noble MI. The effect of carbon dioxide upon myocardial contractile performance, blood flow and oxygen consumption. J Physiol (Lond). 1979;287:149–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzou WS, Korcarz CE, Aeschlimann SE, Morgan BJ, Skatrud JB, Stein JH. Coronary flow velocity changes in response to hypercapnia: assessment by transthoracic Doppler echocardiography. J Am Soc Echocardiogr. 2007;20:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Momen A, Mascarenhas V, Gahremanpour A, et al. Coronary blood flow responses to physiological stress in humans. Am J Physiol Heart Circ Physiol. 2009;296:H854–H861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yokoyama I, Inoue Y, Kinoshita T, Itoh H, Kanno I, Iida H. Heart and brain circulation and CO2 in healthy men. Acta Physiol (Oxf). 2008;193:303–308. [DOI] [PubMed] [Google Scholar]
- 10.Beaudin AE, Brugniaux JV, Vohringer M, et al. Cerebral and myocardial blood flow responses to hypercapnia and hypoxia in humans. Am J Physiol Heart Circ Physiol. 2011;301:H1678–H1686. [DOI] [PubMed] [Google Scholar]
- 11.Eckenhoff JE, Hafkenschiel JH, Landmesser CM. The coronary circulation in the dog. Am J Physiol. 1947;148:582–596. [DOI] [PubMed] [Google Scholar]
- 12.Yang HJ, Yumul R, Tang R, et al. Assessment of myocardial reactivity to controlled hypercapnia with free-breathing T2-prepared cardiac blood oxygen level-dependent MR imaging. Radiology. 2014;272:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito S, Mardimae A, Han J, et al. Non-invasive prospective targeting of arterial PCO2 in subjects at rest. J Physiol (Lond). 2008;586:3675–3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilke N, Simm C, Zhang J, et al. Contrast-enhanced first pass myocardial perfusion imaging: correlation between myocardial blood flow in dogs at rest and during hyperemia. Magn Reson Med. 1993;29:485–497. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Beohar N, Arumana JM, et al. CMR imaging of edema in myocardial infarction using cine balanced steady-state free precession. JACC Cardiovasc Imaging. 2011;4:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall HR, Prato FS, Deans L, Theberge J, Thompson RT, Stodilka RZ. Variable lung density consideration in attenuation correction of whole-body PET/MRI. J Nucl Med. 2012;53:977–984. [DOI] [PubMed] [Google Scholar]
- 17.Vontobel J, Liga R, Possner M, et al. MR-based attenuation correction for cardiac FDG PET on a hybrid PET/MRI scanner: comparison with standard CT attenuation correction. Eur J Nucl Med Mol Imaging. 2015;42:1574–1580. [DOI] [PubMed] [Google Scholar]
- 18.Nakazato R, Dey D, Alexanderson E, et al. Automatic alignment of myocardial perfusion PET and 64-slice coronary CT angiography on hybrid PET/CT. J Nucl Cardiol. 2012;19:482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakazato R, Berman DS, Dey D, et al. Automated quantitative Rb-82 3D PET/CT myocardial perfusion imaging: normal limits and correlation with invasive coronary angiography. J Nucl Cardiol. 2012;19:265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slomka PJ, Nishina H, Berman DS, et al. Automatic quantification of myocardial perfusion stress-rest change: a new measure of ischemia. J Nucl Med. 2004;45:183–191. [PubMed] [Google Scholar]
- 21.Kuhle WG, Porenta G, Huang SC, et al. Quantification of regional myocardial blood flow using 13N-ammonia and reoriented dynamic positron emission tomographic imaging. Circulation. 1992;86:1004–1017. [DOI] [PubMed] [Google Scholar]
- 22.Böttcher M, Czernin J, Sun KT, Phelps ME, Schelbert HR. Effect of caffeine on myocardial blood flow at rest and during pharmacological vasodilation. J Nucl Med. 1995;36:2016–2021. [PubMed] [Google Scholar]
- 23.Guerrero A, Singer JJ, Fay FS. Simultaneous measurement of Ca2+ release and influx into smooth muscle cells in response to caffeine: a novel approach for calculating the fraction of current carried by calcium. J Gen Physiol. 1994;104:395–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn HY, Karaki H, Urakawa N. Inhibitory effects of caffeine on contractions and calcium movement in vascular and intestinal smooth muscle. Br J Pharmacol. 1988;93:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trochu JN, Zhao G, Post H, et al. Selective A2A adenosine receptor agonist as a coronary vasodilator in conscious dogs: potential for use in myocardial perfusion imaging. J Cardiovasc Pharmacol. 2003;41:132–139. [DOI] [PubMed] [Google Scholar]
- 26.Zoghbi GJ, Iskandrian AE. Selective adenosine agonists and myocardial perfusion imaging. J Nucl Cardiol. 2012;19:126–141. [DOI] [PubMed] [Google Scholar]
- 27.Potkin RT, Swenson ER. Resuscitation from severe acute hypercapnia: determinants of tolerance and survival. Chest. 1992;102:1742–1745. [DOI] [PubMed] [Google Scholar]
- 28.Willie CK, Tzeng YC, Fisher JA, Ainslie PN. Integrative regulation of human brain blood flow. J Physiol (Lond). 2014;592:841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spano VR, Mandell DM, Poublanc J, et al. CO2 blood oxygen level-dependent MR mapping of cerebrovascular reserve in a clinical population: safety, tolerability, and technical feasibility. Radiology. 2013;266:592–598. [DOI] [PubMed] [Google Scholar]
- 30.Brian JE., Jr Carbon dioxide and the cerebral circulation. Anesthesiology. 1998;88:1365–1386. [DOI] [PubMed] [Google Scholar]
- 31.Feihl F, Perret C. Permissive hypercapnia: how permissive should we be? Am J Respir Crit Care Med. 1994;150:1722–1737. [DOI] [PubMed] [Google Scholar]
- 32.O’Croinin D, Ni Chonghaile M, Higgins B, Laffey JG. Bench-to-bedside review: permissive hypercapnia. Crit Care. 2005;9:51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Javadi MS, Lautamaki R, Merrill J, et al. Definition of vascular territories on myocardial perfusion images by integration with true coronary anatomy: a hybrid PET/CT analysis. J Nucl Med. 2010;51:198–203. [DOI] [PubMed] [Google Scholar]
- 34.Scheel KW, Ingram LA, Gordey RL. Relationship of coronary flow and perfusion territory in dogs. Am J Physiol. 1982;243:H738–H747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.