Abstract
Background
We recently reported that hesperetin-5,7,3’-O-triacetate (HTA) dually inhibited phosphodiesterase (PDE)3/4 with a therapeutic ratio of 20.8. The application and development of PDE4 inhibitors for treating asthma or COPD are limited by their side effects, such as nausea, vomiting and gastric hypersecretion. PDE4 inhibitors were reported to reverse xylazine/ketamine-induced anesthesia in rats and triggered vomiting in ferrets. Thus the reversing effect of HTA on xylazine/ketamine-induced anesthesia in mice was studied to assess emetic effect of HTA. The aim of this study was to prove the therapeutic effect of HTA without vomiting effect at an effective dose for treating COPD.
Methods
Ten female BALB/c mice in each group were sensitized by ovalbumin (OVA) on days 0 and 14. On day 21, these mice were emphasized the sensitization by Freund’s complete adjuvant. Mice were challenged by 1% OVA nebulization on days 28, 29, and 30. Airway hyperresponsiveness (AHR) was assessed on day 32 in each group, using the FlexiVent system to determine airway resistance (RL) and lung dynamic compliance (Cdyn) in anesthetized ovalbumin (OVA)-sensitized and challenged mice. Each group was orally administered HTA (10 ~ 100 μmol/kg), roflumilast (1 and 5 mg/kg) or vehicles (controls) 2 h before and 6 and 24 h after OVA provocation. For comparison, sham-treated mice were challenged with saline instead of 1% OVA. The ability to reverse xylazine/ketamine-induced anesthesia by HTA or roflumilast for 3 h was determined in normal mice. We used roflumilast, a selective PDE4 inhibitor and bronchodilator for severe COPD approved by the US Food and Drug Administration, as a reference drug.
Results
In the results, HTA (100 μmol/kg, p.o.) or roflumilast (5 mg/kg, p.o.) significantly suppressed all RL values of MCh at 0.78 ~ 25 mg/mL and enhanced Cdyn values of MCh at 3.125 ~ 25 mg/mL compared to OVA-sensitized and -challenged control mice. Orally administered 1, 3 or 10 mg/kg roflumilast, but not 30 or 100 μmol/kg HTA, significantly reversed xylazine/ketamine-induced anesthesia.
Conclusions
In contrast to roflumilast, HTA may ameliorate COPD but induce few side effects of nausea, vomiting and gastric hypersecretion at an effective dose for treating COPD, because HTA did not reverse xylazine/ketamine-induced anesthesia in mice.
Keywords: Airway hyperresponsiveness; Airway resistance; Hesperetin-5,7,3’-O-triacetate; Lung dynamic compliance; Roflumilast; Xylazine/ketamine-induced anesthesia
Background
It is known that phosphodiesterases (PDEs) comprise at least 11 distinct enzyme families that hydrolyze adenosine 3′,5′ cyclic monophosphate (cAMP) and/or guanosine 3′,5′ cyclic monophosphate (cGMP) [1]. PDE3 and PDE4 families are cGMP-inhibited and cAMP-specific, respectively. PDE4 may have high (PDE4H) and low (PDE4L) affinities for rolipram. In general, it is believed that inhibition of PDE4H is associated with adverse responses, such as nausea, vomiting, and gastric hypersecretion, while inhibition of PDE4L is associated with anti-inflammatory and bronchodilating effects. Therefore, the therapeutic ratio of selective PDE4 inhibitors for treating asthma and chronic obstructive pulmonary disease (COPD) is defined as the PDE4H/PDE4L ratio [2].
Hesperetin (5,7,3’-trihydroxy-4’-methoxyflavanone) was reported to selectively inhibit PDE4 activity [3], and is used as a lead compound to synthesize hesperetin-5,7,3’-O-triacetate (HTA), a more-liposoluble derivative of hesperetin. HTA was reported to dually inhibit PDE3/4 with a therapeutic (PDE4H/PDE4L) ratio of 20.8 [4], which is greater than that of roflumilast [5], a selective PDE4 inhibitor. Roflumilast was approved by the European Commission [6], and the US Food and Drug Administration (FDA) [4] as an adjunct to bronchodilator therapy for severe COPD associated with chronic bronchitis in adults with a history of frequent exacerbations. However, dual PDE3/4 inhibitors are reported to have additive or synergistic anti-inflammatory and bronchodilator effects compared to PDE3 or PDE4 inhibitors alone [7]. In other words, the real therapeutic ratio of dual PDE3/4 inhibitors should be greater than that reported [4]. Therefore, we were interested in investigating the suppressive effects of HTA on ovalbumin (OVA)-induced airway hyperresponsiveness (AHR), and clarifying its potential for treating atypical asthma and COPD [8]. In this animal model, the number of neutrophils in the bronchoalveolar lavage fluid of control sensitized and challenged mice was significantly greater than that of eosinophils [8]. AHR was previously assessed by barometric plethysmography [9] using a whole-body plethysmograph in unrestrained animals. However, the determination of enhanced pause does likely not reflect lung mechanics [10, 11]. Thus AHR in the present study was assessed using the FlexiVent system to determine the airway resistance (RL) and lung dynamic compliance (Cdyn) in anesthetized ventilated mice. The application and development of PDE4 inhibitors for treating asthma and COPD are limited by their side effects, such as nausea, vomiting and gastric hypersecretion [2]. PDE4 inhibitors were reported to reverse xylazine/ketamine-induced anesthesia in rats [12] and triggered vomiting in ferrets [13]. Thus the reversing effect of HTA on xylazine/ketamine-induced anesthesia in mice was used to assess emetic effect of HTA. The aim of this study was to prove the therapeutic effect of HTA without vomiting effect at effective dose for treating COPD. To compare the therapeutic and gastrointestinal (GI) side effects of HTA, roflumilast was used as a reference drug.
Methods
Reagents and animals
HTA (mol. wt., 428.27, Fig. 1) was synthesized in accordance with a previously described method [14]. The purity of HTA exceeded 98% and the structure was determined by spectral methods [4]. The reference drug, roflumilast (Daxas® film-coated tablets) was a gift from Takeda Pharmaceutical (Taipei, Taiwan). Aluminum sulfate hexadecahydrate, methacholine (MCh), OVA, urethane, chloralose, ethylenediaminetetraacetic acid (EDTA), dimethyl sulfoxide (DMSO), bis-tris, 3,3′,5,5′-tetramethylbenzidine (TMB) solution, xylazine hydrochloride and (±)-ketamine hydrochloride were purchased from Sigma-Aldrich Chemical (St. Louis, Missouri, USA). Freund’s adjuvant (Mycobacterium butyricum) was purchased from Pierce Biotechnology (Rockford, Illinois, USA). Ethyl alcohol and polyethylene glycol (PEG) 400 were purchased from Merck (Darmstadt, Germany). HTA was dissolved in a mixture of ethyl alcohol and DMSO (1: 1), whereas roflumilast was suspended in phosphate-buffered saline (PBS). Other reagents were dissolved in distilled water. The oral dosages of HTA and roflumilast were expressed as μmol/kg and mg/kg, respectively.
Fig. 1.
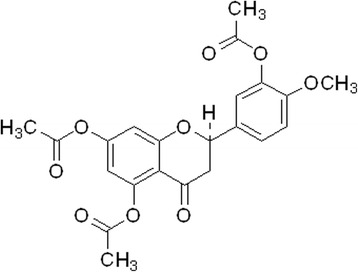
Chemical structure of hesperetin-5,7,3’-O-triacetate (HTA; mol. wt., 428.27)
Female BABL/c mice at 8 ~ 12 weeks old were purchased from the Animal Center of the Ministry of Science and Technology (Taipei, Taiwan), housed in ordinary cages at 22 ± 1 °C with a humidity of 50% ~ 60% under a constant 12/12-h light/dark cycle and provided with OVA-free food and water ad libitum [8]. Under a protocol approved (LAC-100-0152) on May 4, 2012 by the Animal Care and Use Committee of Taipei Medical University, the following experiments were performed.
AHR in vivo
In accordance with a previously published protocol [8], ten female BALB/c mice in each group were sensitized by an intraperitoneal (i.p.) injection of 20 μg of OVA emulsified in 2.25 mg of an aluminum hydroxide gel, prepared from aluminum sulfate hexadecahydrate, in a total volume of 100 μL on days 0 and 14. On day 21, these mice were (i.p.) injected with 100 μL of a mixture of 1% OVA and Freund’s complete adjuvant (1:1). Mice were challenged via the airway using 1% OVA in saline for 30 min on days 28, 29, and 30 by ultrasonic nebulization. After the last OVA challenge [15], AHR was assessed on day 32 (48 h after 1% OVA provocation) in each group. Each group of mice was orally (p.o.) administered HTA (10 ~ 100 μmol/kg), roflumilast (1 and 5 mg/kg) or vehicles (controls) 2 h before and 6 and 24 h after OVA provocation. For comparison, sham-treated mice were challenged with saline instead of 1% OVA (non-challenged). A mixture of DMSO: ethyl alcohol: PEG 400: saline (0.5: 0.5: 1: 8, v/v) or PBS was the vehicle for the control of HTA or roflumilast, respectively. The vehicles were administered (p.o.) at a volume of 0.01 mL/g of body weight. Mice showed no abnormal behavior after oral administration of the vehicle.
In accordance with a previously described method [8], anesthetized (urethane 600 mg/kg and chloralose 120 mg/kg, i.p.), tracheostomized (stainless-steel cannula, 18 G) mice were mechanically ventilated (at 150 breaths/min, with a tidal volume of 10 mL/kg and a positive end-expiratory pressure of 3 cmH2O). Prior to PBS nebulization for 10 s the baseline RL and Cdyn were determined. Then the AHR of mice was assessed by measuring changes in the RL and Cdyn after being challenged with aerosolized MCh (0.78, 1.563, 3.125, 6.25, 12.5, and 25 mg/mL) for 10 s using the FlexiVent system (SCIREQ, Montreal, Quebec, Canada), in which these data were automatically saved for 3 min after 10 s of nebulization.
Xylazine/Ketamine-induced anesthesia
According to previously reported methods [8, 16], after loss of the righting reflex (i.e., when a mouse remains on its back and no longer spontaneously rights itself to a prone position), the duration of anesthesia was measured until its return as the endpoint. The ability to reverse xylazine/ketamine-induced anesthesia by oral administration of HTA, roflumilast or their vehicles for 3 h was determined in female BALB/c mice.
Statistical analysis
Differences among values given as the mean ± standard error of the mean (SEM) were calculated by a one-way analysis of variance (ANOVA), and then determined by Dunnett’s test. The difference between two values, however, was determined by Student’s t-test. Significance was accepted when p < 0.05.
Results
Suppression of AHR in vivo
Baseline RL values of control, non-challenged, and HTA-treated (10, 30, and 100 μmol/kg) groups of sensitized and challenged mice were 1.06 ± 0.08, 0.96 ± 0.07, 1.03 ± 0.06, 0.90 ± 0.10, and 0.85 ± 0.06 cmH2O/mL/s, which did not significantly differ from each other. After PBS nebulization, the RL values of each group were 1.24 ± 0.14, 0.97 ± 0.06, 1.09 ± 0.06, 0.96 ± 0.12, and 0.90 ± 0.13 cmH2O/mL/s, which did not significantly differ from each other or from the respective baseline RL values, suggesting that PBS nebulization did not influence baseline RL values. However, MCh (0.78 ~ 25 mg/mL) concentration-dependently and significantly increased RL values in sensitized and challenged control mice compared to non-challenged mice (Fig. 2a). HTA at 30 μmol/kg (p.o.) significantly suppressed the RL value from 11.46 ± 1.96 to 6.25 ± 0.87 cmH2O/mL/s of MCh at 25 mg/mL. Furthermore, HTA 100 μmol/kg (p.o.) significantly suppressed all RL values from 1.68 ± 0.22 to 1.01 ± 0.06, from 2.14 ± 0.25 to 1.13 ± 0.09, from 2.77 ± 0.37 to 1.32 ± 0.08, from 4.28 ± 0.37 to 1.78 ± 0.14, from 6.24 ± 1.19 to 2.76 ± 0.36, and from 11.46 ± 1.96 to 4.01 ± 0.62 cmH2O/mL/s of MCh at 0.78 ~ 25 mg/mL (Fig. 2a). In contrast, baseline Cdyn values of each group were 0.026 ± 0.0012, 0.030 ± 0.0017, 0.024 ± 0.0005, 0.027 ± 0.0008 and 0.027 ± 0.0022 mL/cmH2O, which did not significantly differ from each other (Fig. 2b). After PBS nebulization, Cdyn values of each group were 0.025 ± 0.0011, 0.029 ± 0.0014, 0.026 ± 0.0031, 0.026 ± 0.0008 and 0.027 ± 0.0021 mL/cmH2O, which did not significantly differ from each other or from the respective baseline Cdyn values, suggesting that PBS nebulization also did not influence baseline Cdyn values. However, MCh (0.78 ~ 25 mg/mL) concentration-dependently and significantly decreased Cdyn values in sensitized and challenged control mice compared to non-challenged mice (Fig. 2b). HTA 100 μmol/kg (p.o.) significantly enhanced Cdyn values from 0.015 ± 0.0015 to 0.021 ± 0.0016, from 0.012 ± 0.0013 to 0.018 ± 0.0014, from 0.009 ± 0.0011 to 0.013 ± 0.0011, and from 0.006 ± 0.0006 to 0.009 ± 0.0007 mL/cmH2O of MCh at 3.125 ~ 25 mg/mL when compared to sensitized and challenged control mice (Fig. 2b).
Fig. 2.
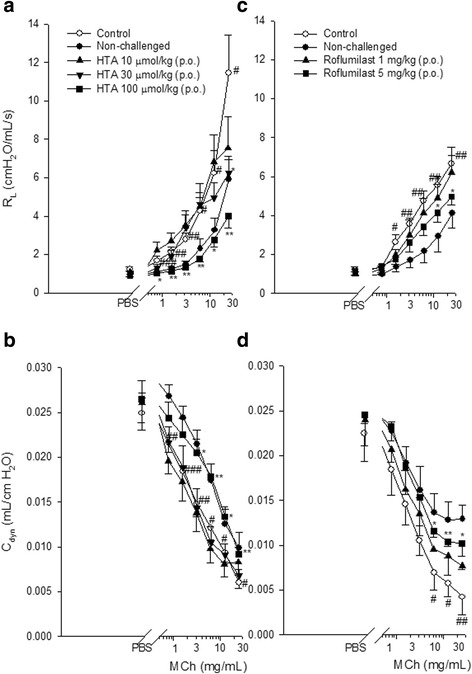
Effect of orally administered HTA (10 ~ 100 μmol/kg) and roflumilast (1 and 5 mg/kg) on the airway resistance (RL) (a, c) and lung dynamic compliance (Cdyn) (b, d) in sensitized and challenged mice which received aerosolized methacholine (MCh, 6.25 ~ 25 mg/mL) 2 days after the last allergen challenge. *p < 0.05, **p < 0.01, ***p < 0.001 compared to the control (vehicle) group. # p < 0.05, ## p < 0.01, ### p < 0.001 compared to the non-challenged group. Each value represents the mean ± SEM (n = 5 ~ 12)
Baseline RL values of control, non-challenged, roflumilast-treated (1 and 5 mg/kg) groups of sensitized and challenged mice were 1.95 ± 0.99, 0.93 ± 0.05, 1.01 ± 0.10 and 1.03 ± 0.10 cmH2O/mL/s, which did not significantly differ from each other. After PBS nebulization, RL values of each group were 1.22 ± 0.14, 0.90 ± 0.06, 1.01 ± 0.11 and 1.15 ± 0.22 cmH2O/mL/s, which did not significantly differ from each other or from the respective baseline RL values, suggesting that PBS nebulization did not influence baseline RL values. However, MCh (1.56 ~ 25 mg/mL) concentration-dependently and significantly increased RL values in sensitized and challenged control mice compared to non-challenged mice (Fig. 2c). Roflumilast at 5 mg/kg (p.o.) significantly suppressed the RL values from 5.56 ± 0.41 to 4.15 ± 0.50, and from 6.65 ± 0.42 to 4.97 ± 0.42 cmH2O/mL/s of MCh at 12.5 and 25 mg/mL. In contrast, respective baseline Cdyn values of each group were 0.004 ± 0.0201, 0.025 ± 0.0009, 0.026 ± 0.0022, and 0.027 ± 0.0026 mL/cmH2O, which did not significantly differ from each other (Fig. 2d). After PBS nebulization, Cdyn values of each group were 0.023 ± 0.0031, 0.025 ± 0.0009, 0.023 ± 0.0020 and 0.026 ± 0.0022 mL/cmH2O, which did not significantly differ from each other or from respective baseline Cdyn values, suggesting that PBS nebulization also did not influence baseline Cdyn values. However, MCh (6.25 ~ 25 mg/mL) concentration-dependently and significantly decreased Cdyn values in sensitized and challenged control mice compared to non-challenged mice (Fig. 2d). Roflumilast at 5 mg/kg (p.o.) significantly enhanced Cdyn values from 0.007 ± 0.002 to 0.012 ± 0.001, from 0.006 ± 0.001 to 0.011 ± 0.001, and from 0.004 ± 0.002 to 0.009 ± 0.001 mL/cmH2O of MCh at 6.25 ~ 25 mg/mL compared to sensitized and challenged control mice (Fig. 2d).
Xylazine/Ketamine-induced anesthesia
Durations of xylazine/ketamine-induced anesthesia in vehicle (control)-treated mice for the HTA- and roflumilast-treated groups were 28.2 ± 4.7 (n = 5) and 28.3 ± 1.7 (n = 8) min, respectively. Oral administration of HTA 300 μmol/kg significantly shortened the duration to 15.4 ± 1.9 (n = 5) min (Fig. 3a), and so did roflumilast 1, 3, and 10 mg/kg to 20.3 ± 2.48, 18.0 ± 4.07, and 10.0 ± 2.94 min, respectively (Fig. 3b).
Fig. 3.
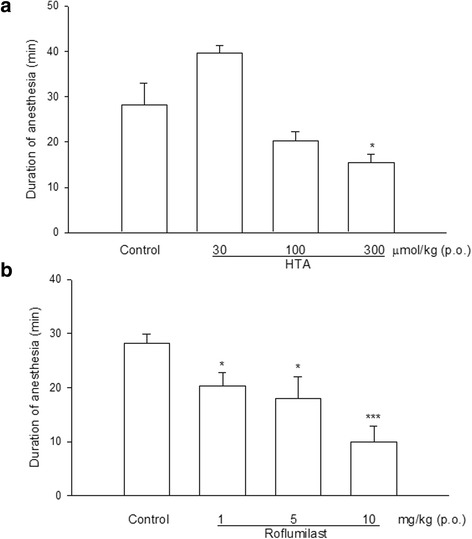
Effects of orally administered HTA (a) and roflumilast (b) on the duration of xylazine (10 mg/kg, i.p.)/ketamine (70 mg/kg, i.p.)-induced anesthesia in mice. * p < 0.05, *** p < 0.001, compared to the control. Each value represents the mean ± SEM. The number of mice in each group was 5 ~ 8
Discussion
HTA dually inhibits PDE3/4, whereas roflumilast selectively inhibits PDE4 activity. Thus degradation of cAMP, an important secondary messenger, is prevented by them and the intracellular cAMP content indirectly increases [15, 17–19]. Increased cAMP activates cAMP-dependent protein kinase, inhibits myosin light-chain kinase, and results in bronchodilation. Thus the RL decreased and the Cdyn was enhanced. These results suggest that HTA would have benefits in treating COPD, although no evidence was found to support it having benefits for treating atypical asthma.
The application and development of PDE4 inhibitors in treating asthma and COPD are limited by their side effects, such as nausea, vomiting and gastric hypersecretion [2]. Rolipram, a first generation PDE4 inhibitor, has a therapeutic ratio of 0.002 [20] and has many side effects. Cilomilast and roflumilast have therapeutic ratios of 1 and 3, respectively [5, 21]. Recently, roflumilast was approved by the European Commission [6] and the US FDA [4] as an add-on to bronchodilator therapy for maintenance treatment of severe COPD associated with chronic bronchitis in adults with a history of frequent exacerbations.
Robichaud et al. reported that MK-912, an α2-adrenoceptor antagonist, reversed xylazine/ketamine-induced anesthesia in rats [12] and triggered vomiting in ferrets [13]. In contrast, clonidine, an α2-adrenoceptor agonist, prevented emesis in ferrets [13]. Thus they suggested that the reversing effect occurred through presynaptic α2-adrenoceptor inhibition [13]. They also found that PDE4 inhibitors reversed xylazine/ketamine-induced anesthesia in rats and ferrets [12, 13]. Thus the reversing effect of PDE4 inhibitors on xylazine/ketamine-induced anesthesia in rats or mice is convenient and could be a surrogate for assessing the emetic effects of these drugs, as rodents have no emetic reflex and we cannot observe emesis. In the present results, orally administered HTA at 300 μmol/kg (approximately 128.5 mg/kg) and roflumilast at 1 ~ 10 mg/kg significantly reversed xylazine/ketamine-induced anesthesia in mice, whereas orally administered HTA at 100 μmol/kg or roflumilast at 5 mg/kg significantly reduced the RL and enhanced the Cdyn. HTA even at 30 μmol/kg also reduced the RL, although did not enhance the Cdyn.
Conclusions
In contrast to roflumilast, HTA may ameliorate COPD but induce few side effects of nausea, vomiting and gastric hypersecretion at a dose effective for treating COPD, because HTA did not reverse xylazine/ketamine-induced anesthesia in mice.
Acknowledgements
We gratefully acknowledge that the reference drug, roflumilast (Daxas® film-coated tablets) was supplied from Takeda Pharmaceutical (Taipei, Taiwan) as a gift.
Funding
This work was supported by a grant (NSC97-2320-B-038-015) from the Ministry of Science and Technology, Taipei, Taiwan.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
YLY and WCK conceived and designed the study. CLC performed the experiments and analyzed the data. CMC synthesized HTA and determined its purity and structure. YLY and WCK wrote the manuscript. All the authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable. This manuscript contains no personal data.
Ethics approval and consent to participate
The study protocol was approved (LAC-100-0152) on May 4, 2012 by the Animal Care and Use Committee of Taipei Medical University.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AHR
Airway hyperresponsiveness
- cAMP
Adenosine 3′,5′ cyclic monophosphate
- Cdyn
Lung dynamic compliance
- cGMP
Guanosine 3′,5′ cyclic monophosphate
- COPD
Chronic obstructive pulmonary disease
- DMSO
Dimethyl sulfoxide
- EDTA
ethylenediaminetetraacetic acid
- FDA
Food and Drug Administration
- GI
Gastrointestinal
- HTA
Hesperetin-5,7,3’-O-triacetate
- MCh
Methacholine
- OVA
Ovalbumin
- PBS
Phosphate-buffered saline
- PDE
Phosphodiesterase
- PDE4H
PDE4 high affinity for rolipram
- PDE4L
PDE4 low affinity for rolipram
- PEG
Polyethylene glycol
- RL
Airway resistance
References
- 1.Lee ME, Markowitz J, Lee JO, Lee H. Crystal structure of phosphodiesterase 4D and inhibitor complex (1) FEBS Lett. 2002;530:53–8. doi: 10.1016/S0014-5793(02)03396-3. [DOI] [PubMed] [Google Scholar]
- 2.Giembycz MA. Phosphodiesterase 4 inhibitors and the treatment of asthma: where are we now and where do we go from here? Drugs. 2000;59:193–212. doi: 10.2165/00003495-200059020-00004. [DOI] [PubMed] [Google Scholar]
- 3.Ko WC, Shih CM, Lai YH, Chen JH, Huang HL. Inhibitory effects of flavonoids on phosphodiesterase isozymes from guinea pig and their structure-activity relationships. Biochem Pharmacol. 2004;68:2087–94. doi: 10.1016/j.bcp.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 4.Hsu HT, Wang WH, Han CY, Chen CN, Chen CM, Ko WC. Inhibitory effects of hesperetin derivatives on guinea pig phosphodiesterases and their ratios between high- and low-affinity rolipram binding. J Pharm Sci. 2013;102:2120–7. doi: 10.1002/jps.23591. [DOI] [PubMed] [Google Scholar]
- 5.Zhao Y, Zhang HT, O’Donnell JM. Inhibitor binding to type 4 phosphodiesterase (PDE4) assessed using [3H]piclamilast and [3H]rolipram. J Pharmacol Exp Ther. 2003;305:565–72. doi: 10.1124/jpet.102.047407. [DOI] [PubMed] [Google Scholar]
- 6.Giembycz MA, Field SK. Roflumilast: first phosphodiesterase 4 inhibitor approved for treatment of COPD. Drug Des Devel Ther. 2010;4:147–58. doi: 10.2147/dddt.s7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbott-Banner KH, Page CP. Dual PDE3/4 and PDE4 inhibitors: novel treatments for COPD and other inflammatory airway diseases. Basic Clin Pharmacol Toxicol. 2014;114:365–76. doi: 10.1111/bcpt.12209. [DOI] [PubMed] [Google Scholar]
- 8.Yang YL, Hsu HT, Wang KH, Han CY, Chen CM, Chen CM, Ko WC. Hesperetin-7,3’-O-dimethylether selectively inhibits phosphodiesterase 4 and effectively suppresses ovalbumin-induced airway hyperresponsiveness with a high therapeutic ratio. J Biomed Sci. 2011;18:84–12. doi: 10.1186/1423-0127-18-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–75. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 10.Hantos Z, Brusasco V. Assessment of respiratory mechanics in small animals: the simpler the better? J Appl Physiol. 2002;93:1196–7. doi: 10.1152/japplphysiol.00526.2002. [DOI] [PubMed] [Google Scholar]
- 11.Lundblad LK, Irvin CG, Adler A, Bates JH. A reevaluation of the validity of unrestrained plethysmography in mice. J Appl Physiol. 2002;93:1198–207. doi: 10.1152/japplphysiol.00080.2002. [DOI] [PubMed] [Google Scholar]
- 12.Robichaud A, Savoie C, Stamatiou PB, Lachance N, Jolicoeur P, Rasori R, Chan CC. Assessing the emetic potential of PDE4 inhibitors in rats. Br J Pharmacol. 2002;135:113–8. doi: 10.1038/sj.bjp.0704457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robichaud A, Savoie C, Stamatiou PB, Tattersall FD, Chan CC. PDE4 inhibitors induce emesis in ferrets via a noradrenergic pathway. Neuropharmacology. 2001;40:262–9. doi: 10.1016/S0028-3908(00)00142-8. [DOI] [PubMed] [Google Scholar]
- 14.Ferte J, Kuhnel JM, Chapuis G, Rolland Y, Lewin G, Schwaller MA. Flavonoid-related modulators of multidrug resistance: synthesis, pharmacological activity, and structure-activity relationships. J Med Chem. 1999;2:478–89. doi: 10.1021/jm981064b. [DOI] [PubMed] [Google Scholar]
- 15.Bourne HR, Lichtenstein LM, Melmon KL, Henney CS, Weinstein Y, Shearer GM. Modulation of inflammation and immunity by cyclic AMP. Science. 1974;184:19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- 16.Robichaud A, Stamatiou PB, Jin SL, Lachance N, MacDonald D, Laliberte F, Liu S, Huang Z, Conti M, Chan CC. Deletion of phosphodiesterase 4D in mice shortens α2-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002;110:1045–52. doi: 10.1172/JCI0215506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuehl FA, Jr, Zanetti ME, Soderman DD, Miller DK, Ham EA. Cyclic AMP-dependent regulation of lipid mediators in white cells. A unifying concept for explaining the efficacy of theophylline in asthma. Am Rev Respir Dis. 1987;136:210–3. doi: 10.1164/ajrccm/136.1.210. [DOI] [PubMed] [Google Scholar]
- 18.Kammer GM. The adenylate cyclase-cAMP-protein kinase A pathway and regulation of the immune response. Immunol Today. 1988;9:222–9. doi: 10.1016/0167-5699(88)91220-0. [DOI] [PubMed] [Google Scholar]
- 19.Moore AR, Willoughby DA. The role of cAMP regulation in controlling inflammation. Clin Exp Immunol. 1995;101:387–9. doi: 10.1111/j.1365-2249.1995.tb03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim E, Chun HO, Jung SH, Kim JH, Lee JM, Suh BC, Xiang MX, Rhee CK. Improvement of therapeutic index of phosphodiesterase type IV inhibitors as anti-Asthmatics. Bioorg Med Chem Lett. 2003;13:2355–8. doi: 10.1016/S0960-894X(03)00405-0. [DOI] [PubMed] [Google Scholar]
- 21.Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297:267–79. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.


