Abstract
Recently, interest in targeted cancer therapies via metabolic pathways has been renewed with the discovery that many tumors become dependent on glucose uptake during anaerobic glycolysis. Also the inability of ketone bodies metabolization due to various deficiencies in mitochondrial enzymes is the major metabolic changes discovered in malignant cells. Therefore, administration of a ketogenic diet (KD) which is based on high in fat and low in carbohydrates might inhibit tumor growth and provide a rationale for therapeutic strategies. So, we conducted this systematic review to assess the effects of KD on the tumor cells growth and survival time in animal studies. All databases were searched from inception to November 2015. We systematically searched the PubMed, Scopus, Google Scholars, Science Direct and Cochrane Library according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. To assess the quality of included studies we used SYRCLE's RoB tool. 268 articles were obtained from databases by primary search. Only 13 studies were eligible according to inclusion criteria. From included studies, 9 articles indicate that KD had a beneficial effect on tumor growth and survival time. Tumor types were included pancreatic, prostate, gastric, colon, brain, neuroblastoma and lung cancers. In conclusions, although studies in this field are rare and inconsistence, recent findings have demonstrated that KD can potentially inhibit the malignant cell growth and increase the survival time. Because of differences physiology between animals and humans, future studies in cancer patients treated with a KD are needed.
Keywords: Cancer, ketogenic diet, survival, tumor growth
Introduction
Efficacy of modified diet and nutritional management has been illustrated in some tumors.[1,2,3,4]
Ketogenic diet (KD) is a high fat, (90% of the calories fulfill from fat) adequate protein, low carbohydrate diet that produces ketone bodies through imitation metabolic changes subsequent starvation.[5,6] In 1920, KD was designed for treatment of epilepsy.[7] However, recent evidence suggests that it is effective on controlling of some metabolic disease.[8] This dietary restriction lowers plasma glucose levels and limits the energy source to some cells while raise circulating blood ketone levels.[9] Also, this diet imposes cells to rely on fat oxidation and mitochondrial respiration compare with glycolysis for energy production.[10] In this regard, these dietary modifications might be expected to reduce tumor size and growth rate which depend on glucose as an energy source during anaerobic glycolysis. Also the inability of ketone bodies metabolization due to various deficiencies in mitochondrial enzymes, is one of the metabolic modification found in malignant cells. Further, ketone bodies possibly result in a decreasing the supply of gluconeogenic precursors by restriction of the branch chain amino acids oxidation.[11]
In this way, KD can provide a rationale for therapeutic strategies via tumor growth inhibition.[12] Although studies about association of mentioned diet and cancers are rare,[13] it was proposed that high fat intake and carbohydrate restriction associated with KD might be useful for cancer by providing sufficient energy substrates for peripheral tissues and as well as interfering with important cancer survival pathway.[14] However, the KD effects on tumor growth and survival are not similar.[9,15,16]
So, in the present literature we attempt to review systematically animal studies for evaluate the impact of KD on tumor cells growth and survival time.
Methods
This review was done base on Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and has been registered at PROSPERO (CRD 42015023956).
Search strategies
By conducting a systematic review to assess the relationship between the KD effects on tumor cells growth and survival time, we have searched PubMed, Scopus, Google Scholars, Science Direct and Cochrane Library. All databases were searched from inception to November 2015. We used the terms “KD” and “Cancer” or “Tumor” or “Neoplasm” without any restriction. Additionally, the reference list of each eligible article was manually reviewed to identify other related researches. All the review process independently was established by three investigators.
Study selection
Generally, articles were included if there were experimental studies on animals that reported effects of KD on tumor cells growth rate and survival time. Excluded studies involved cell lines studies and literatures that didn’t estimate tumor cells growth or survival time after KD. Also, documents that KD combined with other agents such as, hyperbaric oxygen therapy (HBO2T) and radio-chemotherapy were excluded.
268 articles (PubMed 114, Scopus 25, Science Direct 68, Cochrane 1, Google Scholar 60) obtain from databases by primary search. After removing duplicate articles (60) relevant researches were identified from title, abstract and full text. Any disagreement between reviewers was resolved by refer to third reviewer. Finally, thirteen studies were included in this systematic review. The flow chart of the selection of studies is shown in Figure 1.
Figure 1.
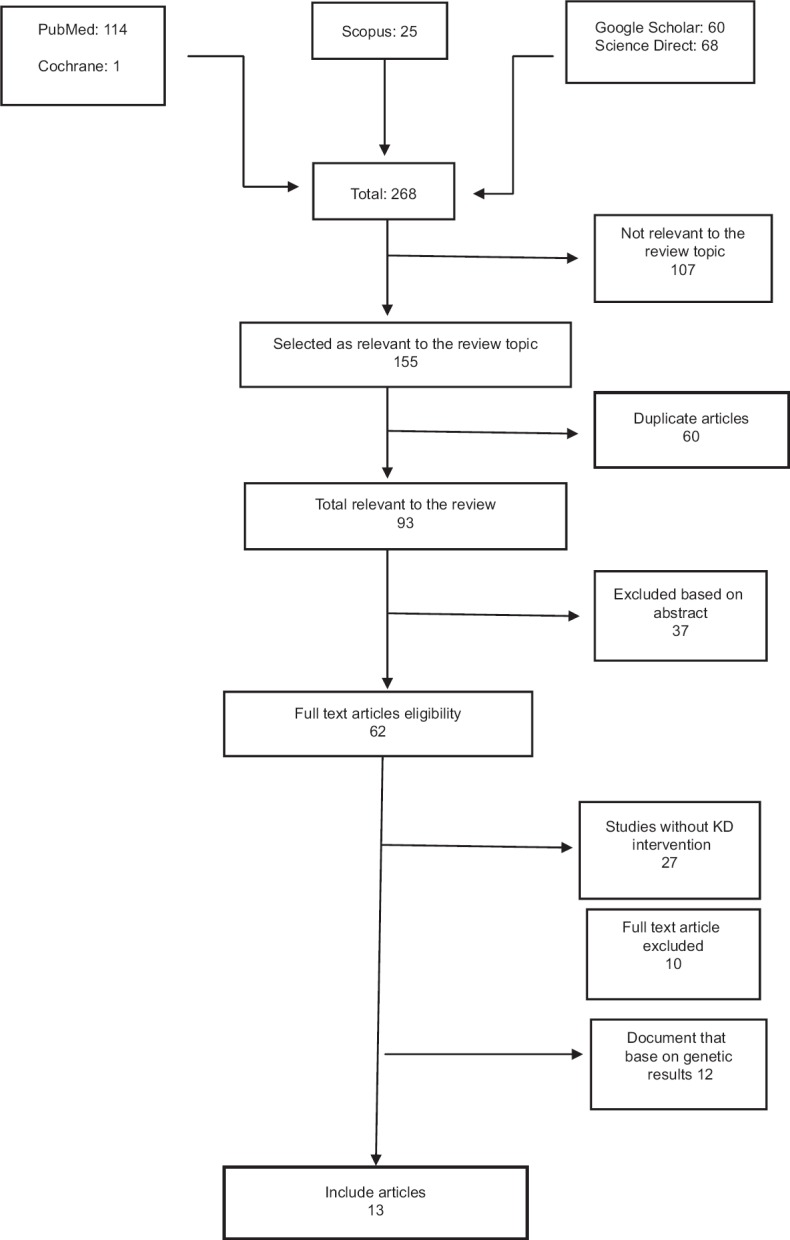
Flowchart of study selection
Data extraction
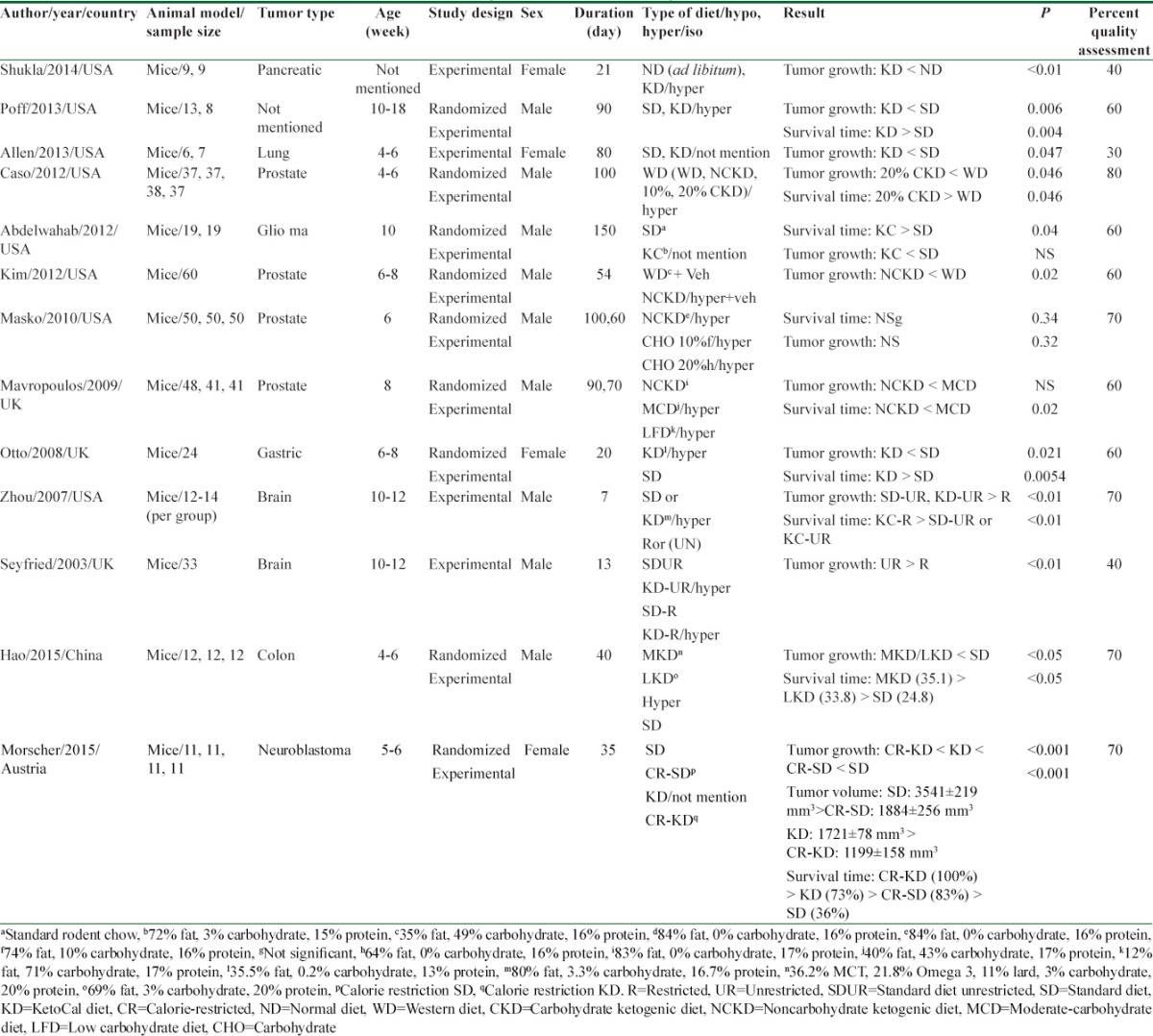
The data extracted from selected studies, included first author, country, year, animal model, cancer type, study design, age, sex, study duration, type of intervention, sample size, main result and P-values were extracted. Characteristics of each study are illustrated in Table 1.
Table 1.
Summary of studies included in systematic review
Quality assessment
To assess the quality of included studies we used SYRCLE's RoB[17] tool that contains 10 items to investigate any important sources of bias such as allocation, adjust for confounder, assignment to the different groups adequately concealed during, animals randomized for housed, blinded investigators, animals random selection for outcome assessment, blinded outcome assessor, addressing incomplete outcome, free of selective outcome reporting and other risk of bias. Quality scores of these studies are shown in Table 1.
Results
From all identified articles, after applying inclusion criteria, 13 studies were selected. All of them assessed the KD effects on tumor growth alone and 9 articles examined both tumor growth and survival time. Study duration, design and their quality were different between them. Most of studies had good quality and the mean score of them was near the 60%.
Poff et al.[11] reported mice with systematic metastatic cancer that used KD represented a significant increase in survival time compare to control group. Also, bioluminescent signal showed a clear trend of reduced tumor growth. By day 7 of intervention, mice in KD group lost about 10% of their body weight and keep that weight for end of intervention. Results revealed a significant correlation between body weight loss with increasing survival time.
This result has been replicated in another study.[12] Mice were injected with gastric tumor cells and then, randomly were divided into two feeding groups; either a KD or a standard diet (SD). The tumor growth and mean survival time in the KD group were significantly reduced and induced respectively, compared to the SD group. The tumor size and tumor volume were assessed with callipers and the ellipsoid formula respectively.
In another study[14] animals were acclimated on the Western diet (WD) for 4 days. After injected prostate tumor cells, animals were randomized in WD, noncarbohydrate ketogenic diet (NCKD) or 10% and 20% carbohydrate KD groups. In this intervention, results showed shortest survival among WD groups. Furthermore, diet containing 20% carbohydrate had a slowest tumor growth and longest survival time. Also, among group that was on 10% carbohydrate diet, nonsignificant tendency for improved survival compared to WD was seen.
Zhou et al.[18] investigated on mice fed with KetoCal (KC) (a new KD that balanced nutritionally for high fat/low carbohydrate containing fat, carbohydrate, protein and fibre comprised 720 g, 30 g, 150 g, and 0 g/kg of the diet; respectively) in either unrestricted (UR) or restricted of caloric intake. The effects of KC on tumor growth and survival time were compared with an UR high carbohydrate SD. When KC diet was consumed in restricted amounts of calorie, decreased tumor growth and enhanced survival time relative to the control groups were seen significantly.
Another result revealed that KC enhanced survival and slowed tumor growth in brain tumor-bearing mice. Following tumor cell implantation, animals were fed SD for 3 days and then were randomized to remain on SD or changed to KC that was providing a 4:1 ratio of fats to carbohydrates plus protein (72% fat, 15% protein, and 3% carbohydrate).[19]
In addition finding from Mavropoulos et al. showed that[20] adherence to a NCKD, without energy restriction, could reduce tumor growth and significantly extended survival relative to a high-fat/moderate carbohydrate or moderate-carbohydrate diet.
In another study, Morscher et al.[21] examined KD and/or calorie restriction on neuroblastoma tumor growth and survival time. After incubating tumor cells, mice were fed four different types of diet for over 5 week: SD, calorie-restricted (CR-SD), KD and CR-KD. Components of these regimes are given in Table 1. Tumors volume of all groups was significantly inhibited compare to SD group. Toward of SD group survival of mice on CR-SD, KD and CR-KD were prominently increased. It should be mentioned that body weight loss on the last day of study in the CR groups was below 20%.
To determine the influence of a KD with different kinds of fatty acids on tumor growth and survival time in mice with colon cancer, Hao et al.[22] split animals into three feeding groups: A KD rich in omega 3 fatty acids and medium-chain triglycerides (MCT) (MKD group), lard only (LKD group) and SD group for 45 days. All animals in KD groups intake the UR-KD and showed a clear increase in body weight in duration of study. However, mean body weight difference was not significant. Mean tumor size in different groups showed retardated tumor growth in MKD and LKD compare with SD group. As well as utilization of this diet with different types of fatty acids significantly increase survival time. Although reduced tumor growth and increased survival time in MKD groups was efficient than other, but this difference between two groups was not significant.
In contrast, another study[16] documented that no significant differences in the tumor volumes and overall survival among NCKD, 10% carbohydrate diet, or 20% carbohydrate diet groups, after animals were fed a WD for a 5-day. Although there were no significant differences in tumor volumes at any time, at days 52 and 59 in 10% carbohydrate group, mice had larger tumors compare with 20% carbohydrate group. In order to avoid from weight loss, NCKD animal were fed about 10% extra calories relative to WD; while the 10% and 20% carbohydrate groups were fed with 7.5% more calories.
In one study, Allen et al. used two kind diets for mice bearing lung cancer. The mice in intervention and control group were fed KC and SD (25% protein, 21% fat, and 54% carbohydrate) respectively for 80 days. This Result shows that KD could slow tumor growth relative to control group.[10,12]
In another experimental study[23] the mouse with brain tumor were randomized and allocated to one of four diet groups: (1) the SD fed ad libitum or unrestricted (SD-UR), (2) the KD fed ad libitum or unrestricted (KD-UR), (3) the SD restricted to 40% (SD-R), or (4) the KD restricted to 40% of the SD (KD-R). Although the tumor grew rapidly in both UR-fed mice groups, tumor growth decreased significantly in SD or KD restricted feeding group. Although total body weight decreased, the restricted fed mice were healthy and more active than the mice in other group. No signs of micro nutrient deficiency were observed in the restricted fed mice.
Another study[15] was conducted on mice that prostate cancer cells were injected sub-cutaneous in the flank. Mice were randomized to three groups: WD, SD and NCKD plus SD. Mice were fed and weighed three times per week. Also, feed was adjusted to maintain body weights similar. Tumor sizes were measured twice a week with calipers once palpable. This research emphasized that NCKD was significantly associated with lower tumor volumes.
Shukla et al. in their study used two type diets: Normal diet and KD for mice induced pancreatic cancer in 3 weeks. After intervention ketone bodies diminish cancer cell growth. In this research in order to assessment of cachexia, muscle weight was recorded. A 45% increase in the muscle weight in KD-fed mice rather than controls was reported.[24]
In order to avoid weight loss in most of these studies KD was administered with high energy level. Therefore, significant changes in weight didn’t observe between groups with different regimes. Body weights remained similar in intervention and control groups despite the major differences in the caloric and composition content of mentioned diets.[10,12,15,16,18,19,20,22,25]
Discussion
This is a systematic review of 13 studies that were evaluated the KD effects on tumor growth and survival time in animal models.
In this study, all included articles indicate that KD had a inhibitory effect on tumor growth[9,10,15,21,22,23,24,25] and 9 researches expressed that KD could enhance survival time.[9,12,19,21,22,23,26] Among these researches, the greatest effect was seen for the KD with omega 3 fatty acids 21.8%, MCT 36.2%, lard 11%, carbohydrate 3% and protein 20% (MKD).
Compliance with animal welfare regulations, long duration of follow up, similar conditions at baseline in the groups, control of body weight and calorie intake are strengths of most of these evidence.
Several limitations need to be taken into account in the interpretation of our findings. Even though animal models still remain a unique source of information, differences in physiology between animal and humans may lead to limited successful translation from animal models to human experimentation. So, these findings must be repeated and confirmed by human studies for applying these dietary restriction regimens in cancer patients.
Secondly, inadequate randomization of animals and small sample size[9,10,25] are limitation in some experiments.[13] In addition, in animal studies, cancer cells incubate to normal tissue, therefore, it is important that the disease was induced before or after randomization.[21] Also, a heterogeneity existed in KD prescribed in different time, before or after of injection tumor. Further, considering the different survival time is an important bias across studies.[15,16] Also, tumor growth assessment by calipers may have a measurement error.[16]
Although the mean score quality for all articles considered was 60%, considering the risk of other bias such as potential conflicts of interest, sample size calculation, appropriateness of dose selection and measurement of outcomes are important.[27] Given the nature of a systematic literature search, consideration of these limitations like publication, reporting, performance and detection biases is also important.
Many studies above the past 60 years have not only supported glucose consumption in tumor cells but also show influence of glucose on cancer survival and metastasis.[28]
A substantial metabolic and molecular distinction between cancerous and normal cells has been known. One great metabolic difference is cellular respiration, glucose metabolism, and energy production.[29] KDs may be work as an adjuvant tumor therapy by two distinct mechanisms that both of them elevate the oxidative stress in tumor cells. Lipid metabolism restricts the availability of glucose for glycolysis; also lipid metabolism forces tissues to take their energy from mitochondrial metabolism.[28] Most of tumor cells have abnormities in number and operation of mitochondria. In the other hand, mitochondria performance is necessary for ketone body oxidation. So, tumor cells unable to metabolize the ketone bodies,[30] and they will be anticipated to experiment oxidative stress induced by limited glucose metabolism in the feeding KD. Similar to fat metabolism, protein forces cells to get their energy from mitochondrial metabolism.[28,31] Further, cancer cells have low adaptability to nutrient change that induced by KD or caloric restriction.[21,32,33] Overall, tumor cells have high levels of glucose uptake, glycolysis and pentose phosphate pathway activation for growth,[22] and use of KD cause an elevation in blood ketones, reduction of blood glucose and restriction of energy supply for cancer tissues.[7]
Serine/threonine kinase Akt most frequently activated in human cancer and cause increasing of cell proliferation and oncogenic mutation rates and inhibition of apoptosis. Reduced Akt levels by KD may be responsible mechanisms for the favorite effects of this diet on cancer.[34]
Reactive oxygen species (ROS) accumulation following by Akt-stimulated,[35] may also help to tumor genesis.[34] KD can be prevented ROS production and increase blood antioxidative capacity.[36] Furthermore, KD can improve mitochondrial function by decreasing of ROS production[18] through restrict glycolysis and increase fatty acid oxidation.
Also, this dietary modification may shift gene expression involved in oxidative stress and inflammation in tumor tissue to a style seen in a normal tissue.[37] Further, reducing of ROS production, inflammatory cytokines and circulating levels of arachidonic acid are multiple possible mechanisms of observed favorite effects by KD on cancer.[38,39,40]
In addition, NCKD lead to lower levels of circulating insulin and insulin-like growth factor-1 (IGF-1), as growth factors in some cancer, and the IGF-I/IGF binding protein (IGFBP)-3 ratio as an indicator of bioactive IGF-I.[12,19] It was shown that lower expression level of IGF-1 are linked with slower tumor growth. Also, high levels of IGFBP-3 may have an anticancer property via proapoptotic pathway activation.[12,22]
In contrast, some studies indicated that KD caused cellular metabolic oxidative stress by elevated ketone levels or chronic exposure to β-hydroxybutyrate.[28] Also, KD may increase the production of lipid peroxidation, protein damage and stress oxidative.[28] It should be noted that in this study various tumor with different characteristics were assessed which lead to different response to KD.
It should be mentioned that KD can create various complications; dehydration is the first most common prevalent during initial fasting course. Gastrointestinal disturbance is the next complication including nausea/vomiting, diarrhea, and constipation that linked to poor toleration of the diet. Furthermore, the KD is an unusually high-fat diet, so changing in lipid profile should be considered.[41] The effect of KD on blood triglyceride to be more evident but KD can have positive effect on decrease of total cholesterol and increment in high-density lipoprotein; if this diet enrichment with ω-3and MCT fatty acids.[42] From rare but serious complication, it can be noted the acute pancreatic and cardiomyopathy. In addition to these complications, hypoproteinemia, hyperuremia, hyponatremia, persistent acidosis, hypomagnesia have observed too.[41,43] In addition, a number of complications on bone and mineral metabolism such as hypercalciuria, urine acidification, hypocitraturia and kidney stones, was related to the KD.[44] However, recent study showed a KD approach is tolerable and can potentially be an adjuvant to standard treatments among cancer patients.[45]
Conclusions
Although in the current review included studies were different in tumor size measurement methods, cancer types, sample size, carbohydrate amount of KD and intervention time; but most of them demonstrated that KD significantly show increase in survival time mean and a clear trend of slower tumor growth in pancreatic, prostate, gastric, brain, lung cancer.[6,9,10,14,15,16,17,18,19]
Further studies with longer duration and well-designed are needed to determine the effects of KD on cancers indices with considering side effects.
Financial support and sponsorship
Nil.
Conflict of interest
There is no conflict of interest.
References
- 1.Rafie N, Golpour Hamedani S, Ghiasvand R, Miraghajani M. Kefir and cancer: A systematic review of literatures. Arch Iran Med. 2015;18:852–7. [PubMed] [Google Scholar]
- 2.Farsinejad-Marj M, Talebi S, Ghiyasvand R, Miraghajani M. Adherence to Mediterranean diet and risk of breast cancer in premenopausal and postmenopausal women. Arch Iran Med. 2015;18:786–92. [PubMed] [Google Scholar]
- 3.Golpour S, Rafie N, Safavi SM, Miraghajani M. Dietary isoflavones and gastric cancer: A brief review of current studies. J Res Med Sci. 2015;20:893–900. doi: 10.4103/1735-1995.170627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jolfaie NR, Mirzaie S, Ghiasvand R, Askari G, Miraghajani M. The effect of glutamine intake on complications of colorectal and colon cancer treatment: A systematic review. J Res Med Sci. 2015;20:910–8. doi: 10.4103/1735-1995.170634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman JM, Kossoff EH. Ketosis and the ketogenic diet, 2010: Advances in treating epilepsy and other disorders. Adv Pediatr. 2010;57:315–29. doi: 10.1016/j.yapd.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, Jain P. The ketogenic diet and other dietary treatments for refractory epilepsy in children. Ann Indian Acad Neurol. 2014;17:253–8. doi: 10.4103/0972-2327.138471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Streijger F, Plunet WT, Lee JH, Liu J, Lam CK, Park S, et al. Ketogenic diet improves forelimb motor function after spinal cord injury in rodents. PLoS One. 2013;8:e78765. doi: 10.1371/journal.pone.0078765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paoli A, Bianco A, Damiani E, Bosco G. Ketogenic diet in neuromuscular and neurodegenerative diseases. Biomed Res Int. 2014;2014:474296. doi: 10.1155/2014/474296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poff AM, Ari C, Seyfried TN, D’Agostino DP. The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer. PLoS One. 2013;8:e65522. doi: 10.1371/journal.pone.0065522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen BG, Bhatia SK, Buatti JM, Brandt KE, Lindholm KE, Button AM, et al. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clin Cancer Res. 2013;19:3905–13. doi: 10.1158/1078-0432.CCR-12-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tisdale MJ, Brennan RA, Fearon KC. Reduction of weight loss and tumour size in a cachexia model by a high fat diet. Br J Cancer. 1987;56:39–43. doi: 10.1038/bjc.1987.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, Wittig R, et al. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer. 2008;8:122. doi: 10.1186/1471-2407-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klement RJ. Restricting carbohydrates to fight head and neck cancer-is this realistic? Cancer Biol Med. 2014;11:145–61. doi: 10.7497/j.issn.2095-3941.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klement RJ. Calorie or carbohydrate restriction? The ketogenic diet as another option for supportive cancer treatment. Oncologist. 2013;18:1056. doi: 10.1634/theoncologist.2013-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim HS, Masko EM, Poulton SL, Kennedy KM, Pizzo SV, Dewhirst MW, et al. Carbohydrate restriction and lactate transporter inhibition in a mouse xenograft model of human prostate cancer. BJU Int. 2012;110:1062–9. doi: 10.1111/j.1464-410X.2012.10971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masko EM, Thomas JA, 2nd, Antonelli JA, Lloyd JC, Phillips TE, Poulton SH, et al. Low-carbohydrate diets and prostate cancer: How low is “low enough”? Cancer Prev Res (Phila) 2010;3:1124–31. doi: 10.1158/1940-6207.CAPR-10-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab (Lond) 2007;4:5. doi: 10.1186/1743-7075-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, et al. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS One. 2012;7:e36197. doi: 10.1371/journal.pone.0036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mavropoulos JC, Buschemeyer WC, 3rd, Tewari AK, Rokhfeld D, Pollak M, Zhao Y, et al. The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer Prev Res (Phila) 2009;2:557–65. doi: 10.1158/1940-6207.CAPR-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morscher RJ, Aminzadeh-Gohari S, Feichtinger RG, Mayr JA, Lang R, Neureiter D, et al. Inhibition of neuroblastoma tumor growth by ketogenic diet and/or calorie restriction in a CD1-Nu mouse model. PLoS One. 2015;10:e0129802. doi: 10.1371/journal.pone.0129802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao GW, Chen YS, He DM, Wang HY, Wu GH, Zhang B. Growth of human colon cancer cells in nude mice is delayed by ketogenic diet with or without omega-3 fatty acids and medium-chain triglycerides. Asian Pac J Cancer Prev. 2015;16:2061–8. doi: 10.7314/apjcp.2015.16.5.2061. [DOI] [PubMed] [Google Scholar]
- 23.Seyfried TN, Sanderson TM, El-Abbadi MM, McGowan R, Mukherjee P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. Br J Cancer. 2003;89:1375–82. doi: 10.1038/sj.bjc.6601269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shukla SK, Gebregiworgis T, Purohit V, Chaika NV, Gunda V, Radhakrishnan P, et al. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer Metab. 2014;2:18. doi: 10.1186/2049-3002-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caso J, Masko EM, Ii JA, Poulton SH, Dewhirst M, Pizzo SV, et al. The effect of carbohydrate restriction on prostate cancer tumor growth in a castrate mouse xenograft model. Prostate. 2013;73:449–54. doi: 10.1002/pros.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, an effective alternative therapy for malignant brain cancer. Nutr Metab. 2007;4:1. doi: 10.1186/1743-7075-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krauth D, Woodruff TJ, Bero L. Instruments for assessing risk of bias and other methodological criteria of published animal studies: A systematic review. Environ Health Perspect. 2013;121:985–92. doi: 10.1289/ehp.1206389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen BG, Bhatia SK, Anderson CM, Eichenberger-Gilmore JM, Sibenaller ZA, Mapuskar KA, et al. Ketogenic diets as an adjuvant cancer therapy: History and potential mechanism. Redox Biol. 2014;2:963–70. doi: 10.1016/j.redox.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maroon JC, Seyfried TN, Donohue JP, Bost J. The role of metabolic therapy in treating glioblastoma multiforme. Surg Neurol Int. 2015;6:61. doi: 10.4103/2152-7806.155259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seyfried TN, Kiebish MA, Marsh J, Shelton LM, Huysentruyt LC, Mukherjee P. Metabolic management of brain cancer. Biochim Biophys Acta. 2011;1807:577–94. doi: 10.1016/j.bbabio.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Liang BC, Grootveld M. The importance of mitochondria in the tumourigenic phenotype: Gliomas as the paradigm (review) Int J Mol Med. 2011;27:159–71. doi: 10.3892/ijmm.2010.579. [DOI] [PubMed] [Google Scholar]
- 32.Maroon J, Bost J, Amos A, Zuccoli G. Restricted calorie ketogenic diet for the treatment of glioblastoma multiforme. J Child Neurol. 2013;28:1002–8. doi: 10.1177/0883073813488670. [DOI] [PubMed] [Google Scholar]
- 33.Seyfried TN, Shelton LM, Mukherjee P. Does the existing standard of care increase glioblastoma energy metabolism? Lancet Oncol. 2010;11:811–3. doi: 10.1016/S1470-2045(10)70166-2. [DOI] [PubMed] [Google Scholar]
- 34.Robey RB, Hay N. Is Akt the “Warburg kinase”? – Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol. 2009;19:25–31. doi: 10.1016/j.semcancer.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDaniel SS, Rensing NR, Thio LL, Yamada KA, Wong M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia. 2011;52:e7–11. doi: 10.1111/j.1528-1167.2011.02981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woolf EC, Stafford P, Abdelwahab MG, Fenton K, Preul MC, Scheck AC. The ketogenic diet potentiates radiation therapy in a mouse model of glioma: Effects on inflammatory pathways and reactive oxygen species. Cancer Res. 2013;73(8 Suppl):4441. [Google Scholar]
- 37.Stafford P, Abdelwahab MG, Kim DY, Preul MC, Rho JM, Scheck AC. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr Metab (Lond) 2010;7:74. doi: 10.1186/1743-7075-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dupuis N, Curatolo N, Benoist JF, Auvin S. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. 2015;56:e95–8. doi: 10.1111/epi.13038. [DOI] [PubMed] [Google Scholar]
- 39.Ruskin DN, Kawamura M, Masino SA. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PLoS One. 2009;4:e8349. doi: 10.1371/journal.pone.0008349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim DY, Hao J, Liu R, Turner G, Shi FD, Rho JM. Inflammation-mediated memory dysfunction and effects of a ketogenic diet in a murine model of multiple sclerosis. PLoS One. 2012;7:e35476. doi: 10.1371/journal.pone.0035476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang HC, Chung DE, Kim DW, Kim HD. Early- and late-onset complications of the ketogenic diet for intractable epilepsy. Epilepsia. 2004;45:1116–23. doi: 10.1111/j.0013-9580.2004.10004.x. [DOI] [PubMed] [Google Scholar]
- 42.Paoli A, Moro T, Bosco G, Bianco A, Grimaldi KA, Camporesi E, et al. Effects of n-3 polyunsaturated fatty acids (ω-3) supplementation on some cardiovascular risk factors with a ketogenic Mediterranean diet. Mar Drugs. 2015;13:996–1009. doi: 10.3390/md13020996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moriyama K, Watanabe M, Yamada Y, Shiihara T. Protein-losing enteropathy as a rare complication of the ketogenic diet. Pediatr Neurol. 2015;52:526–8. doi: 10.1016/j.pediatrneurol.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 44.Hawkes CP, Levine MA. Ketotic hypercalcemia: A case series and description of a novel entity. J Clin Endocrinol Metab. 2014;99:1531–6. doi: 10.1210/jc.2013-4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bozzetti F, Zupec-Kania B. Toward a cancer-specific diet. Clin Nutr. 2016;35:1188–95. doi: 10.1016/j.clnu.2015.01.013. [DOI] [PubMed] [Google Scholar]


