Abstract
Urinary bladder carcinoma (UBC) is the ninth most common malignancy and the second most common urological malignancy after prostate cancer in men. Thoracic metastases occur in more than half of those with muscle-invasive disease, and these generally assume the form of multiple solid parenchymal lesions characteristic of hematogenous seeding of the lung. Unusual patterns of thoracic spread of UBC have also been described albeit sporadically in the form of case reports and series. The aim of our case series is to provide illustrations of several atypical patterns of thoracic involvement by UBC such as isolated mediastinal lymphadenopathy, cavitary lung metastases, malignant pleural effusion, endobronchial disease, and pulmonary tumor embolism. This review is meant to highlight the intersection of the fields of urological oncology and thoracic radiology in the care of patients with UBC.
Keywords: Urinary bladder carcinoma, transitional cell carcinoma, thoracic metastasis, urothelial cell carcinoma

Introduction
Urinary bladder carcinoma (UBC), also known as transitional cell carcinoma of the urinary system, is the ninth most common cancer worldwide and afflicts men much more often than women. In the United States, the estimated annual prevalence of UBC is close to 600,000 and the annual incidence is over 60,000.[1] It is primarily a smoking-related malignancy in Western countries.[2] UBC can be divided into two distinct phenotypes based on propensity for invasion.[3] Approximately, 80% of cases present with low-grade exophytic papillary lesions that are amenable to local resection and although prone to recurrence generally remain localized. The other phenotype is that of muscle-invasive disease, which can be confined to the bladder but produces distant metastases in up to 70% of patients.[4] Liver (47%), lung (45%), and bone (32%) are the three most frequent sites of distant spread.[5] The typical pattern of thoracic metastases from UBC is that of multiple randomly distributed solid lung nodules characteristic of hematogenous dissemination [Figure 1]. Diffuse osseous metastases affecting the ribs or thoracic vertebrae also occur frequently. While radical cystectomy remains the standard approach to the treatment of nonmetastatic muscle-invasive disease in operative candidates, cisplatin-based chemotherapy has been the traditional choice for metastatic UBC. Recently, the combination of gemcitabine and docetaxel has shown promising results as the second-line treatment of metastatic UBC, particularly in cases with lung involvement.[6] Curative pulmonary metastasectomy of small solitary metastatic lesions has been reported to yield 5-year progression-free survival of about 25%.[7]
Figure 1.
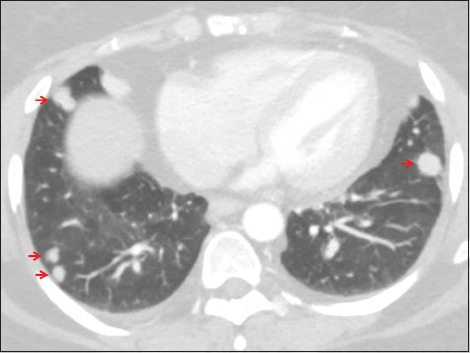
A 47-year-old female with a history of urinary bladder carcinoma presented for follow-up. Axial computed tomography of the chest set to lung windows demonstrates multiple randomly distributed solid nodules (arrows), a pattern typical of hematogenous dissemination of malignancy.
Various unusual patterns of thoracic metastases from UBC have been reported as individual cases or small series. Herein, we illustrate and discuss such atypical manifestations using representative cases from our institutions.
Case Reports
Case 1: Isolated mediastinal lymphadenopathy
A 68-year-old male, formerly a heavy smoker, was evaluated in the pulmonary clinic for an abnormal chest radiograph (CR). He had been diagnosed with high-grade invasive UBC with focal squamous differentiation 2 years before this evaluation and had been treated with serial transurethral resections as he had refused cystectomy. At the clinic visit, he reported recent weight loss and swelling on the left side of the neck. Physical examination was significant for left posterior cervical lymphadenopathy, scattered wheezes on lung auscultation, and lower extremity pitting edema. Routine laboratory evaluation was unremarkable. CR showed a widened right paratracheal stripe [Figure 2a]. Computed tomography (CT) of the chest without intravenous contrast demonstrated multicompartment mediastinal lymphadenopathy, including the right lower paratracheal (4R) and subcarinal (7) lymph node stations [Figure 2b]. There were no parenchymal lesions. The patient underwent bronchoscopy with core transbronchial needle aspiration (TBNA) of the 4R lymph node, which revealed metastatic carcinoma histologically compatible with urothelial origin with squamous differentiation similar to that seen on the patient's prior bladder biopsies. Fine-needle aspiration of the enlarged left posterior cervical lymph node yielded similar morphology. The patient refused to undergo chemotherapy and was subsequently lost to follow-up.
Figure 2.
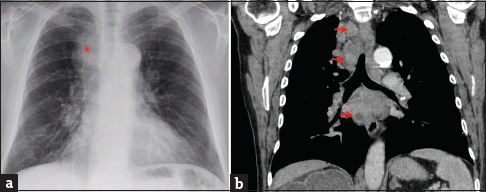
A 68-year-old male with a history of high-grade invasive urinary bladder carcinoma presented with weight loss and left-sided neck swelling. (a) Chest radiograph (posterior-anterior projection) showed a widened right paratracheal stripe (asterisk). The diaphragmatic contours are flattened, suggesting lung hyperinflation. (b) Chest computed tomography set to soft tissue windows (coronal reconstruction) demonstrates bulky paratracheal and subcarinal lymphadenopathy with areas of hypodensity suggestive of necrosis (arrows). Transbronchial needle aspiration of the right paratracheal lymph node revealed metastatic carcinoma histologically compatible with urothelial origin.
Case 2: Cavitary/cystic lung metastases
An 85-year-old female with kyphoscoliosis, chronic obstructive pulmonary disease, and recurrent low-grade papillary UBC presented for evaluation of an abnormal CR performed elsewhere that was suggestive of bilateral cavitary lung lesions. She had previously undergone multiple transurethral resections of bladder tumor. She was a former 40 pack-year smoker. She denied constitutional, pulmonary, or genitourinary (GU) symptoms. Physical examination revealed a thin woman with kyphoscoliosis. There were faint end-expiratory wheezes. No abdominal masses were palpable. Chest CT revealed numerous cavitary nodules and masses, some with thick [Figure 3a] and others with thin [Figure 3b] walls. CT-guided biopsy of the lesion in Figure 3a was consistent with metastatic urothelial carcinoma [Figure 3c and d]. The patient was referred to oncology for consideration of chemotherapy, which she declined.
Figure 3.
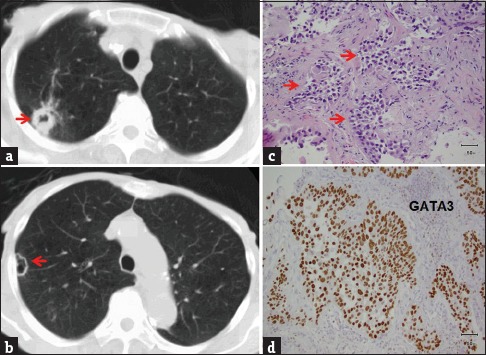
An 85-year-old female with chronic obstructive pulmonary disease and recurrent low-grade papillary urinary bladder carcinoma presented for the evaluation of an abnormal chest radiograph. Axial chest computed tomography set to lung windows reveals both thick-walled (a) and thin-walled (b) cavitary nodules and masses (arrows). Computed tomography-guided needle biopsy of the lesion in panel A showing multiple nests of malignant cells (c, arrows) that stain positive for GATA-3 on immunohistochemistry (d), features consistent with metastatic urothelial carcinoma.
Case 3: Malignant pleural effusion
A 54-year-old female presented to the emergency department with dyspnea and chest discomfort. There was no cough, sputum production, or fever. Her medical history was significant for UBC metastatic to the liver, lungs, subcutaneous tissue, and left eye. On initial evaluation, she was tachycardic and tachypneic with an oxygen saturation of 82% on room air. Notable physical examination findings included left eye proptosis, multiple subcutaneous nodules, and decreased breath sounds over the lung bases bilaterally. There was no edema or jugular venous distention. CT angiography of the chest was performed to exclude pulmonary embolism, which was not present, but the scan demonstrated moderate bilateral pleural effusions with compressive atelectasis [Figure 4a]. There was an increase in the number of pulmonary and subcutaneous nodules as compared to a prior study. Ultrasound-guided right thoracentesis yielded 800 ml of serosanguinous pleural fluid and resulted in relief of her symptoms. The fluid cytology specimen was consistent with metastatic UBC [Figure 4b and c]. Her poor functional status precluded further therapeutic measures, and she was discharged home with hospice services.
Figure 4.
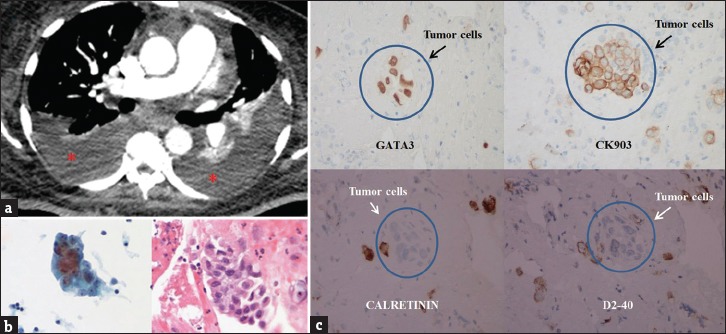
A 54-year-old female with a history of metastatic urinary bladder carcinoma presented with shortness of breath and hypoxemia. (a) Axial chest computed tomography set to soft tissue windows demonstrates bilateral pleural effusions (asterisks) with compressive atelectasis. (b) Cytology from the right pleural effusion demonstrates tumor cells with enlarged nuclei, increased nuclear to cytoplasmic ratio, and irregular nuclear membrane. (c) Immunohistochemistry demonstrates positive staining for GATA-3 and CK903 and negative staining for calretinin and D2-40, a pattern consistent with metastatic urothelial carcinoma.
Case 4: Endobronchial lesion
A 79-year-old female with UBC diagnosed and resected 7 months previously presented with cough and progressive shortness of breath and wheezing over a 2-month period. Examination revealed inspiratory and expiratory wheezing over the right anterior chest. Investigations led to a CR which was consistent with right middle and lower lobe atelectasis [Figure 5a]. CT of the chest demonstrated a mass partially occluding the bronchus intermedius, right middle lobe bronchus, and right lower lobe bronchus [Figure 5b]. Multiple lung nodules were present as well (not shown). The patient was brought to the bronchoscopy suite for flexible bronchoscopy, endobronchial tumor removal, and lymph node biopsy. Pathology was consistent with high-grade UBC. The patient tolerated the procedure well, and she was discharged with plans to follow-up in oncology clinic; however, she was admitted to an outside hospital 2 weeks later with postobstructive pneumonia. Rigid bronchoscopy with mechanical debridement and ablative therapy was performed but was complicated by cardiac arrest, and the patient expired.
Figure 5.
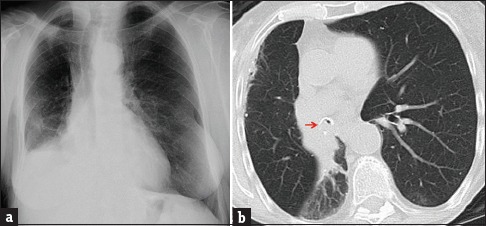
A 79-year-old female with urinary bladder carcinoma diagnosed and resected 7 months previously presented with shortness of breath and focal right-sided wheezing. (a) Chest radiograph (anterior-posterior projection) showing volume loss in the right hemithorax with rightward tracheal deviation and silhouetting of the right heart border and right diaphragm, suggestive of right lower and right middle lobe collapse. (b) Axial chest computed tomography set to lung windows showing endobronchial disease occluding the bronchus intermedius (arrow). The patient underwent bronchoscopy with endobronchial tumor debulking; pathology was indicative of high-grade high-grade metastatic urothelial carcinoma.
Case 5: Pulmonary tumor thromboemboli
A 47-year-old male with morbid obesity and hypertension was transferred from an outside institution for elective surgical management of UBC diagnosed through transurethral resection of bladder tumor. His initial course was complicated by hypoxia to 94% with an increased arterial-alveolar gradient, prompting CT angiography of the chest which demonstrated filling defects in multiple left- and right-sided segmental arteries [Figure 6]. Anticoagulation was started and an inferior vena cava (IVC) filter was placed. One week later, the patient underwent radical cystoprostatectomy with neobladder reconstruction and rectosigmoid colon resection as tumor was attached to the descending colon. Pathologic examination of the tumor revealed UBC with sarcomatoid features. His postoperative course was complicated by failure to wean from the ventilator due to persistent hypoxemia despite anticoagulation. The patient and his family eventually elected for comfort measures only and he died shortly thereafter. Major findings at autopsy included extensive tumor within the abdominal cavity and retroperitoneum, diffuse pulmonary metastases, and intravascular tumor thrombi. The IVC filter was also plugged with tumor thrombus, and microscopic examination revealed the tumor to be identical to the previously resected high-grade UBC with sarcomatoid features.
Figure 6.
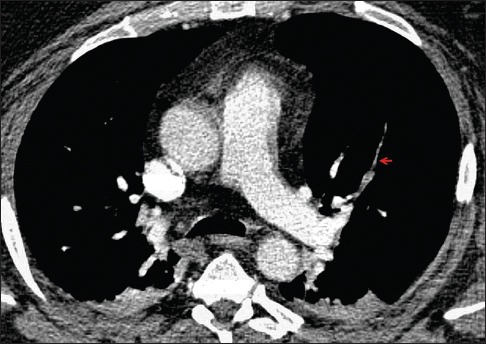
A 47-year-old man with morbid obesity presented for transurethral resection of a bladder mass. Preoperative hypoxemia prompted computed tomography angiography of the chest, which demonstrates multiple filling defects, including in the anterior segmental artery of the left upper lobe (arrow). Treatment with anticoagulation was started and the patient underwent surgical removal of the bladder tumor, which revealed urothelial carcinoma with sarcomatoid features. His postoperative course was complicated by persistent hypoxemia and progression of disease, and the patient ultimately expired. Autopsy findings demonstrated intravascular tumor thrombi identical on microscopic examination to the previously diagnosed tumor.
Discussion
Isolated mediastinal lymphadenopathy
Primary lung carcinoma and lymphoma are by far the most common causes of malignant intrathoracic lymphadenopathy. Intrathoracic lymph node metastases from extrathoracic primary sites are a rare occurrence. In the era before routine CT scanning, review of 1071 cases of extrathoracic malignancy evaluated at Massachusetts General Hospital (MGH) yielded only 25 cases (2.3%) with CR evidence of mediastinal and/or hilar lymphadenopathy.[8] More recently, Song et al., reported that approximately 10% of the 536 patients who underwent endobronchial ultrasound-guided TBNA (EBUS-TBNA) of intrathoracic lymph nodes over an 18-month period did so for suspected involvement by extrathoracic malignancy.[9] UBC is among the least common extrathoracic sites to give rise to intrathoracic lymph node spread, accounting for 4%–13% of extrathoracic malignancies diagnosed by intrathoracic lymph node sampling.[9,10] Only 1 out of 34 patients with UBC in the aforementioned MGH series (3%) had intrathoracic lymphadenopathy detected on CR.[8] Among GU cancers, renal cell carcinoma and testicular tumors appear to produce intrathoracic lymph node involvement more frequently than UBC. Overall, gastrointestinal, breast, and head and neck malignancies are the extrathoracic malignancies most commonly encountered in intrathoracic lymph node samples.
Cavitary/cystic lung metastases
UBC has long been recognized as an etiology of cavitary lung metastases. Among the 16 cases with such metastases in the seminal series reported by Dodd and Boyle from MD Anderson Cancer Center before the advent of CT, one was a patient with UBC.[11] In that series, nearly 70% of the cavitary lesions were of squamous histology, which is the cell type most strongly associated with lung cavitation. The characteristic tendency of squamous cell carcinoma (SCC) to cavitate in the lung has led to the theory that central necrosis is promoted by tumor histology and is not simply a function of inadequate perfusion to the core of the mass.[11] In the era of CT imaging, cavitary lung metastases from UBC have been reported only sporadically and have been described with and without squamous differentiation, both with superficial and invasive primary tumors, and in the setting of established UBC and as its initial presentation.[12,13,14,15,16] Curiously, a comprehensive description of the radiology of metastatic UBC by Goldman et al., from Johns Hopkins that featured 28 cases of lung involvement by CR did not report a single instance of cavitation.[17] Nonneoplastic etiologic categories for cavitary lung lesions-more relevant in the absence of overt metastatic malignancy-include bacterial and atypical infection, septic embolization, vasculitis, and necrobiotic nodules. Decreasing wall thickness of the lung cavity correlates positively with the likelihood of benign disease.[18] Certain diagnostic challenges remain even in the context of established malignancy. For one, chemotherapy itself has been associated with secondary cavitation.[19] In addition, in the era of heavy reliance on needle sampling, distinguishing high-grade UBC, especially in the presence of squamous differentiation, from either primary lung or metastatic SCC can present a challenge for pathologists. It has recently been shown that these two cell types exhibit largely nonoverlapping immunohistochemistry (IHC) staining patterns for CK7, CK20, GATA-3, and Uroplakin III, with positivity of these markers reliably favoring UBC.[20,21]
Malignant pleural effusion
Malignancy is the third most common cause of pleural effusions in the United States after congestive heart failure and infection, with lung cancer, breast cancer, and lymphoma responsible for about 75% of malignant pleural effusions (MPEs).[22] While the GU tract overall is the fourth most common primary tumor location identified, UBC per se rarely gives rise to MPE.[23,24] Involvement of the pleura usually occurs in the setting of widespread metastatic disease though isolated pleural involvement has also been reported.[24,25] Multiple mechanisms contribute to the formation of MPE, including increased pleural capillary permeability and lymphatic outflow obstruction by tumor.[22] Cytological examination of pleural fluid obtained by thoracentesis is the preferred method of confirming the diagnosis of MPE, but its yield is only approximately 50%–60% in proven malignant disease.[26] Tumor type and burden affect yield as do the quantity of fluid and the experience of the cytopathologist. When present, intracytoplasmic eosinophilic inclusions (Melamed-Wolinska bodies) suggest a urothelial primary, but the cytomorphological features are ultimately nonspecific and overlap with more common etiologies.[27] Accurate diagnosis rests on the combination of clinical history, cytomorphology, and IHC.[24] MPE portends a poor prognosis, and treatment is invariably palliative. Along with chemotherapy for appropriate candidates, symptomatic fluid recurrence can be addressed by repeated thoracenteses, chemical pleurodesis, or indwelling tunneled pleural catheter placement. The modality of choice depends on an individual patient's expected survival, functional status, and preference.
Endobronchial lesions
Endobronchial mass lesions are likely the rarest form of metastatic spread of UBC to the thoracic cavity, which is not surprising given the overall rarity of this pattern among extrathoracic cancers, though frequency estimates are highly variable even among autopsy studies depending on the population and definition of endobronchial involvement.[28,29] Summation of the available series, some of them relatively large, would yield under a dozen cases of endobronchial disease in UBC, and such cases are entirely absent from a few of these reports.[30,31,32,33,34,35] Breast, colorectal, and renal cell are the most common primary sites associated with endobronchial spread.[34] This tends to be a late manifestation of the respective primary tumor and is almost always a metachronous occurrence.[35] At the time of presentation with endobronchial metastasis, involvement of other distant sites is usually detectable as well. The typical symptoms of endobronchial malignancy are cough, dyspnea, hemoptysis, and focal wheezing. The diagnosis is apparent bronchoscopically, and sampling is readily accomplished by this route if necessary. Since endobronchial lesions are generally part of a widely metastatic clinical course, the vast majority of patients are approached palliatively. Chemotherapy, radiation, and interventional bronchoscopic techniques can all be applied with the aim of restoration of airway patency to relieve dyspnea in symptomatic cases and improve the quality of remaining life.[36]
Pulmonary tumor thromboemboli
Pulmonary tumor emboli can be defined as the presence of tumor cells within the pulmonary arterial vasculature not contiguous with metastatic foci.[37,38] It occurs in four main types: (1) large tumor emboli occluding main, lobar, or segmental branches, (2) isolated microvascular disease of the small arteries or arterioles, (3) pulmonary microvascular invasion in the setting of generalized lymphatic dissemination, or (4) some combination of these.[39,40,41] The thrombi consist of varying proportions of tumor and thrombus. Pulmonary thrombotic microangiopathy (PTTM) represents a subtype of microvascular disease and occurs when tumor cells induce local activation of fibrocellular intimal proliferation through various molecules such as vascular endothelial growth factor and tissue factor, leading to pulmonary hypertension and cor pulmonale.[38,42] The reported incidence of tumor thromboembolism in metastatic malignancy varies widely among studies, from <3% to as high as 44%.[38,40] It is under-recognized given the shared risk factors and clinical presentation with venous thromboembolism. No clinical findings are specific for tumor emboli, but lack of response to anticoagulation for pulmonary embolism should prompt consideration of this diagnosis.[37] Testing such as ventilation-perfusion scanning or CT angiography are unlikely to distinguish the two, but pulmonary wedge aspiration cytology and EBUS are two potential techniques that may yield the diagnosis, though the majority of cases are identified post-mortem.[43,44] Breast, lung, and prostate cancers are malignancies most commonly associated with tumor emboli, which likely reflects their overall prevalence. Gastric cancer may impart a particular predisposition to PTTM,[45] whereas UBC appears to be a rare cause: review of the literature reveals only several published case reports.[39,40,46,47,48] No evidence exists that antemortem diagnosis changes outcomes, but it may prevent unnecessary anticoagulation and testing. Large central tumor emboli may be surgically removed, but palliative chemotherapy is the usual course of treatment.
Summary
In this case series, we have illustrated examples of metastatic UBC occurring in all five compartments of the thorax: the mediastinum, the lung parenchyma (where it is capable of causing cavitation), the pleural space, the airway lumen, and the pulmonary vasculature. As UBC and primary lung carcinoma are both predominantly smoking-related malignancies, they afflict a similar patient demographic, and therefore, it is important to be aware of the differences and similarities in the behavior of the two malignancies in the chest. It is our hope that this case-based presentation will prompt consideration of metastatic UBC to the thorax in the appropriate clinical setting, even when it manifests atypically.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp?2017/7/1/23/207064
References
- 1.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–93. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelucchi C, Bosetti C, Negri E, Malvezzi M, La Vecchia C. Mechanisms of disease: The epidemiology of bladder cancer. Nat Clin Pract Urol. 2006;3:327–40. doi: 10.1038/ncpuro0510. [DOI] [PubMed] [Google Scholar]
- 3.Dinney CP, McConkey DJ, Millikan RE, Wu X, Bar-Eli M, Adam L, et al. Focus on bladder cancer. Cancer Cell. 2004;6:111–6. doi: 10.1016/j.ccr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Jewett HJ, Strong GH. Infiltrating carcinoma of the bladder; relation of depth of penetration of the bladder wall to incidence of local extension and metastases. J Urol. 1946;55:366–72. doi: 10.1016/S0022-5347(17)69924-5. [DOI] [PubMed] [Google Scholar]
- 5.Wallmeroth A, Wagner U, Moch H, Gasser TC, Sauter G, Mihatsch MJ. Patterns of metastasis in muscle-invasive bladder cancer (pT2-4): An autopsy study on 367 patients. Urol Int. 1999;62:69–75. doi: 10.1159/000030361. [DOI] [PubMed] [Google Scholar]
- 6.Naiki T, Kawai N, Hashimoto Y, Okamura T, Ando R, Yasui T, et al. Gemcitabine and docetaxel, an effective second-line chemotherapy for lung metastasis of urothelial carcinoma. Int J Clin Oncol. 2014;19:516–22. doi: 10.1007/s10147-013-0574-1. [DOI] [PubMed] [Google Scholar]
- 7.Matsuguma H, Yoshino I, Ito H, Goya T, Matsui Y, Nakajima J, et al. Is there a role for pulmonary metastasectomy with a curative intent in patients with metastatic urinary transitional cell carcinoma? Ann Thorac Surg. 2011;92:449–53. doi: 10.1016/j.athoracsur.2011.03.097. [DOI] [PubMed] [Google Scholar]
- 8.McLoud TC, Kalisher L, Stark P, Greene R. Intrathoracic lymph node metastases from extrathoracic neoplasms. AJR Am J Roentgenol. 1978;131:403–7. doi: 10.2214/ajr.131.3.403. [DOI] [PubMed] [Google Scholar]
- 9.Song JU, Park HY, Jeon K, Koh WJ, Suh GY, Chung MP, et al. The role of endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of mediastinal and hilar lymph node metastases in patients with extrapulmonary malignancy. Intern Med. 2011;50:2525–32. doi: 10.2169/internalmedicine.50.5834. [DOI] [PubMed] [Google Scholar]
- 10.Sanz-Santos J, Cirauqui B, Sanchez E, Andreo F, Serra P, Monso E, et al. Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of intrathoracic lymph node metastases from extrathoracic malignancies. Clin Exp Metastasis. 2013;30:521–8. doi: 10.1007/s10585-012-9556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dodd GD, Boyle JJ. Excavating pulmonary metastases. Am J Roentgenol Radium Ther Nucl Med. 1961;85:277–93. [PubMed] [Google Scholar]
- 12.Angulo JC, Lopez JI, Flores N. Cavitation of lung metastases from bladder cancer. Report of two cases. Tumori. 1993;79:141–3. doi: 10.1177/030089169307900213. [DOI] [PubMed] [Google Scholar]
- 13.Ocakli B, Karakurt Z, Sulu E, Turker H, Kuzu H, Durucu M, et al. Cystic pulmonary metastasis of bladder cancer: Report of two cases. Turk Respir J. 2002;3:116–21. [Google Scholar]
- 14.Dougherty DW, Gonsorcik VK, Harpster LE, Trussell JC, Drabick JJ. Superficial bladder cancer metastatic to the lungs: Two case reports and review of the literature. Urology. 2009;73:210.e3–5. doi: 10.1016/j.urology.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 15.Fiorelli A, Vicidomini G, Messina G, Santini M. Metastasis from transitional cell carcinoma of urinary bladder as cystic pulmonary lesion. J Thorac Dis. 2011;3:71–3. doi: 10.3978/j.issn.2072-1439.2010.11.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurian A, Lee J, Born A. Urothelial bladder cancer with cavitary lung metastases. Can Respir J. 2011;18:e46–7. doi: 10.1155/2011/273241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldman SM, Fajardo AA, Naraval RC, Madewell JE. Metastatic transitional cell carcinoma from the bladder: Radiographic manifestions. AJR Am J Roentgenol. 1979;132:419–25. doi: 10.2214/ajr.132.3.419. [DOI] [PubMed] [Google Scholar]
- 18.Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: Diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135:1269–71. doi: 10.2214/ajr.135.6.1269. [DOI] [PubMed] [Google Scholar]
- 19.Thalinger AR, Rosenthal SN, Borg S, Arseneau JC. Cavitation of pulmonary metastases as a response to chemotherapy. Cancer. 1980;46:1329–32. doi: 10.1002/1097-0142(19800915)46:6<1329::aid-cncr2820460605>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.Cantley RL, Kapur U, Truong L, Cimbaluk D, Barkan GA, Wojcik E, et al. Fine-needle aspiration diagnosis of metastatic urothelial carcinoma: A review. Diagn Cytopathol. 2012;40:173–8. doi: 10.1002/dc.21612. [DOI] [PubMed] [Google Scholar]
- 21.Gruver AM, Amin MB, Luthringer DJ, Westfall D, Arora K, Farver CF, et al. Selective immunohistochemical markers to distinguish between metastatic high-grade urothelial carcinoma and primary poorly differentiated invasive squamous cell carcinoma of the lung. Arch Pathol Lab Med. 2012;136:1339–46. doi: 10.5858/arpa.2011-0575-OA. [DOI] [PubMed] [Google Scholar]
- 22.Light RW. Pleural effusions. Med Clin North Am. 2011;95:1055–70. doi: 10.1016/j.mcna.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Thomas JM, Musani AI. Malignant pleural effusions: A review. Clin Chest Med. 2013;34:459–71. doi: 10.1016/j.ccm.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Huang CC, Attele A, Michael CW. Cytomorphologic features of metastatic urothelial carcinoma in serous effusions. Diagn Cytopathol. 2013;41:569–74. doi: 10.1002/dc.22896. [DOI] [PubMed] [Google Scholar]
- 25.McGrath SM, Rana DN, Lynch M, Desai M. Metastatic transitional cell carcinoma causing a unilateral pleural effusion: A case report. Acta Cytol. 2008;52:351–3. doi: 10.1159/000325521. [DOI] [PubMed] [Google Scholar]
- 26.Porcel JM, Light RW. Pleural effusions. Dis Mon. 2013;59:29–57. doi: 10.1016/j.disamonth.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Renshaw AA, Madge R, Granter SR. Intracytoplasmic eosinophilic inclusions (Melamed-Wolinska bodies).Association with metastatic transitional cell carcinoma in pleural fluid. Acta Cytol. 1997;41:995–8. doi: 10.1159/000332778. [DOI] [PubMed] [Google Scholar]
- 28.Rosenblatt MB, Lisa JR, Trinidad S. Pitfalls in the clinical histologic diagnosis of bronchogenic carcinoma. Dis Chest. 1966;49:396–404. doi: 10.1378/chest.49.4.396. [DOI] [PubMed] [Google Scholar]
- 29.Braman SS, Whitcomb ME. Endobronchial metastasis. Arch Intern Med. 1975;135:543–7. [PubMed] [Google Scholar]
- 30.Shepherd MP. Endobronchial metastatic disease. Thorax. 1982;37:362–5. doi: 10.1136/thx.37.5.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heitmiller RF, Marasco WJ, Hruban RH, Marsh BR. Endobronchial metastasis. J Thorac Cardiovasc Surg. 1993;106:537–42. [PubMed] [Google Scholar]
- 32.Salud A, Porcel JM, Rovirosa A, Bellmunt J. Endobronchial metastatic disease: Analysis of 32 cases. J Surg Oncol. 1996;62:249–52. doi: 10.1002/(SICI)1096-9098(199608)62:4<249::AID-JSO4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Katsimbri PP, Bamias AT, Froudarakis ME, Peponis IA, Constantopoulos SH, Pavlidis NA. Endobronchial metastases secondary to solid tumors: Report of eight cases and review of the literature. Lung Cancer. 2000;28:163–70. doi: 10.1016/s0169-5002(99)00134-8. [DOI] [PubMed] [Google Scholar]
- 34.Sørensen JB. Endobronchial metastases from extrapulmonary solid tumors. Acta Oncol. 2004;43:73–9. doi: 10.1080/02841860310018053. [DOI] [PubMed] [Google Scholar]
- 35.Marchioni A, Lasagni A, Busca A, Cavazza A, Agostini L, Migaldi M, et al. Endobronchial metastasis: An epidemiologic and clinicopathologic study of 174 consecutive cases. Lung Cancer. 2014;84:222–8. doi: 10.1016/j.lungcan.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Ost DE, Ernst A, Grosu HB, Lei X, Diaz-Mendoza J, Slade M, et al. Therapeutic bronchoscopy for malignant central airway obstruction: Success rates and impact on dyspnea and quality of life. Chest. 2015;147:1282–98. doi: 10.1378/chest.14-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bassiri AG, Haghighi B, Doyle RL, Berry GJ, Rizk NW. Pulmonary tumor embolism. Am J Respir Crit Care Med. 1997;155:2089–95. doi: 10.1164/ajrccm.155.6.9196119. [DOI] [PubMed] [Google Scholar]
- 38.Lammi M, Wurzel J, Criner GJ. Pulmonary tumor embolism. Lung. 2010;188:441–3. doi: 10.1007/s00408-010-9249-0. [DOI] [PubMed] [Google Scholar]
- 39.Roberts KE, Hamele-Bena D, Saqi A, Stein CA, Cole RP. Pulmonary tumor embolism: A review of the literature. Am J Med. 2003;115:228–32. doi: 10.1016/s0002-9343(03)00305-x. [DOI] [PubMed] [Google Scholar]
- 40.Dhillon SS, Singh DJ, Dass B, Schaub CR. Transitional cell carcinoma manifesting as acute cor pulmonale: Cause of microscopic tumor embolism. South Med J. 2001;94:1030–2. [PubMed] [Google Scholar]
- 41.Hibino M, Akazawa K, Hikino K, Oe M. Pulmonary tumor embolism secondary to uterine corpus carcinosarcoma mimicking pulmonary thromboembolism. Intern Med. 2012;51:2603–7. doi: 10.2169/internalmedicine.51.7220. [DOI] [PubMed] [Google Scholar]
- 42.Salinas PD, Toth LN, Manning HL. A rare cause of postoperative hypotension. Chest. 2015;147:e175–80. doi: 10.1378/chest.14-2245. [DOI] [PubMed] [Google Scholar]
- 43.Desai NR, Greenhill SR, Vance M, Kovitz KL. Pulmonary tumor embolism diagnosed by endobronchial ultrasound. J Bronchology Interv Pulmonol. 2015;22:e16–9. doi: 10.1097/LBR.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 44.Buser M, Felizeter-Kessler M, Lenggenhager D, Maeder MT. Rapidly progressive pulmonary hypertension in a patient with pulmonary tumor thrombotic microangiopathy. Am J Respir Crit Care Med. 2015;191:711–2. doi: 10.1164/rccm.201501-0004IM. [DOI] [PubMed] [Google Scholar]
- 45.Uruga H, Fujii T, Kurosaki A, Hanada S, Takaya H, Miyamoto A, et al. Pulmonary tumor thrombotic microangiopathy: A clinical analysis of 30 autopsy cases. Intern Med. 2013;52:1317–23. doi: 10.2169/internalmedicine.52.9472. [DOI] [PubMed] [Google Scholar]
- 46.Chakeres DW, Spiegel PK. Fatal pulmonary hypertension secondary to intravascular metastatic tumor emboli. AJR Am J Roentgenol. 1982;139:997–1000. doi: 10.2214/ajr.139.5.997. [DOI] [PubMed] [Google Scholar]
- 47.Rüchardt A, Gruber B, Trenkwalder P. Intrapulmonary tumor cell embolism from cancer of the bladder as the cause of a subacute cor pulmonale. Dtsch Med Wochenschr. 2001;126:847–50. doi: 10.1055/s-2001-16015. [DOI] [PubMed] [Google Scholar]
- 48.Fitzpatrick TM, Covelli HD, Tenholder MF. The acute and insidious onset of pulmonary metastatic transitional cell carcinoma. Chest. 1991;99:498–500. doi: 10.1378/chest.99.2.498. [DOI] [PubMed] [Google Scholar]


