Abstract
Background:
Barr body is formed from random inactivation and condensation of one of the two female chromosomes in virtually all the somatic cells of female mammals. Buccal smears have been reported to be potential sources of Barr bodies.
Aim:
This study was done to assess the efficacy of acriflavine (AF) Schiff and Papanicolaou (PAP) stains in sex determination by identifying Barr bodies in buccal smears of both sexes.
Materials and Methods:
Two samples of buccal smears, collected from thirty males and thirty females in the age group of 16–60 years were used to demonstrate Barr bodies using AF Schiff and PAP stains, respectively. Hundred cells were examined for Barr body positive nucleus, and its mean percentage was calculated and statistically analyzed.
Results:
In females, AF Schiff stained positive cells ranged from 16% to 53% and PAP stained positive cells ranged from 9% to 38%. In males, 0–9% AF positive Barr bodies and 0–5% PAP stained Barr bodies were identified.
Conclusion:
Sex determination using buccal smear is a simple and reliable method. AF Schiff stain is better both qualitatively and quantitatively when compared to PAP stain, thus aids in more accurate sex determination.
Keywords: Acriflavine, Barr body, buccal smear, Papanicolaou, sex determination
Introduction
Sex determination forms an important aspect in the forensic investigation of assault, theft, and sexual offences to identify the culprit. In cases of sexual offences, buccal mucosal cells along with saliva stains are found in various parts of the body and at the scene of the crime. Buccal smears help in detecting the gender and identifying the person in train and aircraft accidents, natural disasters and in cases with decomposed primary sex organs. Gender determination of living person is required in doubtful cases like in sports, with altered physical and sexual features and to decide certain civil rights reserved for one sex.[1]
Sex determination can be done by karyotyping, polymerase chain reaction, fluorescent body (Y chromatin) demonstration in male somatic cells, Davidson body in female neutrophils, and Barr body in female somatic cells.[1] Barr body is formed from random inactivation and condensation of one of the two female chromosomes in virtually all the somatic cells of female mammals and was named after Barr and Bertram in 1949.[2] It appears as a facultative heterochromatic dark-stained peripheral nuclear structure visible during interphase in normal female somatic cells.[3]
Barr bodies can be demonstrated from buccal smears, vaginal smears, hair root, and skin biopsy. Studies have proven to recognize sex from buccal scrapes and hair up to 4 months with decreasing frequencies under the dried condition, but both the X chromatin and Y chromatin disappeared in 6 hours under wet conditions.[4,5,6,7]
Though many stains are used in identifying Barr bodies, only one study is reported in the literature using acriflavine (AF) Schiff on buccal smears.[4] A comparative study using Papanicolaou (PAP) and AF Schiff stains for Barr body identification is not yet reported. The objective of the present study was to assess the efficiency and reliability of PAP and AF Schiff stains in sex determination by identifying Barr bodies in buccal smears of both sexes.
Materials and Methods
Sample collection and preparation
The present study has been reviewed by the Institutional Ethical Committee and has therefore been performed in accordance with the ethical standards laid down in the 1965 Declaration of Helsinki. A total of thirty men and thirty women in the age group of 16–60 years were selected randomly for the study. A written informed consent was obtained from all the participants. The participants were first asked to rinse the mouth with water. Two buccal mucosal smears were collected from every participant by scraping with a clean flat wooden spatula after labelling the slide. The slides were air dried and fixed in 95% isopropyl alcohol for 30 min before staining.
Staining method
Acriflavine Schiff staining method
Following washing in running tap water for few seconds, the samples were hydrolyzed in preheated 1N hydrochloric acid at 60°C for 5 min. After washing in distilled water for few seconds, the slides treated with freshly prepared fluorescent Schiff-type reagents (AF hydrochloride 0.1 g dissolved in 100 ml 90% isopropyl alcohol) for 20 min, washed twice in acid alcohol (12N hydrochloric acid 1 ml and 70% ethyl alcohol 100 ml) for 1 min, and dehydrated in absolute alcohol.[4,8]
Papanicolaou staining method
The samples were stained in Harris's hematoxylin after rehydration, followed by dehydration in isopropyl alcohol and stained in OG 6 for 2 min. After rinsing in 95% isopropyl alcohol twice for 2 min each, they are stained in EA 50 for 3 min followed by rinse in 95% isopropyl alcohol for 1 minute.[9]
Barr body count
Cells with the intact nuclear membrane, without any crenation or depression, and the chromatins without clumps were selected and counted. The frequency of Barr body was examined by observing 100 nuclei per specimen under oil immersion. The Barr bodies that were found directly inside and attaching to the nuclear membrane were regarded as positive. PAP-stained Barr bodies were found as dark violet spot using research microscope (Lawrence and Mayo) and AF-stained Barr body appeared as bright greenish yellow spot using a fluorescence microscope (Lawrence and Mayo). The mean and standard deviation were determined for the percentage of Barr-body-positive cells in both the genders. Statistical analysis was done using the statistical software SPSS software version 16.0 (IBM Corporation, New York, USA) and P < 0.05 was considered to be statistically significant.
Results
Both in PAP and AF stained samples, females showed statistically significant increase in Barr bodies than males. Compared to PAP, AF staining showed more number of Barr bodies in both females and males [Table 1]. No correlation was found between the percentage of Barr-body-positive cells and the age of the individual in both males and females.
Table 1.
Barr body positive cells in males and females using Papanicolaou and acriflavine Schiff stains

In females, all the samples showed Barr bodies in the nucleus using AF stain and PAP stain. The frequencies of Barr bodies were 16–53% using AF stain and 9–38% using PAP stain [Table 1 and Figures 1, 2].
Figure 1.
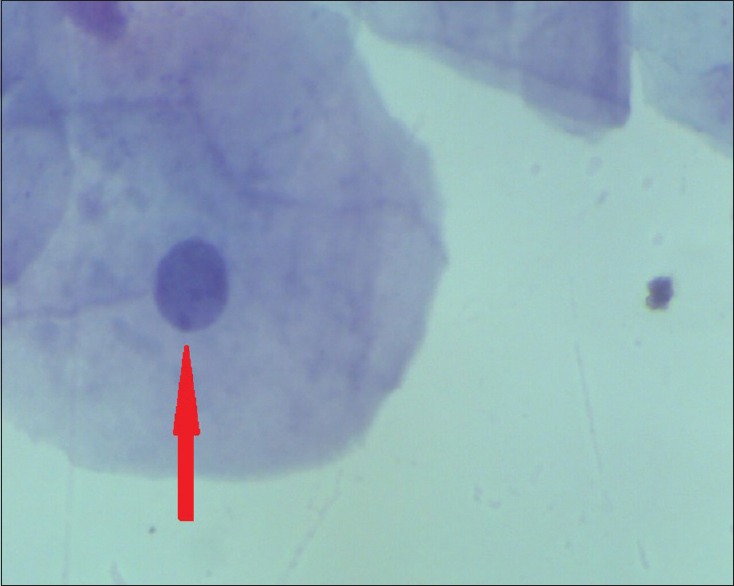
Barr body in the buccal smear of a female (Papanicolaou stain, ×100)
Figure 2.

Barr body in the buccal smear of a female (Acriflavine stain, ×100)
In males, 86% showed the presence of Barr bodies using AF stain and the range was 0–9%, while 60% showed Barr bodies using PAP stain with a range of 0–6% [Table 1 and Figures 3, 4].
Figure 3.
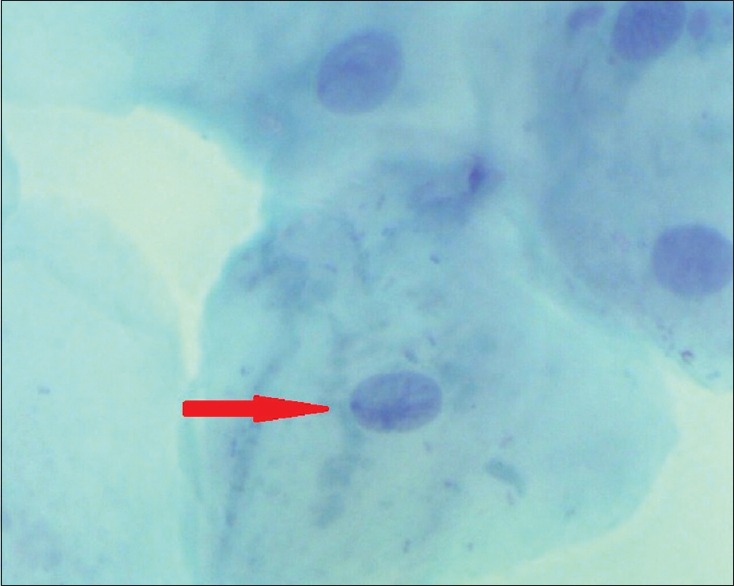
Barr body in the buccal smear of a male (Papanicolaou stain, ×100)
Figure 4.

Barr body in the buccal smear of a male (Acriflavine stain, ×100)
The positive and negative predictive values for the detection of Barr bodies using AF stain were calculated as 53% and 100%, respectively.
Discussion
The buccal smear technique to identify sex was first introduced by Moore and Barr in 1955. The process of inactivation of X chromatin is known as lyonization, the process named after the scientist Lyon. In 1961, Lyon outlined the X-inactivation, also known as the Lyon hypothesis. It states that only one of the X chromosomes is genetically active in females while the other X of either maternal or paternal origin undergoes random heteropyknosis and is inactive. This occurs at among all the cells of the blastocyst in females on or about the 16th day of embryonic life. Inactivation of the same X chromosome persists in all the cells derived from each precursor cell. Thus, normal women are in reality mosaics and have two populations of cells, one with an inactivated maternal X and the other with an inactivated paternal X.[10]
The positivity for Barr bodies in males is due to the inheritance of males to carry primary sex organs of both the sexes. The process of inactivation is incompletely understood, but it has been suggested that it is under the control of inactivation center, located at Xq13. XIST, a gene which is transcribed from the inactive X, is necessary for initiation and propagation of X inactivation and does so by coating the inactive X. As inactive X is turned off by XIST allele, up to 21% of genes on Xp, and 3% on Xq may escape X inactivation.[11]
The frequency of Barr body is decreased during pregnancy, as well as in women on oral contraceptives.[12] Low frequency of Barr body was observed in newborn females and their mothers on the 1st postpartum day increased gradually on the 2nd and 3rd day, which stabilized on the 5th day and became similar in both mothers and the children.[13] Reactivation of X chromosome was observed whenever the body was under physiological stress.[14] Low frequency suggestive of reactivation of inactive X chromosome is associated with malignancy and is confirmed by enhanced glucose-6-phosphate dehydrogenase activity.[15,16,17,18] Barr bodies appear as basophilic structures with varying morphology which can be spherical, rectangular, planoconvex, biconvex, or triangular measuring around 0.8 × 1.1 microns. In electron microscopy, it resembles various alphabetical letters such as V, W, S, or X.[19,20]
Since Barr bodies are present within the nuclear material, special stains for nucleus such as PAP stain, feulgen and guard stain, orcein, hematoxylin and eosin, cresyl violet, carbol fuchsin, and fluorescent stains are used to visualize them. Methods which stain the chromatin deeply and trace the cytoplasm and the nucleoli are considered superior. Feulgen stain requires hydrochloric acid hydrolysis before the actual staining, and it has the advantage that Barr body will not be confused with other structures as in buccal smear contaminated with microorganisms. AF Schiff (3, 6- diamino-lO-methyl acridine) is one of the most interesting stains for Barr body demonstration because of its fluorescence properties.[21] The spectral characteristics (yellow fluorescence emission at blue excitation) and the very high quantum efficiency of the fluorescence have led to an extensive use of this fluorescence technique.[22,23]
The main objective of the present study was to assess efficiency and reliability of AF Schiff and PAP stains in sex determination by identifying Barr bodies in buccal smears of both sexes. There are several methods for fluorescence staining of X chromatin including acridine orange, acridine yellow, AF, auramine O, and coriphosphine O. The fluorescence of these stain preparations disappears in a short period except AF hydrochloride which proved to retain fluorescence the longest.[7] In this study, we have seen that fluorescence using AF Schiff stain was retained for 4 days when kept covered. After the feulgen reaction, the X chromatin bodies were successfully stained as bright fluorescent greenish yellow spots directly inside the nuclear membrane.
In the present study, there is a difference in the range and the mean percentage of Barr bodies among women when compared to other studies. Nagamori and Takeda found a significant difference in the number of Barr bodies between male and female hair samples using AF. The frequency ranged from 27% to 70% (average 45.8%) in female samples and 0–8% (average 2.9%) in male samples.[7] Nagamori et al. detected Barr bodies in both buccal smears and hair cortex using AF Schiff stain and found that in female samples the frequencies were 58–87% (71.3% in average) in the cells of buccal mucosa and 59–85% (67.1% in average) in the nuclei of the hair cortex. The frequencies were 0–4% (1.2% in average) in the nuclei of buccal mucosa and 0–5% (1.3% in average) in the nuclei of the hair cortex for male samples.[4]
Mittal et al. demonstrated Barr bodies in the buccal mucosal cells of 1.1% of males and 39.2% of females using PAP stain.[1] A Nigerian study in 2012 showed that Barr body positivity in female buccal smears were 25% with cresyl fast violet, 33% with aceto-orcein (AO) and 40% with a combination of stains.[24] A study using AO and PAP stain on buccal smears showed that percentage of Barr bodies in AO stained slides ranged from 5% to 32% among females and from 0% to 8% in males, while with PAP stain the percentage was 4–20% in females and 0–5% in males.[25] Another study using acridine orange demonstrated a high range of Barr-body-positive mucosal cells (18–72%) in females compared to males (0–3%) under confocal microscopy.[26]
Using PAP stain, Barr bodies were not as distinct as with AF stain. Unlike PAP, AF did not stain inflammatory cells and fungi. PAP stain was retained for many days compared to AF stain. In our study, both AF and PAP stained samples were read without mounting. Optimum hydrolysis time is essential for feulgen reaction and standardization of hydrolysis time is crucial for AF stain. Initially, Nagamori's method of conventional stain preparation was tried, but improved AF stain preparation was found to be better. This method offered the following advantages: (a) simplified the preparation of the AF-feulgen reagent (b) the cytoplasm was less stained while the nuclei stained bright green (c) eliminated poorly defined reagents from the staining solutions.[8]
The results of this experiment demonstrated that the frequencies of Barr body are distinguishable between the male and female samples in buccal mucosal scrapes. The frequencies of Barr body in both sexes showed discrete distributions. After reviewing literature and from the present study, it is clear that Barr body count varies with different stains and with the same stain in different studies. This could be due to sample size variation, microscopy variation, cell selection bias, or the presence of microorganisms.
Conclusion
Identifying Barr bodies in the buccal cells is a simple method of gender determination. AF proved to be a better and more specific stain than PAP for visualizing nuclear details as it stains only after feulgen reaction and its staining method was remarkably simple. This stain demonstrates more specificity in sex determination compared to PAP stain.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mittal T, Saralaya KM, Kuruvilla A, Achary C. Sex determination from buccal mucosa scrapes. Int J Legal Med. 2009;123:437–40. doi: 10.1007/s00414-008-0285-8. [DOI] [PubMed] [Google Scholar]
- 2.Barr ML, Bertram EG. A morphological distinction between neurones of the male and female, and the behaviour of the nucleolar satellite during accelerated nucleoprotein synthesis. Nature. 1949;163:676. doi: 10.1038/163676a0. [DOI] [PubMed] [Google Scholar]
- 3.Caspersson T, Zech L, Modest EJ, Foley GE, Wagh U, Simonsson E. DNA- binding flourochromes for the study of the organization of the metaphase nucleus. Exp Cell Res. 1969;58:141–52. doi: 10.1016/0014-4827(69)90124-4. [DOI] [PubMed] [Google Scholar]
- 4.Nagamori H, Ohno Y, Uchima E, Kajiwara M, Nakazato M, Une Y, et al. Sex determination from buccal mucosa and hair root by the combined treatment of quinacrine staining and the fluorescent feulgen reaction using a single specimen. Forensic Sci Int. 1986;31:119–28. doi: 10.1016/0379-0738(86)90195-7. [DOI] [PubMed] [Google Scholar]
- 5.Nagamori H. Sex determination from plucked human hairs without epithelial root sheath. Forensic Sci Int. 1978;12:167–73. doi: 10.1016/0379-0738(78)90001-4. [DOI] [PubMed] [Google Scholar]
- 6.Nagamori H, Takeda K. Sex determination from plucked human hairs without epithelial root sheath. II. Depigmentation of melanin in the hair cortex before feulgen reaction. Forensic Sci Int. 1980;15:169–75. doi: 10.1016/0379-0738(80)90157-7. [DOI] [PubMed] [Google Scholar]
- 7.Nagamori H, Takeda K. Sex determination from plucked human hairs without epithelial root sheath. III. Fluorescent feulgen reaction using acriflavine. Forensic Sci Int. 1981;17:85–90. doi: 10.1016/0379-0738(81)90193-6. [DOI] [PubMed] [Google Scholar]
- 8.Levinson JW, Retzel S, McCormick JJ. An improved acriflavine-feulgen method. J Histochem Cytochem. 1977;25:355–8. doi: 10.1177/25.5.68068. [DOI] [PubMed] [Google Scholar]
- 9.Bancroft JD. Theory and Practice of Histological Techniques. 6th ed. London, UK: Churchill-Livingstone; 2008. pp. 127–8. [Google Scholar]
- 10.Lyon MF. Lyonisation of the X chromosome. Lancet. 1963;2:1120–1. doi: 10.1016/s0140-6736(63)92895-2. [DOI] [PubMed] [Google Scholar]
- 11.Lyon MF. X-chromosome inactivation spreads itself: Effects in autosomes. Am J Hum Genet. 1998;63:17–9. doi: 10.1086/301940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chakravarty A, Purandare H, Mehta L, Chakravarty BP. Effect of synthetic and natural sex steroids on X-chromatin. Indian J Med Res. 1978;68:785–9. [PubMed] [Google Scholar]
- 13.Roy S, Das AK, Khare VN, Roy B. Frequency of sex chromatin in the buccal smear of newborn females and their mothers during first six days after delivery. Acta Cytol. 1976;20:83–6. [PubMed] [Google Scholar]
- 14.Atkin NB. Symposium on Nuclear Sex. London. Vol. 68. London: Heinemann; 1952. (1958) [Google Scholar]
- 15.Camargo M, Wang N. Cytogenetic evidence for the absence of an inactivated X chromosome in a human female (XX) breast carcinoma cell line. Hum Genet. 1980;55:81–5. doi: 10.1007/BF00329131. [DOI] [PubMed] [Google Scholar]
- 16.Smethurst M, Bishun NP, Fernandez D, Allen J, Burn JI, Alaghband-Zadeh J, et al. Steroid hormone receptors and sex chromatin frequency in breast cancer. J Endocrinol Invest. 1981;4:455–7. doi: 10.1007/BF03348311. [DOI] [PubMed] [Google Scholar]
- 17.Therman E, Denniston C, Nieminen U, Buchler DA, Timonen S. X chromatin, endomitoses, and mitotic abnormalities in human cervical cancer. Cancer Genet Cytogenet. 1985;16:1–11. doi: 10.1016/0165-4608(85)90072-x. [DOI] [PubMed] [Google Scholar]
- 18.Straub DG, Lucas LA, McMahon NJ, Pellett OL, Teplitz RL. Apparent reversal of X-condensation mechanism in tumors of the female. Cancer Res. 1969;29:1233–43. [PubMed] [Google Scholar]
- 19.Hamerton J.L. Human Cytogenetics: Clinical Cytogenetics. Vol. 2. New York: Academic Press; 1971. [Google Scholar]
- 20.Moore KL. The sex chromatin. Philadelphia: WB Saunders; 1966. [Google Scholar]
- 21.Böhm N, Sprenger E. Fluorescence cytophotometry: A valuable method for the quantitative determination of nuclear feulgen-DNA. Histochemie. 1968;16:100–18. doi: 10.1007/BF00280607. [DOI] [PubMed] [Google Scholar]
- 22.Cornelisse CJ, Ploem JS. A new type of two-color fluorescence staining for cytology specimens. J Histochem Cytochem. 1976;24:73–81. doi: 10.1177/24.1.56393. [DOI] [PubMed] [Google Scholar]
- 23.Lerman LS. Structural considerations in the interaction of DNA and acridines. J Mol Biol. 1961;3:18–30. doi: 10.1016/s0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- 24.G.I.N. Ndubuka. Aceto-orecin aceto-cresyl fast violet technique for the demonstration and enumeration of Barr bodies in buccal smears. Afr J Med Phy, Biomed Eng & Sc. 2012;3:52–57. [Google Scholar]
- 25.Datar U, Angadi PV, Hallikerimath S, Kale AD. Cytological assessment of Barr bodies using aceto-orcein and Papanicolaou stains in buccal mucosal smears and their sex estimation efficacy in an Indian sample. Acta Cytol. 2013;57:516–21. doi: 10.1159/000353216. [DOI] [PubMed] [Google Scholar]
- 26.Reddy DS, Sherlin HJ, Ramani P, Prakash PA. Determination of sex by exfoliative cytology using acridine orange confocal microscopy: A short study. J Forensic Dent Sci. 2012;4:66–9. doi: 10.4103/0975-1475.109887. [DOI] [PMC free article] [PubMed] [Google Scholar]


