Abstract
OBJECTIVE
We evaluated the longitudinal effects of home-based asthma education combined with medication adherence feedback (adherence monitoring with feedback [AMF]) and asthma education alone (asthma basic care [ABC]) on asthma outcomes, relative to a usual-care (UC) control group.
METHODS
A total of 250 inner-city children with asthma (mean age: 7 years; 62% male; 98% black) were recruited from a pediatric emergency department (ED). Health-outcome measures included caregiver-frequency of asthma symptoms, ED visits, hospitalizations, and courses of oral corticosteroids at baseline and 6-, 12-, and 18-month assessments. Adherence measures included caregiver-reported adherence to inhaled corticosteroid (ICS) therapy and pharmacy records of ICS refills. Multilevel modeling was used to examine the differential effects of AMF and ABC compared with UC.
RESULTS
ED visits decreased more rapidly for the AMF group than for the UC group, but no difference was found between the ABC and UC groups. The AMF intervention led to short-term improvements in ICS adherence during the active-intervention phase relative to UC, but this improvement decreased over time. Asthma symptoms and courses of corticosteroids decreased more rapidly for the ABC group than for the UC group. Hospitalization rates did not differ between either intervention group and the UC group. No differences were found between the ABC and AMF groups on any outcome.
CONCLUSIONS
Asthma education led to improved adherence and decreased morbidity compared with UC. Home-based educational interventions may lead to modest short-term improvements in asthma outcomes among inner-city children. Adherence feedback did not improve outcomes over education alone.
Keywords: asthma, adherence, inner-city youth, psychosocial intervention, randomized, controlled trial
Asthma is the leading chronic illness among children in the United States. Low-income, minority children have disproportionately higher prevalence, morbidity, and mortality from asthma.1,2 Research suggests that inappropriate asthma management practices among low-income patients, including overreliance on emergency care and poor adherence with therapy, are important contributing behavioral factors.3 Poor adherence contributes to increased asthma morbidity and mortality4 and is associated with increased health care use and costs and decreased treatment effectiveness.5–7 Studies suggest that medication adherence with preventive therapies is ~50% for children with asthma,8 with minority children at particular risk for nonadherence.9
Previous interventions aimed at improving asthma self-management among minority children have produced mixed results, with some reporting decreased symptoms10,11 and emergency department (ED) visits and hospitalizations12,13 and others reporting minimal intervention effects.14,15 Feedback of electronically monitored adherence shows promise as a strategy to increase medication adherence in adults with asthma16 and HIV.17 Onyirimba et al16 found that feedback of objectively measured adherence resulted in adherence of >70% in the intervention group compared with <50% in the control group. Feedback resulted in improvements in adherence among inner-city children with asthma in a pilot study18; however, no randomized, controlled trial has evaluated the effects of this adherence promotion strategy among inner-city children with asthma.
The aim of this randomized, controlled trial was to test the efficacy of asthma education combined with a medication adherence feedback intervention (adherence monitoring with feedback [AMF]) and an asthma education intervention alone (asthma basic care [ABC]) in reducing ED visits, compared with usual care (UC) among inner-city children with asthma. Secondary outcomes included asthma medication adherence, asthma symptom frequency, hospitalizations, and courses of oral steroids. We further hypothesized that the improvement in asthma morbidity and adherence would be significantly greater for children who were randomly assigned to the AMF intervention than for children in the ABC group.
METHODS
Study Design
This randomized, 3-arm, parallel-group, controlled trial was reviewed and approved by the Johns Hopkins Medical Institutions institutional review board. Written informed consent was obtained from the child’s primary caregiver. To mask staff to group assignment during recruitment, the statistician created block randomization schema and placed the randomization assignments into sealed envelopes, which were opened after families completed baseline surveys. All participants were contacted for follow-up surveys at 6, 12, and 18 months after randomization. Trained research assistants who were blinded to study assignments conducted surveys by telephone. Pharmacy refill data were requested at baseline and 12 months from all pharmacies identified by the caregiver.
Participants
Children with asthma were recruited from the pediatric ED by weekly review of discharge records between 2001 and 2003. Children were eligible for randomization when they were between 2 and 12 years of age, had physician-diagnosed asthma, had 2 ED visits or 1 hospitalization for asthma in the preceding year, resided in Baltimore city, and were prescribed an asthma controller medication. Several attempts were made to contact all potentially eligible participants by telephone, with at least 10 attempts made at varying times and days.
Intervention Description*
UC Group
Participants who were randomly assigned to the UC group received an asthma education booklet and resource guide that provided information about low-cost asthma care providers, social services, legal services, and other resources. Regardless of group assignment, participants were regularly encouraged to receive care from their primary care provider.
ABC Intervention
Families who were randomly assigned to the ABC intervention received five 30- to 45-minute home visits by trained asthma educators (AEs) 1, 2, 3, 4, and 8 weeks after randomization. The ABC intervention is a home-based asthma education program with 5 core components: (1) review of the prescribed asthma regimen and training in medication, spacer, and peak flow technique; (2) development of an asthma action plan; (3) identification of barriers to accessing health care and problem solving to reduce barriers; (4) discussion of beliefs and concerns about asthma and medications; and (5) provision of written asthma education materials.
AMF Intervention
Families who were randomly assigned to the AMF intervention received home visits by AEs at the schedule described in the previous section. AMF contains the ABC content plus the following:
Objective feedback of medication adherence: Electronic medication monitors were used for adherence feedback. Monitors included the Doser CT (Meditrac, Inc, Hudson, MA) and MEMs caps (AARDEX Ltd, Union City, CA). The AEs were trained to provide nonthreatening, supportive feedback on adherence to encourage a partnership with the family.
Goal-setting: Families were encouraged to set asthma control goals (eg, no coughing at night) and weekly adherence goals. The AEs assisted families in setting age-appropriate expectations regarding the child’s ability to self-manage asthma.
Reinforcement for attaining adherence goals: The importance of positive reinforcement, such as verbal praise and low-cost rewards, was discussed with the caregiver. When the child attained the adherence goal, the AE provided a small reward (eg, crayons). When it was not achieved, the AE worked with the family to identify barriers and taught problem-solving skills.
Strategies for self-monitoring medication use: Families were taught to monitor adherence and asthma symptoms by using behavioral charts and symptom diaries. When possible, the AE highlighted the relationship between improvements in adherence and asthma outcomes.
Measures
Self-reported Adherence
Caregivers reported how the child administered the medication (eg, 2 puffs twice a day), then estimated how often the child missed doses in the previous 7 days. Percentage self-reported adherence was calculated as use/prescribed use × 100%.
Pharmacy-based Adherence
By using a protocol described by Butz et al,19 the number of inhaled corticosteroid (ICS) refills per quarter was abstracted from pharmacy records for the year before and after randomization. The number of canisters dispensed was converted to therapeutically equivalent values so that 1 canister represented a 1-month supply of ICS.20,21 The quarterly ICS refill rates were defined as the number of ICS canisters dispensed quarterly. These analyses included the quarter before enrollment (the baseline value) through the fourth quarter after randomization. An ICS refill rate of 3.0 is equivalent to 100% adherence for the quarter.
Asthma Morbidity Measures
Caregiver reports of asthma symptoms (cough, wheeze, shortness of breath, or chest tightness/discomfort), nighttime awakenings, ED visits, hospitalizations, and courses of oral corticosteroids in the previous 6 months were collected. Total numbers of days and nights (combining day and night symptoms) were calculated for each child with a range of 0 to 60 for day or night symptom counts reported during the previous 30 days for an asthma symptom frequency variable.22,23
Statistical Analyses
Baseline characteristics were compared across the groups. Intent-to-treat analyses were conducted to compare each intervention group with the UC group at each time point for outcome measures. Hierarchical Linear Modeling (HLM 6; Lincolnwood, IL) was used to estimate average trajectories and group differences in trajectories for the asthma outcomes during the 18-month period while adjusting for insurance type and baseline level of each asthma outcome. For each outcome, the individual intercept (the adjusted mean at the baseline assessment) and linear time slope (the rate of change over time) were estimated as level 1 parameters. Dummy-coded intervention effects were then modeled as level 2 predictors of those level 1 intercepts and slopes. For ICS refills and asthma symptoms, quadratic time slopes were estimated to calculate curvilinear changes over time. For binary, count, and continuous outcomes, a Poisson with log-link function, Bernoulli, and normal models were specified, respectively. All models were estimated with full information maximum likelihood (for continuous outcomes) or penalized quasi-likelihood methods (for all count and binary outcomes), to use all available data.
RESULTS
Sample Characteristics
Of the 414 families who consented to participate, 377 (91%) completed the baseline questionnaire and 250 were randomly assigned to the UC, ABC, or AMF groups (Fig 1). The remaining 127 were excluded because they were not prescribed asthma controller medications. A total of 216 (86%) of 250 children had valid and complete pharmacy records available. Because only 61 (24%) of 250 children were on leukotriene modifiers and 44 (72%) of 61 were on both ICS and leukotriene modifiers, only ICS adherence data are presented; children who were on only leukotriene modifiers were removed from additional adherence analyses. Children who were on leukotriene modifiers only (n = 17) were more likely to be male (P =.005) and less likely to be on Medicaid (P =.011). Of the participants who were randomly assigned to the ABC or AMF interventions, 112 (67%) completed all 5 visits, with no differences in the average number of visits completed (ABC: 4.0 visits; AMF: 3.8 visits). Participants who completed all follow-up surveys (82%) reported more baseline ED visits than those who did not (P = .008) but were similar on all other baseline characteristics.
FIGURE 1.
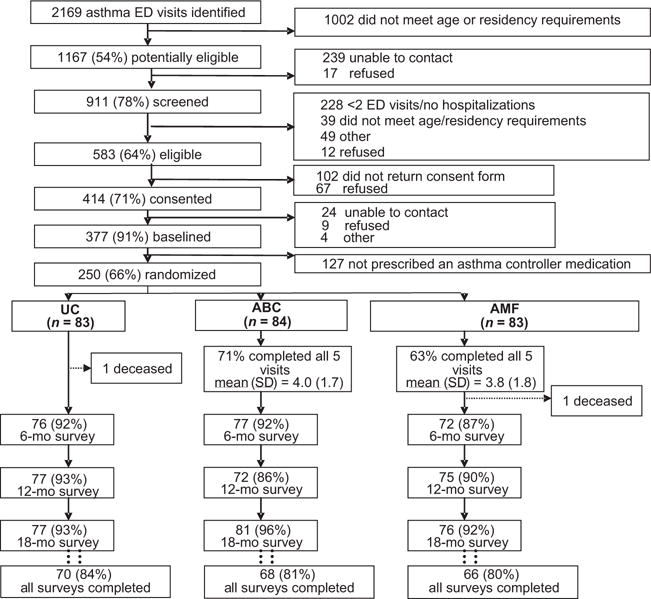
Consolidated Standards of Reporting Trials (CONSORT) diagram of flow of participants through the study.
Table 1 presents the baseline demographic characteristics: ABC, AMF, and UC groups did not differ on demographic characteristics, except that more UC participants reported having Medicaid than did the ABC group (P = .001). Thus, the dummy-coded insurance type (1 = Medicaid, 0 = all others) was included as a covariate in all subsequent analyses. Self-reported adherence to ICS therapy was high throughout (average adherence of >80%; Table 2) and did not change significantly over time. In contrast, pharmacy-based ICS adherence was low, with the average quarterly ICS refill rate of <1 at all assessments, suggesting that children obtained less than one third of the expected number of refills (Table 2). Only 3.2% participants refilled ≥80% of their ICS medication for the year after randomization. See Table 3 for health-outcomes data.
TABLE 1.
Baseline Demographic Characteristics According to Group
| Characteristic | ABC (n = 84) | AMF (n = 83) | UC (n = 83) | Pa |
|---|---|---|---|---|
| Child | ||||
| Age, mean ± SD, y | 7.05 ± 3.37 | 6.54 ± 3.43 | 7.35 ± 3.30 | .300 |
| Male gender, n (%) | 52 (62.0) | 54 (65.0) | 49 (59.0) | .730 |
| Black race, n (%) | 84 (100.0) | 81 (98.0) | 80 (96.0) | .240 |
| Medicaid health insurance, n (%) | 62 (74.0) | 74 (89.0) | 78 (94.0) | .001 |
| Has regular source for nonurgent asthma care, n (%) | 84 (100.0) | 83 (100.0) | 83 (100.0) | .990 |
| Household, n (%) | ||||
| Mother is primary caregiver | 75 (89.0) | 80 (96.0) | 73 (880) | .120 |
| Caregiver completed high school | 58 (69.0) | 58 (70.0) | 56 (68.0) | .940 |
| Household income < $10 000/y | 30 (37.0) | 33 (41.3) | 29 (35.4) | .730 |
| Smoker in the home | 23 (27.4) | 22 (26.8) | 23 (27.7) | .990 |
| Concern about neighborhood safety | 57 (67.9) | 54 (65.1) | 59 (71.1) | .710 |
P values are based on the χ2 test (categorical variables) or analysis of variance (continuous variables).
TABLE 2.
Descriptive Statistics for the Adherence Outcomes at Each Assessment
| Outcome | All | ABC | AMF | UC |
|---|---|---|---|---|
| Self-reported adherence to ICS therapy in previous 6 mo, % | ||||
| 0 mo of follow-up | 84.91 ± 27.88 | 86.87 ± 27.29 | 83.16 ± 29.69 | 84.87 ± 26.77 |
| 6 mo of follow-up | 86.01 ± 27.23 | 81.62 ± 32.33 | 89.40 ± 19.34 | 87.51 ± 27.96 |
| 12 mo of follow-up | 84.11 ± 34.03 | 80.63 ± 44.48 | 85.30 ± 28.51 | 86.37 ± 26.85 |
| 18 mo of follow-up | 89.18 ± 21.63 | 85.38 ± 24.72) | 87.33 ± 25.24 | 94.96 ± 10.78 |
| No. of ICS canister refills in previous quarter | ||||
| 0 mo of follow-up | 0.74 ± 0.94 | 0.78 ± 0.94 | 0.75 ± 0.97 | 0.69 ± 0.94 |
| 3 mo of follow-up | 0.79 ± 1.14 | 0.81 ± 1.15 | 0.92 ± 1.36 | 0.65 ± 0.88 |
| 6 mo of follow-up | 0.62 ± 0.95 | 0.68 ± 1.02 | 0.69 ± 0.98 | 0.50 ± 0.83 |
| 9 mo of follow-up | 0.59 ± 0.99 | 0.67 ± 1.10 | 0.61 ± 0.89 | 0.50 ± 0.97 |
| 12 mo of follow-up | 0.57 ± 0.84 | 0.60 ± 0.88 | 0.58 ± 0.86 | 0.53 ± 0.80 |
Data are means ± SD.
TABLE 3.
Descriptive Statistics for the Health Outcomes at Each Assessment
| Outcome | All (N = 250) | ABC (n = 84) | AMF (n = 83) | UC (n = 83) |
|---|---|---|---|---|
| Asthma symptoms per month, mean ± SD | ||||
| 0 mo of follow-up | 6.39 ± 6.84 | 7.38 ± 7.00 | 6.38 ± 6.89 | 5.41 ± 6.56 |
| 6 mo of follow-up | 4.59 ± 5.63 | 4.09 ± 5.14 | 5.09 ± 6.41 | 4.61 ± 5.35 |
| 12 mo of follow-up | 4.58 ± 5.96 | 3.75 ± 4.64 | 5.50 ± 6.97 | 4.47 ± 5.95 |
| 18 mo of follow-up | 4.53 ± 6.32 | 3.36 ± 4.67 | 5.98 ± 7.83 | 4.35 ± 5.95 |
| ED visits In previous 6 mo, mean ± SD | ||||
| 0 mo of follow-up | 2.84 ± 3.10 | 2.63 ± 2.99 | 3.10 ± 3.00 | 2.81 ± 3.32 |
| 6 mo of follow-up | 1.35 ± 1.88 | 1.19 ± 1.31 | 1.72 ± 2.48 | 1.16 ± 1.68 |
| 12 mo of follow-up | 1.11 ± 1.77 | 0.86 ± 1.38 | 1.11 ± 1.77 | 1.35 ± 2.08 |
| 18 mo of follow-up | 0.88 ± 1.39 | 0.81 ± 1.04) | 0.74 ± 1.25) | 1.10 ± 1.78 |
| Hospitalization In previous 6 mo, n (%) | ||||
| 0 mo of follow-up | 104 (41.6) | 35 (41.7) | 35 (42.2) | 34 (41.0) |
| 6 mo of follow-up | 46 (20.4) | 13 (16.9) | 19 (26.4) | 14 (18.4) |
| 12 mo of follow-up | 41 (18.3) | 10 (13.9) | 16 (21.3) | 15 (19.5) |
| 18 mo of follow-up | 34 (14.5) | 12 (14.8) | 12 (15.8) | 10 (13.0) |
| Courses of oral steroids in previous 6 mo, mean ± SD | ||||
| 0 mo of follow-up | 2.41 ± 2.48 | 2.60 ± 3.15 | 2.20 ± 1.81 | 2.43 ± 2.29 |
| 6 mo of follow-up | 1.21 ± 1.63 | 0.94 ± 1.08 | 1.40 ± 1.90 | 1.32 ± 1.79 |
| 12 mo of follow-up | 1.08 ± 1.55 | 0.81 ± 1.02 | 1.13 ± 1.66 | 1.27 ± 1.81 |
| 18 mo of follow-up | 1.09 ± 2.31 | 0.74 ± 0.91 | 0.96 ± 1.59 | 1.57 ± 3.56 |
General Time Trends for All Groups
Overall, children in all 3 groups demonstrated statistically significant improvements on all asthma morbidity outcomes (Tables 2 and 3, and Figs 2 and 3). There was a curvilinear reduction in ED visits across groups, with a 33% decrease in ED visits every 6 months (P < .001; Fig 3B) and a quarterly ICS refill rate increase of 16% every 3 months (P = .05; Fig 2). Because of lack of change over time, we were unable to model self-reported ICS adherence. To account for seasonal fluctuations in morbidity, we included dummy-coded seasons as a covariate. Season did not account for the variability in outcomes or change the within-person model estimates; therefore, it was excluded from the final analyses.
FIGURE 2.
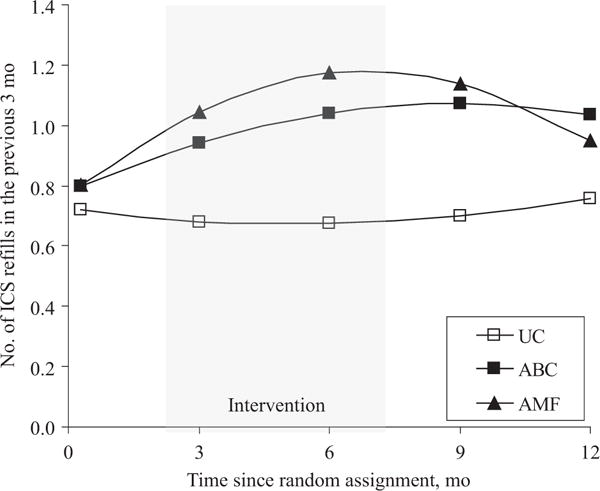
Trajectories of ICS refills obtained per 3 months according to randomized groups. Mean values are adjusted for baseline values and type of health insurance.
FIGURE 3.
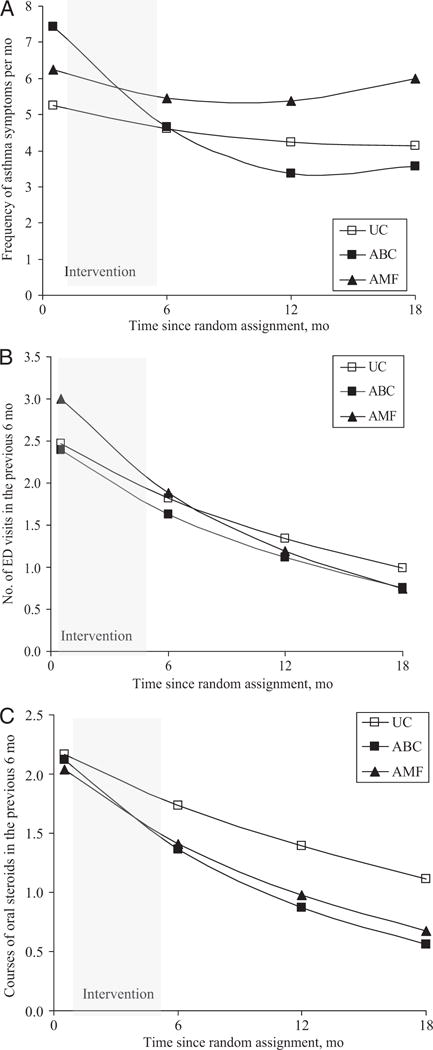
Trajectories of the health outcome frequencies: asthma symptoms (A), ED visits (B), and courses of oral steroids (C), by groups. Adjusted for baseline values and type of health insurance.
Comparison of the AMF With the UC Group
To test our primary hypothesis, we compared the longitudinal trajectories of our primary outcome (ED visits) and secondary outcomes (ICS adherence, asthma symptom frequency, hospitalizations, and oral steroid use) for each intervention group with those for the UC group. ED visits decreased faster for the AMF than for the UC group, with a 15% greater decrease in the number of ED visits per 6 months (P = .02; Table 4). ICS refill rates increased 52% faster for the AMF than for the UC group per quarter (P = .03) but slowed by 9% per quarter (P = .03). Oral corticosteroid use decreased 13% more in the AMF than in the UC group (P =.06); however, the trajectories of asthma symptom frequency and hospitalizations did not differ between the 2 groups.
TABLE 4.
HLM Estimates of Asthma Outcomes Over Time
| Parameter | Estimatea | 95% CI | P |
|---|---|---|---|
| AMF compared with UC | |||
| Quarterly ICS refill rate, linear | 1.52 | 1.05 to 2.19 | .03 |
| Quarterly ICS refill rate, quadratic | 0.91 | 0.83 to 0.99 | .03 |
| Asthma symptoms, linear | −0.43 | −2.92 to 2.06 | .74 |
| Asthma symptoms, quadratic | 0.68 | 0.08 to 1.44 | .09 |
| ED visits | 0.85 | 0.74 to 0.97 | .02 |
| Hospitalization | 1.04 | 0.61 to 1.76 | .90 |
| Oral corticosteroid use | 0.86 | 0.74 to 1.01 | .06 |
| ABC compared with UC | |||
| Quarterly ICS refill rate, linear | 1.32 | 0.91 to 1.93 | .15 |
| Quarterly ICS refill rate, quadratic | 0.94 | 0.86 to 1.03 | .22 |
| Asthma symptoms, linear | −2.90 | −3.92 to −1.88 | .001 |
| Asthma symptoms, quadratic | 0.46 | −0.15 to 0.77 | .004 |
| ED visits | 0.92 | 0.81 to 1.06 | .25 |
| Hospitalization | 1.04 | 0.60 to 1.79 | .90 |
| Oral steroid use | 0.80 | 0.69 to 0.94 | .006 |
| AMF compared with ABC | |||
| Quarterly ICS refill rate, linear | 1.11 | 0.77 to 1.61 | .56 |
| Quarterly ICS refill rate, quadratic | 0.97 | 0.88 to 1.05 | .43 |
| Asthma symptoms, linear | 2.09 | 0.08 to 4.27 | .06 |
| Asthma symptoms, quadratic | −0.32 | −1.02 to −0.39 | .36 |
| ED visits | 0.92 | 0.81 to 1.05 | .22 |
| Hospitalization | 1.01 | 0.60 to 1.70 | .97 |
| Oral steroid use | 1.08 | 0.93 to 1.25 | .32 |
| Intervention (AMF + ABC) compared with UC | |||
| Quarterly ICS refill rate, linear | 1.42 | 1.03 to 1.97 | .03 |
| Quarterly ICS refill rate, quadratic | 0.93 | 0.86 to 1.00 | .05 |
| Asthma symptoms, linear | −1.56 | −3.76 to 0.64 | .16 |
| Asthma symptoms, quadratic | 0.45 | 0.22 to 1.12 | .18 |
| ED visits | 0.88 | 0.78 to 0.99 | .03 |
| Hospitalization | 1.04 | 0.65 to 1.65 | .88 |
| Oral corticosteroid use | 0.83 | 0.73 to 0.95 | .008 |
HLM indicates Hierarchical Linear Modeling.
Parameter estimates for ED visits, ICS refill rate, and oral steroid use are based on incidence rate ratio. The estimates for asthma symptoms are based on unstandardized regression coefficient. The estimates for hospitalization are based on odds ratio.
Comparison of the ABC With the UC Group
The rate of reduction in ED visits did not differ between the ABC and UC groups (P = .25; Table 4). Improvement in asthma symptom frequency was faster for the ABC than for the UC group, with 2.90 fewer asthma symptoms every 6 months, but the rate of reduction decreased over time (P < .001 for linear slope and P = .004 for quadratic slope). Similarly, the courses of oral corticosteroids in the ABC group decreased 20% more than in the UC group (P = .006) every 6 months; however, the change in hospitalizations and ICS refill rates did not differ between the 2 groups.
Comparison of the AMF With the ABC Group
Identical sets of analyses were repeated comparing AMF and ABC (as reference group); no significant differences in their trajectories were found for any of the outcomes (Table 4).
Comparison of Combined Intervention Groups With the UC Group
The analyses were repeated comparing the combined intervention (ABC and AMF) and UC groups to examine the aggregate effect of receiving any intervention. ED visits (P = .03) and courses of oral steroids (P = .008) decreased faster for the combined intervention groups than for the UC group (12% and 17% greater decrease per 6 months, respectively). ICS refill rates increased faster for the combined intervention groups than for the UC group (P = .03), but the rate decreased over time (P =.05). The combined intervention groups and the UC group did not differ on asthma symptom frequency or hospitalizations (Table 4).
DISCUSSION
In this randomized, controlled trial, we tested the efficacy of 2 asthma education interventions for children of inner-city families in reducing the number of ED visits and secondary outcomes of medication adherence, asthma symptoms, hospitalizations, and courses of oral steroids. Although we found that home-based asthma education with and without objective feedback of medication adherence data compared with a UC control condition resulted in significant improvements in selected asthma outcomes, these results were inconsistent across outcome measures. Although the number of oral corticosteroid courses decreased in both intervention groups, only the AMF intervention resulted in a significant decrease in the rate of ED visits and improvement in ICS refill rates; however, only the ABC group demonstrated improvements in asthma symptoms. Furthermore, no significant differences were evident in any outcome between the AMF and ABC groups. Thus, although both home-based interventions showed significant benefit for select asthma outcomes, our findings do not support using AMF in asthma education over providing asthma education alone for improving asthma symptoms or urgent care use. Our results are consistent with a recent meta-analysis that found that asthma education, although a necessary part of asthma care, does not affect all outcomes equally.24
Both interventions emphasized medication adherence to improve asthma control; however, the AMF group received behavioral shaping (feedback and positive reinforcement) specifically targeting adherence. Although we did observe short-term improvements in pharmacy-based ICS adherence in the AMF intervention, overall adherence remained low and improvement was short-lived. In the AMF group, the rate of increase in ICS refills peaked at 40% (1.2 canisters per quarter) during active intervention; however, when feedback and reinforcement were removed, the behavior change did not generalize and medication adherence declined to 19% (0.58 canisters per quarter) by 12 months. Previous smaller scale studies that provided feedback found similar changes in adherence during active interventions but did not include longer term follow-up assessment once the intervention was withdrawn.16,18 That most participants in this study, regardless of group assignment, had markedly low ICS refill adherence, even during active intervention, may account for the modest improvement in symptoms and health care use that we observed.
The presence of the UC control group allowed us to document the change in asthma outcomes after an ED visit absent any additional intervention. As is common in studies that target populations with high morbidity, we found statistically significant improvement on all health and use outcomes during the course of the study across all groups.14 This observation has implications for future asthma intervention research. First, studies should be powered to evaluate the efficacy of interventions beyond clinical improvements that are likely to occur naturally over time or are the result of UC. Second, researchers should be cautious in concluding that an intervention is efficacious when using a pre–post study design or when findings show that ≥2 active-intervention groups show the same degree of improvement. Without a UC group, it is difficult to determine whether benefits are attributable to intervention effect, usual medical care, regression to the mean, or secular time trends. Absent the UC group, we may have concluded inaccurately that both interventions resulted in dramatic improvements compared with baseline.
This study targeted inner-city children who were at high risk for poor asthma outcomes. Inner-city families are often faced with economic stress, poverty, and violence in their neighborhoods, as well as higher prevalence of environmental asthma triggers.21,25,26 It is likely that the brevity of our intervention plus the focus on medication adherence did not match the complex life circumstances of these families. In such an environment, resolution of impeding crises often supersedes undertaking preventive approaches to manage children’s asthma; therefore, the modest changes in asthma outcomes may be attributable to the discordance between the interventions and the complex and competing needs of these families. Improvements in adherence were seen only during active intervention, suggesting that longer term interventions may lead to more sustainable change.
Finally, this is 1 of the first studies to use hierarchical linear and nonlinear modeling to compare the rates of changes in asthma outcomes. Given that asthma is cyclical, with symptoms and morbidity fluctuating over time, our ability to model curvilinear changes during the follow-up period is critical. Because of estimation methods used in hierarchical modeling, participants with more data contribute more to the parameter estimates than those with missing data, resulting in less bias than pairwise or listwise deletion methods.
Despite these methodologic strengths, our findings should be considered in light of their limitations. We did not use Bonferroni correction for interpreting the outcomes; as a result, we may risk committing type I error. We did not use electronic monitors as an objective measure of adherence, because they were used for AMF. Prescription records do indicate drug availability and are reliable sources of drug exposure27 but do not show adherence patterns. We depended on caregiver report of health care use; however several studies have found it to be accurate.28,29 Furthermore, despite our success in screening, recruiting, and retaining participants, we enrolled only a subset of all eligible patients and were unable to collect data on nonparticipants to assess the generalizability of our results to the larger population. Finally, although we found modest improvements in asthma outcomes, we did not collect information that would have permitted evaluation of the cost-effectiveness of the interventions.
CONCLUSIONS
Our findings support previous research that suggests that any home-based educational intervention as a supplement to UC leads to modest short-term improvements in asthma outcomes and adherence among inner-city children24; however, we did not find support for the superior effects of asthma education plus AMF compared with asthma education alone. Additional studies with more intensive interventions (eg, ongoing case management combined with asthma education and adherence-promoting interventions) may be required to effect sustainable changes in asthma outcomes in this high-risk population.
WHAT’S KNOWN ON THIS SUBJECT
Previous interventions aimed at improving asthma self-management among minority children have produced mixed results. Feedback of electronically monitored adherence shows promise as a strategy for increasing medication adherence in adults with asthma and HIV.
WHAT THIS STUDY ADDS
The aim of this randomized, controlled trial was to test the efficacy of asthma education combined with medication adherence feedback intervention and an asthma education intervention alone in reducing ED visits, compared with UC among inner-city children with asthma.
Acknowledgments
This research was supported by National Heart, Lung, and Blood Institute grant HL063333.
ABBREVIATIONS
- ED
emergency department
- AMF
adherence monitoring with feedback
- ABC
asthma basic care
- UC
usual care
- AE
asthma educator
- ICS
inhaled corticosteroid
Footnotes
This trial has been registered at www.clinicaltrials.gov (identifier NCT00233181).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
Intervention materials are available from the author.
References
- 1.McDaniel M, Paxson C, Waldfogel J. Racial disparities in childhood asthma in the United States: evidence from the National Health Interview Survey, 1997 to 2003. Pediatrics. 2006;117(5) doi: 10.1542/peds.2005-1721. Available at: www.pediatrics.org/cgi/content/full/117/5/e868. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami L. The state of childhood asthma, United States, 1980–2005. Adv Data. 2006;(381):1–24. [PubMed] [Google Scholar]
- 3.Rand CS, Butz AM, Kolodner K, Huss K, Eggleston P, Malveaux F. Emergency department visits by urban African American children with asthma. J Allergy Clin Immunol. 2000;105(1 pt 1):83–90. doi: 10.1016/s0091-6749(00)90182-9. [DOI] [PubMed] [Google Scholar]
- 4.Bauman LJ, Wright E, Leickly FE, et al. Relationship of adherence to pediatric asthma morbidity among inner-city children. Pediatrics. 2002;110(1 pt 1) doi: 10.1542/peds.110.1.e6. Available at: www.pediatrics.org/cgi/content/full/109/1/e6. [DOI] [PubMed] [Google Scholar]
- 5.Anis AH, Lynd LD, Wang XH, et al. Double trouble: impact of inappropriate use of asthma medication on the use of health care resources. CMAJ. 2001;164(5):625–631. [PMC free article] [PubMed] [Google Scholar]
- 6.Cleemput I, Kesteloot K. Economic implications of non-compliance in health care. Lancet. 2002;359(9324):2129–2130. doi: 10.1016/S0140-6736(02)09114-6. [DOI] [PubMed] [Google Scholar]
- 7.Hepke KL, Martus MT, Share DA. Costs and utilization associated with pharmaceutical adherence in a diabetic population. Am J Manag Care. 2004;10(2 pt 2):144–151. [PubMed] [Google Scholar]
- 8.Bender B, Wamboldt FS, O’Connor SL, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol. 2000;85(5):416–421. doi: 10.1016/s1081-1206(10)62557-4. [DOI] [PubMed] [Google Scholar]
- 9.McQuaid EL, Kopel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: reasoning, responsibility, and behavior. J Pediatr Psychol. 2003;28(5):323–333. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- 10.Bonner S, Zimmerman BJ, Evans D, Irigoyen M, Resnick D, Mellins RB. An individualized intervention to improve asthma management among urban Latino and African-American families. J Asthma. 2002;39(2):167–179. doi: 10.1081/jas-120002198. [DOI] [PubMed] [Google Scholar]
- 11.Evans R, III, Gergen PJ, Mitchell H, et al. A randomized clinical trial to reduce asthma morbidity among inner-city children: results of the National Cooperative Inner-City Asthma Study. J Pediatr. 1999;135(3):332–338. doi: 10.1016/s0022-3476(99)70130-7. [DOI] [PubMed] [Google Scholar]
- 12.Harish Z, Bregante AC, Morgan C, et al. A comprehensive inner-city asthma program reduces hospital and emergency room utilization. Ann Allergy Asthma Immunol. 2001;86(2):185–189. doi: 10.1016/S1081-1206(10)62689-0. [DOI] [PubMed] [Google Scholar]
- 13.Kelly CS, Morrow AL, Shults J, Nakas N, Strope GL, Adelman RD. Outcomes evaluation of a comprehensive intervention program for asthmatic children enrolled in Medicaid. Pediatrics. 2000;105(5):1029–1035. doi: 10.1542/peds.105.5.1029. [DOI] [PubMed] [Google Scholar]
- 14.Walders N, Kercsmar C, Schluchter M, Redline S, Kirchner HL, Drotar D. An interdisciplinary intervention for undertreated pediatric asthma. Chest. 2006;129(2):292–299. doi: 10.1378/chest.129.2.292. [DOI] [PubMed] [Google Scholar]
- 15.Guendelman S, Meade K, Benson M, Chen YQ, Samuels S. Improving asthma outcomes and self-management behaviors of inner-city children: a randomized trial of the Health Buddy interactive device and an asthma diary. Arch Pediatr Adolesc Med. 2002;156(2):114–120. doi: 10.1001/archpedi.156.2.114. [DOI] [PubMed] [Google Scholar]
- 16.Onyirimba F, Apter A, Reisine S, et al. Direct clinician-to-patient feedback discussion of inhaled steroid use: its effect on adherence. Ann Allergy Asthma Immunol. 2003;90(4):411–415. doi: 10.1016/S1081-1206(10)61825-X. [DOI] [PubMed] [Google Scholar]
- 17.Smith SR, Rublein JC, Marcus C, Brock TP, Chesney MA. A medication self-management program to improve adherence to HIV therapy regimens. Patient Educ Couns. 2003;50(2):187–199. doi: 10.1016/s0738-3991(02)00127-1. [DOI] [PubMed] [Google Scholar]
- 18.Bartlett SJ, Lukk P, Butz A, Lampros-Klein F, Rand CS. Enhancing medication adherence among inner-city children with asthma: results from pilot studies. J Asthma. 2002;39(1):47–54. doi: 10.1081/jas-120000806. [DOI] [PubMed] [Google Scholar]
- 19.Butz AM, Tsoukleris M, Donithan M, et al. Patterns of inhaled antiinflammatory medication use in young underserved children with asthma. Pediatrics. 2006;118(6):2504–2513. doi: 10.1542/peds.2006-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mudd K, Bollinger ME, Hsu VD, Donithan M, Butz A. Pharmacy fill patterns in young urban children with persistent asthma. J Asthma. 2006;43(8):597–600. doi: 10.1080/02770900600878537. [DOI] [PubMed] [Google Scholar]
- 21.Simons E, Curtin-Brosnan J, Buckley T, Breysse P, Eggleston PA. Indoor environmental differences between inner city and suburban homes of children with asthma. J Urban Health. 2007;84(4):577–590. doi: 10.1007/s11524-007-9205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartlett SJ, Krishnan JA, Riekert KA, Butz AM, Malveaux FJ, Rand CS. Maternal depressive symptoms and adherence to therapy in inner-city children with asthma. Pediatrics. 2004;113(2):229–237. doi: 10.1542/peds.113.2.229. [DOI] [PubMed] [Google Scholar]
- 23.Butz AM, Riekert KA, Eggleston P, Winkelstein M, Thompson RE, Rand C. Factors associated with preventive asthma care in inner-city children. Clin Pediatr (Phila) 2004;43(8):709–719. doi: 10.1177/000992280404300804. [DOI] [PubMed] [Google Scholar]
- 24.Coffman JM, Cabana MD, Halpin HA, Yelin EH. Effects of asthma education on children’s use of acute care services: a meta-analysis. Pediatrics. 2008;121(3):575–586. doi: 10.1542/peds.2007-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright RJ, Mitchell H, Visness CM, et al. Community violence and asthma morbidity: the Inner-City Asthma Study. Am J Public Health. 2004;94(4):625–632. doi: 10.2105/ajph.94.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore K, David TJ, Murray CS, Child F, Arkwright PD. Effect of childhood eczema and asthma on parental sleep and well-being: a prospective comparative study. Br J Dermatol. 2006;154(3):514–518. doi: 10.1111/j.1365-2133.2005.07082.x. [DOI] [PubMed] [Google Scholar]
- 27.Lau HS, de BA, Beuning KS, Porsius A. Validation of pharmacy records in drug exposure assessment. J Clin Epidemiol. 1997;50(5):619–625. doi: 10.1016/s0895-4356(97)00040-1. [DOI] [PubMed] [Google Scholar]
- 28.Bender B, Zhang L. Negative affect, medication adherence, and asthma control in children. J Allergy Clin Immunol. 2008;122(3):490–495. doi: 10.1016/j.jaci.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 29.D’Souza-Vazirani D, Minkovitz CS, Strobino DM. Validity of maternal report of acute health care use for children younger than 3 years. Arch Pediatr Adolesc Med. 2005;159(2):167–172. doi: 10.1001/archpedi.159.2.167. [DOI] [PubMed] [Google Scholar]


