Abstract
Brown adipose tissue (BAT) in adults has been shown to have a meaningful impact on energy expenditure and cold-induced thermogenesis. Data from rodent research have suggested that exercise may be a promising method of increasing BAT activity, with potential applications to the treatment and prevention of obesity and diabetes. However, emerging human research using positron emission tomography (PET) with [18F] Fluorodeoxyglucose (FDG) has identified lower BAT activity in endurance-trained athletes compared to sedentary controls, despite similar metabolic rate responses to cold exposure. Here we report a similar incidental finding in a pilot study that included a sample of 2 endurance athletes and 10 untrained individuals. This incidental finding motivated a retrospective analysis of the data aimed at assessing the potential confounding influence of muscle FDG uptake on BAT estimation. Results indicated that athletes skewed the relationship between body mass index (BMI) and supraclavicular fat (sFAT) FDG uptake, while a non-significant inverse relationship between muscle FDG uptake and sFAT FDG uptake was also observed. The current retrospective analysis provides preliminary evidence suggesting that BAT estimation may be biased in endurance-trained individuals, which may relate to skeletal muscle FDG uptake. These results point to important methodological considerations for estimating BAT activity via FDG uptake, for which we propose potential solutions that facilitate unbiased estimation of BAT activity in groups that differ in terms of lean body mass and physical activity level.
Keywords: Magnetic resonance imaging, Physical activity, Obesity, Non-shivering thermogenesis, Fluorodeoxyglucose
Introduction
Brown adipose tissue (BAT) has been recognized as a source of body heat in small mammals and human infants for decades, but it was previously believed that BAT content was negligible in adult humans. In 2007, researchers first acknowledged physiologically meaningful amounts of BAT in adults (1). In the years since, research has been conducted to uncover the physiological implications of BAT in adult humans, and to identify factors related to intra- and inter-individual variation in BAT content. Acute cold exposure is the primary stimulus for BAT activation, which markedly increases metabolic activity and glucose uptake in the tissue (2). As such, the most widely used methodology for in vivo detection of BAT is positron emission tomography (PET) performed in combination with the infusion of a glucose analog radiotracer, [18F] Fluorodeoxyglucose (FDG), after the application of a cold stimulus to increase the metabolic activity of BAT. In FDG-PET examinations, activity and volume of BAT is then estimated based on the magnitude and extent of [18F]FDG uptake in common human BAT depots, typically in the supraclavicular region.
Human research utilizing FDG-PET has indicated that BAT activity is not uniform across all adult populations. Previous investigations have suggested that BAT activity may be higher in the young compared to the elderly (3), and in lean individuals compared to obese (4). Given the high prevalence of obesity and its associated comorbidities, this observed disparity in BAT activity between lean and obese individuals has received much attention. The uncoupled respiration observed with BAT activation results in a pronounced increase in energy expenditure (5), which may help to promote negative energy balance and facilitate the prevention or treatment of obesity in humans, as it does in mice. While studies utilizing PET have identified an inverse relationship between body mass index (BMI) and/or body fat percentage with BAT activity (4), the direction of causation is not fully understood (6,7). In addition, the magnitude by which lean mass differences influence this relationship is not fully understood. Individuals with high BMI are more likely to have larger amounts of muscle tissue and lean mass; skeletal muscle is the body’s major site of glucose disposal, which has been shown to take up the overwhelming majority of an infused FDG dose (8).
In addition to exploring characteristics that correlate with BAT activity, researchers have sought to identify interventions that may increase BAT activity. It has been hypothesized that exercise training may have chronic effects on BAT activity by increasing catecholamine secretion, uncoupling protein-1 (UCP-1) gene transcription, mitochondrial biogenesis, hyperplasia of BAT, and the conversion of white adipocytes to a more brown-like phenotype (9). Previous research has also identified a number of exercise-related endocrine factors that are thought to influence BAT activity, including irisin, interleukin-6 (IL-6), cardiac natriuretic peptides, fibroblast growth factor 21 (FGF21), and β-aminoisobutyric acid (BAIBA) (9). Preliminary research in rodent models has suggested that exercise may increase the activity of classical BAT (10) or promote the “browning” of subcutaneous white adipose tissue (11,12), although there is ongoing debate whether exercise increases UCP-1 expression in classical BAT in the absence of cold exposure, high-fat diet, or low baseline UCP-1 levels (13). The theoretical potential for exercise to enhance uncoupled respiration in brown adipocytes, or BAT thermogenesis, is further supported by rodent data indicating that irisin and IL-6 can alter energy expenditure, glucose homeostasis, and uncoupled respiration (14,15).
Despite promising results in animal models, human studies have not identified consistent positive relationships between physical activity level and BAT volume or activity. A recent cross-sectional study identified a positive correlation between habitual physical activity and BAT activity (16). However, the use of a sample comprised of cancer patients significantly limits the generalizability of the results, and patients were not cooled before or during imaging. A study comparing lean, endurance-trained male athletes to lean, healthy controls found that trained individuals had significantly lower BAT activity, despite a similar increase in energy expenditure, during cold exposure (17). More recently, female athletes were found to have lower BAT volume and activity than non-athletes (18). These reports of unexpectedly low BAT activity in highly active subjects challenge data from animal models indicating that exercise may increase BAT activity. To explore the potential relationship between exercise and BAT, more data are needed to quantify BAT activity in endurance-trained athletes, and to evaluate the relationship between BAT activity and cold-induced thermogenesis in this population.
Previous research has demonstrated that a significant amount of infused FDG is taken up by skeletal muscle during cold exposure (8), that acute exercise increases muscle glucose uptake (19–21), and that longitudinal aerobic exercise training increases the amount of FDG taken up by skeletal muscle, while reducing uptake in adipose tissue (22). These findings are problematic for FDG-based estimation of BAT activity, because standardized FDG doses are typically administered without regard to body size, lean mass, or physical activity level. Increased muscle uptake of FDG may result in underestimation of BAT activity in individuals with high amounts of lean mass, such as athletes or obese individuals, or high levels of aerobic exercise. To date, the effects of high physical activity levels on BAT activity are not fully understood, and it is possible that methodologies utilizing FDG for BAT identification may require researchers to account for skeletal muscle FDG uptake related to lean mass and exercise volume. Here we report an incidental finding of very low FDG uptake in BAT of two endurance-trained athletes compared to healthy volunteers of similar BMI and age. We hypothesize that the low FDG uptake found in endurance-trained athletes may relate to skeletal muscle FDG uptake, which can confound the estimation of BAT activity, and discuss potential methodological considerations for future research in this area.
Methods
Experimental design
This study was approved by the Institutional Review Board of the University of North Carolina at Chapel Hill. Data from twelve healthy men and women (Mean ± SD; Age = 27.3 ± 8.6 years; Height = 175.4 ± 5.2 cm; Weight = 74.9 ± 8.3 kg; BMI = 24.3 ±2.0 kg·m−2) were available for retrospective analysis of previously collected data. At the time of enrollment, two subjects self-identified as recreational endurance athletes that were engaged in rigorous training for competitions involving long-distance running and/or cycling (one marathon, one triathlon). Upon imaging, both participants were found to have unusually low FDG uptake in the supraclavicular region, prompting further analysis. The endurance-trained subsample consisted of one male and one female; the untrained subsample consisted of eight males and two females that were sampled from the general population and did not appear or report to be engaged in rigorous endurance-training programs. All participants underwent a PET/MR imaging session, following a standardized cooling protocol. To allow for the identification of BAT in the supraclavicular region, participants were exposed to cold for 1 hour before and about 90 minutes after receiving an intravenous injection of 5mCi of FDG. Imaging began approximately one hour after tracer injection, to provide sufficient time for the tracer to accumulate in body tissues without extensive radioactive decay. Six of the participants also completed resting metabolic assessments in thermoneutral conditions, and during cold exposure, within 24 hours of imaging to estimate resting metabolic rate (RMR). All testing was performed following at least six hours of fasting, and at least 24 hours of abstention from exercise. Testing sessions were performed in the morning hours, with no visits beginning later than 12:00.
Cooling Protocol
An individualized cooling protocol was used to activate BAT thermogenesis and stimulate FDG uptake within BAT in the supraclavicular region, in accordance with methodological considerations outlined by van der Laans, et al. (23). For imaging, the cooling protocol took place inside the PET/MR scanner to minimize artifact from movement and repositioning. Participants were cooled using an ArcticSun 2000 temperature management system (Medivance, Louisville, CO), which consists of four water-perfused pads. Two of the pads were wrapped around each of the subject’s thighs, while the other two were wrapped around the subject’s torso. Subjects were first acclimated to a thermoneutral condition for thirty minutes, with the water flowing through the pads set at 30° C. After this 30-minute acclimation period, the water temperature was reduced until the subject began to shiver. Shivering status was monitored by visual inspection by laboratory staff, and by verbal discussion with the participant. Once the subject reached the shivering threshold, the water temperature was increased by 1–4° C, until shivering completely subsided. The absence of shivering during maximal stimulation of NST was also confirmed by the absence of small motion artifacts in the MR images. The individualized cooling procedure was necessary to guarantee maximal stimulation of BAT thermogenesis while avoiding muscle shivering, as recently reported by van der Lans et al. (23). After 60 minutes of exposure to this cold condition, an intravenous injection of 5 mCi of FDG was administered; participants remained under cold condition for approximately 90 minutes from the time of the FDG injection to the end of the imaging scans. For the metabolic study, which occurred within 24 hours of the imaging scan, a similar cooling protocol was followed.
Imaging
A hybrid PET/MR scanner (Biograph mMR, Siemens Healthcare, Germany) was used to simultaneously acquire all PET and MR images. Fat fraction maps were acquired using a two-echo chemical-shift Dixon protocol with a repetition time of 10 ms, echo time of 2.46 ms, flip angle of 13°, field of view of 500 × 370 × 120 mm, matrix of 512 × 384, resolution of 0.98 × 0.96 × 1.00 mm, and with 96 slices of 1 mm thickness. Static PET images were acquired in 3D mode with a resolution of 4.1 mm × 2.6 mm × 3.1 mm, using 3–4 bed positions to cover the area from the head to kidneys, and took approximately six minutes per bed position.
PET/MR images were analyzed using MIM software (MIM software Inc., Cleveland, OH). To correct for slight movement of subjects between scans, intensity-based deformation software was applied to each water component image. Fat fraction (FF) images (Figure 1) were created from deformed water component images (W) and co-deformed fat component images (F) using the formula FF = F·(F+W)−1. Within the supraclavicular region, a fat fraction threshold of 40% was used to identify and measure the volume of the supraclavicular fat depot (sFAT) while excluding blood vessels, muscle and bones; this method and 40% FF threshold is consistent with previous work published by Lundstrom et al. (24). Images were analyzed to determine peak and mean SUV (Standardized uptake value; a weight-normalized ratio of radioactivity in a region of interest to the total injected dose of radioactivity) and total volume of the supraclavicular fat depot. Total sFAT activity was calculated as the product of the mean SUV of supraclavicular adipose tissue and the total volume. Small regions of interest were selected to measure muscle FDG uptake, similarly to the method previously described by Vosselman et al. (17). Spherical regions of interest were placed bilaterally in the superficial sternocleidomastoid muscles, which is the same muscle utilized by Singhal et al. (18). Mean SUV values within these superficial muscle regions were used to approximate muscle FDG uptake. Muscle SUV was calculated as the average between bilateral ROIs placed within the superficial neck musculature.
Figure 1.
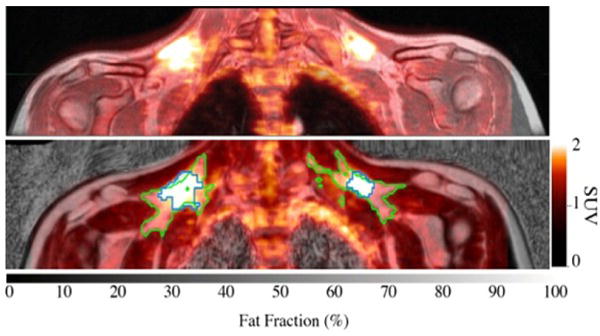
Top: Fused FDG-PET/MR image of the upper torso. Bottom: Glucose uptake map overlaid onto a fat fraction MR map. Green line contours adipose tissue within supraclavicular area with a fat fraction >40%; blue line contours sFAT with SUV values above 1.5.
Metabolic Rate Assessment
Within 24 hours of imaging, resting metabolic rate (RMR) was measured. Metabolic rate was assessed using a portable indirect calorimetry device (Fitmate Pro, COSMED, Italy), which analyzes expired respiratory gases to estimate energy expenditure. Participant age, height, weight, and sex were entered into the device; respiratory gases were collected for fifteen minutes as each participant rested in a seated position, with the first five minutes discarded from analysis by default. Resting metabolic rate was calculated using the device’s default software. This device has previously been shown to have acceptable validity and reliability (25,26), with an intra-day intra-class correlation coefficient (ICC) of 0.981 and standard error of measurement (SEM) of 70.6 kcal, and inter-day values of ICC = 0.946 and SEM = 116.9 kcal (25). To evaluate cold-induced thermogenesis by comparing RMR at room temperature to RMR during cold exposure, metabolic rate was first assessed under thermoneutral condition and then under cold condition. Subjects rested in a seated position for 30 minutes prior to each RMR measurement to ensure that energy expenditure from locomotion would not increase RMR values, and to allow participants to acclimate to the temperature being applied. After this 30-minute acclimation period, RMR testing began. For measurements taken in the cold condition, body cooling was performed using the same device and same water temperature setting used for metabolic imaging. Values are presented as raw values (kcal·day−1) and as a percentage of the individual’s predicted RMR, using the Harris-Benedict equation to predict an individual’s RMR based on sex, height, weight, and age (27).
Statistical Analysis
Due to small sample size, analyses for the current study are primarily descriptive and exploratory in nature. Variables of interest included mean SUV in supraclavicular adipose tissue (sFAT mean SUV), total SUV in supraclavicular adipose tissue (sFAT total SUV, commonly referred to as total BAT activity), mean muscle SUV, RMR, and BMI. A series of Pearson bivariate correlations were used to explore linear relationships between sFAT FDG uptake, muscle FDG uptake, BMI, and RMR measures, and to examine the influence of training status on these relationships. All analyses were completed using R software (Version 3.2.2, R Foundation for Statistical Computing, Vienna, Austria).
Results
FDG uptake in supraclavicular adipose tissue
Mean SUV of sFAT was 2.66 ± 2.17 SUV. Large differences between individuals were observed, with values ranging from 0.37 to 7.37 SUV. Four subjects had mean sFAT SUV values below 1.0 SUV, including both endurance athletes (Figure 2). Total sFAT activity also revealed a wide range of values (38.4 to 790.0 total SUV), with a mean value of 282.8 ± 256.8 total SUV. Values for total sFAT activity followed a similar pattern, with both endurance athletes accounting for the two lowest values observed in the sample (Figure 3). Peak SUV for the sample was 11.05 ± 8.96 SUV (range = 1.77 – 24.40 SUV), and volume of the supraclavicular fat depot was 110.10 ± 55.43 mL (range = 38.76 – 216.41 mL). An example of markedly different sFAT FDG uptake between athletes and non-athletes is provided in Figure 4.
Figure 2.
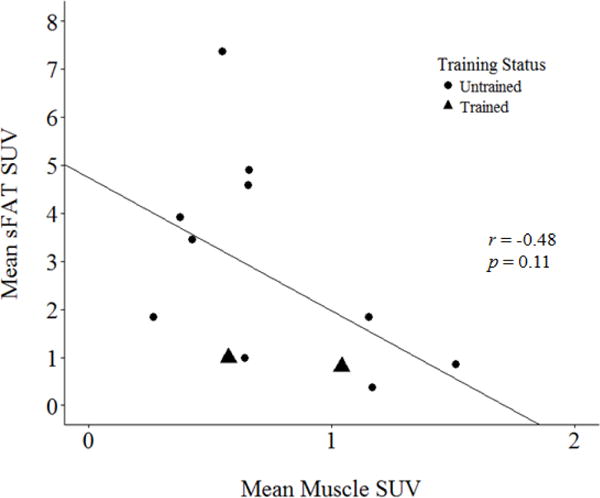
Mean standardized uptake values (SUV) in supraclavicular adipose tissue vs. mean SUV in bilateral spherical regions of the sternocleidomastoid muscle.
Figure 3.
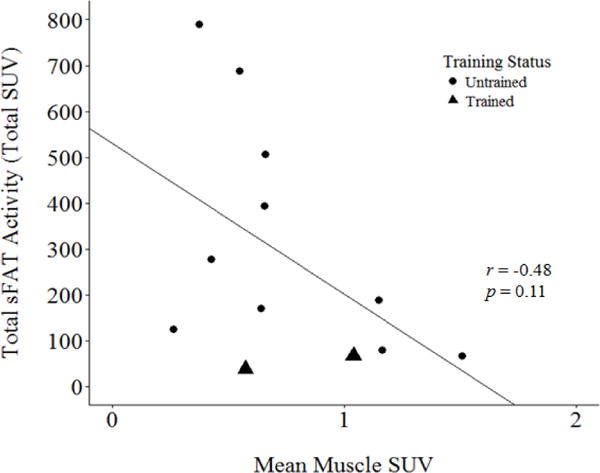
Total supraclavicular fat (sFAT) activity, calculated as the product of supraclavicular adipose tissue volume and mean SUV of supraclavicular adipose tissue, vs. mean SUV in bilateral spherical regions of the sternocleidomastoid muscle.
Figure 4.
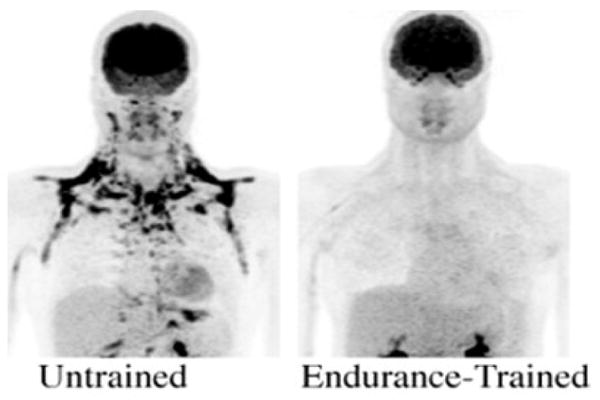
Maximum Intensity projection FDG maps of an untrained subject and endurance-trained subject of similar age (20, 25 years) and BMI (24.4, 24.0 kg·m−2). Reduced FDG uptake is seen in the sFAT of the endurance-trained subject.
FDG uptake in musculature of the neck
Standardized uptake values in the superficial neck musculature (sternocleidomastoid) ranged from 0.27 to 1.51 SUV, with a mean of 0.75 ± 0.38 SUV. Values for the endurance athletes were each within one standard deviation of the mean (0.58 and 1.04 SUV, respectively). There appeared to be an inverse relationship between mean sFAT SUV and muscle SUV (Figure 2), and between total sFAT activity and muscle SUV (Figure 3). Both correlations resulted in a Pearson correlation coefficient of r = − 0.48, but were not statistically significant (p = 0.11).
FDG uptake and BMI
When excluding the endurance athletes from analysis, a nonsignificant inverse relationship was observed between BMI and mean sFAT SUV (r = −0.50, p = 0.14; Figure 5A). When evaluating this relationship with endurance athletes included, the strength of this association was reduced (r = −0.19, p = 0.55; Figure 5B).
Figure 5.
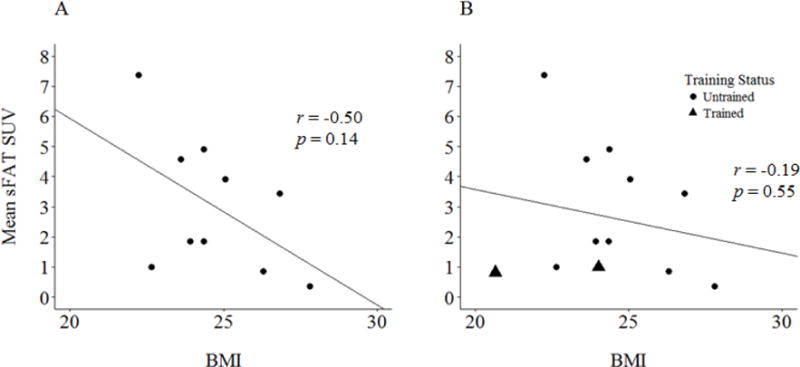
(A) Mean SUV in sFAT vs. body mass index (BMI), with endurance athletes excluded. (B) Mean SUV in sFAT vs. body mass index (BMI), with the full sample.
When excluding the endurance athletes from analysis, a nonsignificant inverse relationship was observed between BMI and total sFAT activity (r = −0.39, p = 0.27; Figure 6A). When evaluating this relationship with endurance athletes included, the strength of this association was reduced (r = −0.10, p = 0.75; Figure 6B).
Figure 6.
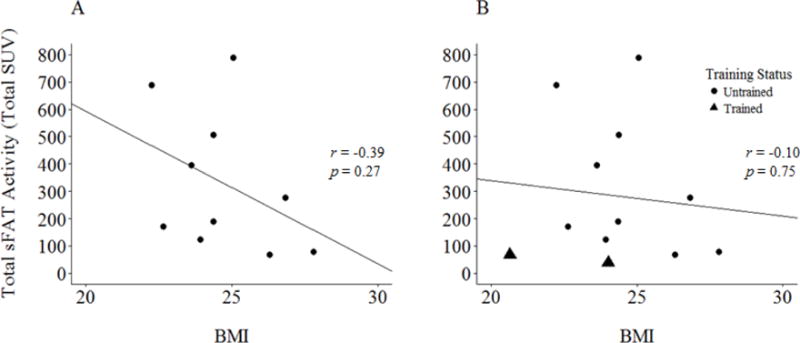
(A) Total sFAT activity vs. BMI, with endurance athletes excluded. (B) Total sFAT activity vs. BMI, with the full sample.
Metabolic testing
Resting metabolic rate during cold exposure (2230 ± 459 kcal·day−1) was higher than RMR during thermoneutral conditions (1963 ± 321 kcal·day−1). On average, RMR during cold exposure rose by 268 ± 223 kcal·day−1 in comparison to thermoneutral conditions, which represents an increase of 13.3 ± 9.7%.
One of the six subjects that underwent metabolic testing was an athlete; this athlete registered the lowest change from thermoneutral to cold conditions when expressed as percent change (8.1%). In absolute terms, the endurance athlete displayed the third-highest increase in energy expenditure during cold exposure (+192 kcal·day−1), and also had the second-highest RMR during cold exposure (2566 kcal·day−1). When expressed as a percentage of predicted BMR using the Harris-Benedict prediction equation (27), mean RMR for the sample rose from 111.2 ± 13.4% (thermoneutral) to 126.7 ± 22.5% (cold). During cold exposure, the endurance athlete had the second-highest metabolic rate as a percentage of the predicted value (139%; Figure 7). Despite low statistical power, RMR during cold exposure, expressed as the percentage of predicted RMR, appeared to show a strong relationship with total sFAT activity in untrained subjects (r = 0.85, p = 0.07). This relationship was weakened by the inclusion of the endurance-trained athlete (r = 0.65, p = 0.16; Figure 7).
Figure 7.
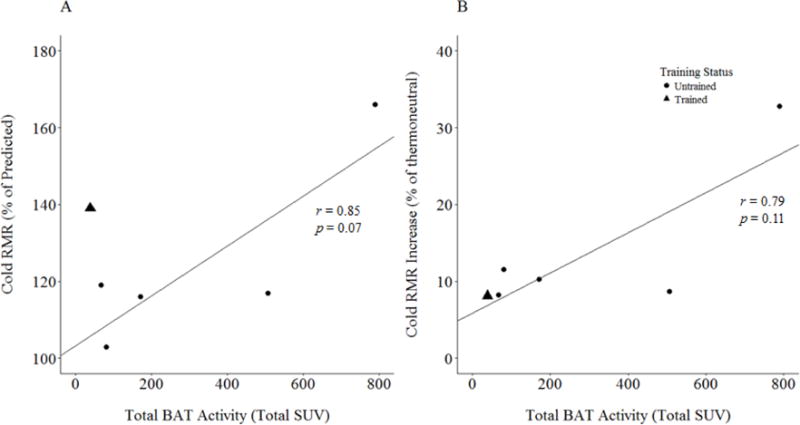
(A) Resting metabolic rate (RMR) during cold exposure, presented as a percentage of predicted RMR, vs. total sFAT activity (total SUV). (B) Cold RMR, presented as the percent increase from the thermoneutral condition, vs. total sFAT activity (total SUV). Best fit lines calculated from untrained subjects only. Predicted values were determined based on sex, weight, height, and age using the Harris-Benedict equation.
Discussion
Data from the current pilot study support previous reports of an inverse relationship between BMI and sFAT activity (4), and demonstrate a positive relationship between RMR during cold exposure and sFAT activity. A marked change in RMR was observed during acute cold exposure, yielding an increase of 268 ± 223 kcal·day−1 (13.3 ± 9.7% increase) in comparison to thermoneutral conditions, as is expected from BAT activity in adult humans (28). Interestingly, the inclusion of endurance-trained athletes in the analysis weakened the relationship between sFAT FDG uptake and BMI, as well as the relationship between cold exposure RMR and sFAT FDG uptake. Although values for mean SUV in sFAT (Figure 2) and total sFAT activity (Figure 3) were quite variable across participants, both endurance athletes showed lower mean and total sFAT SUV than would be expected based on their BMI (Figure 5B, 6B). These results are supported by previous findings of lower mean and total FDG uptake in supraclavicular BAT of athletes when compared to controls, despite similar BMI and cold-induced changes in energy expenditure (17,18). Although preliminary in nature, the current results combined with previous literature suggest that additional methodological considerations may be required when assessing BAT in highly active populations.
Results from metabolic testing do not appear to support the conclusion that the low mean and total sFAT SUV observed in endurance athletes is the result of a reduced capacity for cold-induced non-shivering thermogenesis. While the endurance athlete tested demonstrated the lowest percent change in RMR from thermoneutral to cold conditions (8.1%), half of the sample changed by less than 9%, with 5 of the 6 subjects changing by less than 11.6%. More importantly, the endurance athlete’s change score was likely influenced by a high RMR measured in thermoneutral conditions (2374 kcal·day−1; 128% of the predicted RMR value), which supports previous research showing a trend for higher resting energy expenditure in athletes compared to nonathletes in thermoneutral conditions (18). When evaluating cold-induced thermogenesis in absolute terms, the endurance athlete displayed the third-highest increase in RMR from thermoneutral to cold conditions, and had the second highest RMR during cold exposure, both in absolute terms (2566 kcal·day−1) and expressed as a percentage of predicted RMR (139%; Figure 7). While the current analysis utilized a small sample size, Vosselman et al. (17) also observed a similar trend, with athletes having higher absolute energy expenditure in thermoneutral and cold conditions compared to untrained controls, and a similar magnitude of change in RMR from thermoneutral to cold, despite lower FDG-based estimates of BAT activity. Taken together, results do not indicate that low observed sFAT activity in endurance-trained athletes can be attributed to impaired cold-induced thermogenesis. It is also possible that lower values in athletes may relate to a blunted sympathetic nervous system (SNS) response to cold stress, as endurance training has been shown to reduce SNS activity in response to a number of other stimuli (29). However, this theory is not supported by the data of Vosselman et al. (17), in which endurance-trained athletes displayed significantly lower BAT volume and activity, despite similar heart rate and blood pressure responses to the cold condition.
Reduced glucose uptake in sFAT of athletes may, on the other hand, be caused by an increase in glucose uptake in skeletal muscle. It is known that a substantial percentage of an intravenous FDG dose will be taken up by skeletal muscle (8). When given a standardized dose of a tracer, increased uptake from skeletal muscle will logically result in reduced tracer concentration in other tissues, such as BAT, resulting in underestimated BAT activity. Lean mass may also contribute to the inverse relationship observed with sFAT activity and BMI (Figure 5A, Figure 6A), as individuals with higher BMI generally tend to have higher absolute amounts of lean body mass and skeletal muscle. For example, in a previous study reporting lower BAT activity in overweight/obese subjects compared to lean subjects (4), lean and overweight groups differed by over 8 kg of lean mass, but were given the same standardized dose of FDG. It is also known that longitudinal aerobic exercise training increases insulin-stimulated muscle FDG uptake (22). Exercise training increases insulin sensitivity (20,21) and glucose uptake of the trained musculature (19); in theory, these glucose-partitioning effects of exercise could divert a larger proportion of the FDG dose toward skeletal muscle and away from supraclavicular BAT.
Contrary to this hypothesis, and similar to the findings of Vosselman et al. (17), the endurance athletes did not display higher muscle FDG uptake when compared to untrained participants. This unexpected finding could be due, in part, to overlooked methodological considerations involving skeletal muscle uptake calculations. In Vosselman et al. (17), for example, average muscle SUV values were calculated from select regions that included the deltoids, biceps, and triceps. These muscles have been shown to express low mean SUV values (8), which is likely to reduce variability between individuals. In addition, the trained group had an addition 3.6 kg of lean mass in comparison to the control group (17); a similar average muscle SUV value would therefore suggest a greater absolute uptake of FDG by muscle tissue. In the current study, the sternocleidomastoid was selected for muscle uptake estimation because it has been shown to display relatively high uptake of FDG during cold exposure (8), it has been used previously in similar research (18), and imaging was only conducted on the area of the body above kidney-level. While previous studies have shown increased muscle FDG uptake following longitudinal exercise training (22), exercise-induced increases in muscle uptake are likely most pronounced in the musculature being trained (19). It is plausible that analysis of the musculature that is consistently trained by the endurance athletes in the sample, such as the knee flexors and extensors during running and cycling, may be necessary to identify potential differences in muscle FDG uptake. It is also pertinent to note that studies quantifying BAT typically require anywhere from 24–48 hours of abstention from exercise prior to FDG-based imaging (8,17,22), but it is possible that longer abstention periods may be required. The acute effects of a single bout of exercise on glucose transport, insulin sensitivity, and glycogen replenishment may persist for at least 48 hours post-exercise (21), and chronic effects of consistent exercise training likely persist even longer (20). The muscle uptake analysis of the current study is limited by a narrow region of interest and small sample size, but future investigators are encouraged to consider measuring regions of interest that more globally approximate muscle FDG uptake, with special attention paid to muscle groups that are relevant to the individual’s exercise habits.
Results of the current analysis must be interpreted within the context of its limitations. Due to low sample size, statistical tests for the current study are underpowered, and generalizability is limited. As a result, the reported observations should be interpreted as preliminary in nature, and the purpose of this analysis is to provide data to inform the design of future research in this area. Assessment of muscle uptake was confined to the sternocleidomastoid muscle, while a more global analysis of muscle uptake would be preferred. In the future, researchers should investigate more global assessments of muscle FDG uptake during cold exposure, with special interest paid to the muscle groups that are habitually trained by the athletes sampled. Similarly, the assessment of BAT activity was limited to the supraclavicular region; if exercise indeed induces the “browning” of white adipocytes that are broadly dispersed outside of the supraclavicular region, quantification of uncoupled respiration in these adipocytes would be challenging to reliably identify and quantify using PET imaging with FDG. Body fat percentage was not calculated for the current sample; future studies should include this measurement to further evaluate the role that lean body mass plays in the relationship between BMI and FDG uptake in the supraclavicular fat depot, particularly in trained athletes, and to potentially allow for mathematical adjustments or FDG dosing adjustments that account for skeletal muscle mass and physical activity level.
While the current study featured a limited sample size, the retrospective analysis of this small sample was primarily intended to investigate the plausibility and likelihood that skeletal muscle FDG uptake plays a confounding role in BAT activity estimation. The resultant findings provide preliminary but compelling justification for further research evaluating methodological strategies to account for lean mass and physical activity level when using FDG-based imaging to estimate BAT activity. Future research should seek to determine how FDG doses can be appropriately scaled to account for lean body mass, skeletal muscle mass, and physical activity level to ensure unbiased estimates of BAT activity, and to determine if mathematical adjustment can allow for unbiased estimation of BAT activity in individuals engaged in large amounts of physical activity or exercise training.
Conclusions
In agreement with previous reports (17,18), the current pilot data demonstrate unexpectedly low sFAT activity in endurance-trained athletes compared to untrained individuals with similar BMI. Interestingly, the current data do not indicate that endurance athletes have abnormally low energy expenditure during cold exposure, nor do the data published by Vosselman et al. (17). These data and the literature currently available indicate that studies attempting to estimate BAT volume and activity using PET/FDG methods should carefully consider the effects of skeletal muscle mass and physical activity on BAT FDG uptake.
Based on these preliminary observations, future research is encouraged to determine if this methodology can be improved upon to allow for unbiased estimation of BAT activity across adult populations. Potential methodological improvements may include scaling FDG doses to lean body mass or skeletal muscle mass, increasing the duration of abstention from exercise prior to imaging, or developing mathematical methods to correct for skeletal muscle FDG uptake in trained or highly active individuals. Addressing these methodological considerations may be a critical step in assessing population-based differences in BAT activity, and in moving toward interventions to increase BAT activity in the management of obesity and diabetes.
Acknowledgments
This work was supported by the NIH grant number R01DK108231 and by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR001109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest: No conflicts declared.
References
- 1.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 2.Carey AL, Formosa MF, Van Every B, Bertovic D, Eikelis N, Lambert GW, Kalff V, Duffy SJ, Cherk MH, Kingwell BA. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia. 2013;56:147–155. doi: 10.1007/s00125-012-2748-1. [DOI] [PubMed] [Google Scholar]
- 3.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 5.Golozoubova V, Cannon B, Nedergaard J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am J Physiol Endocrinol Metab. 2006;291:E350–357. doi: 10.1152/ajpendo.00387.2005. [DOI] [PubMed] [Google Scholar]
- 6.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijgen GH, Bouvy ND, Teule GJ, Brans B, Hoeks J, Schrauwen P, van Marken Lichtenbelt WD. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2012;97:E1229–1233. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- 8.Blondin DP, Labbe SM, Phoenix S, Guerin B, Turcotte EE, Richard D, Carpentier AC, Haman F. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol. 2015;593:701–714. doi: 10.1113/jphysiol.2014.283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez-Delgado G, Martinez-Tellez B, Olza J, Aguilera CM, Gil A, Ruiz JR. Role of Exercise in the Activation of Brown Adipose Tissue. Ann Nutr Metab. 2015;67:21–32. doi: 10.1159/000437173. [DOI] [PubMed] [Google Scholar]
- 10.Slocum N, Durrant JR, Bailey D, Yoon L, Jordan H, Barton J, Brown RH, Clifton L, Milliken T, Harrington W, Kimbrough C, Faber CA, Cariello N, Elangbam CS. Responses of brown adipose tissue to diet-induced obesity, exercise, dietary restriction and ephedrine treatment. Exp Toxicol Pathol. 2013;65:549–557. doi: 10.1016/j.etp.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 11.De Matteis R, Lucertini F, Guescini M, Polidori E, Zeppa S, Stocchi V, Cinti S, Cuppini R. Exercise as a new physiological stimulus for brown adipose tissue activity. Nutr Metab Cardiovasc Dis. 2013;23:582–590. doi: 10.1016/j.numecd.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Xu X, Ying Z, Cai M, Xu Z, Li Y, Jiang SY, Tzan K, Wang A, Parthasarathy S, He G, Rajagopalan S, Sun Q. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1115–1125. doi: 10.1152/ajpregu.00806.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flouris AD, Dinas PC, Valente A, Andrade CM, Kawashita NH, Sakellariou P. Exercise-induced effects on UCP1 expression in classical brown adipose tissue: a systematic review. Horm Mol Biol Clin Investig. 2017 doi: 10.1515/hmbci-2016-0048. [DOI] [PubMed] [Google Scholar]
- 14.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knudsen JG, Murholm M, Carey AL, Bienso RS, Basse AL, Allen TL, Hidalgo J, Kingwell BA, Febbraio MA, Hansen JB, Pilegaard H. Role of IL-6 in exercise training- and cold-induced UCP1 expression in subcutaneous white adipose tissue. PLoS One. 2014;9:e84910. doi: 10.1371/journal.pone.0084910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinas PC, Nikaki A, Jamurtas AZ, Prassopoulos V, Efthymiadou R, Koutedakis Y, Georgoulias P, Flouris AD. Association between habitual physical activity and brown adipose tissue activity in individuals undergoing PET-CT scan. Clin Endocrinol (Oxf) 2015;82:147–154. doi: 10.1111/cen.12620. [DOI] [PubMed] [Google Scholar]
- 17.Vosselman MJ, Hoeks J, Brans B, Pallubinsky H, Nascimento EB, van der Lans AA, Broeders EP, Mottaghy FM, Schrauwen P, van Marken Lichtenbelt WD. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int J Obes (Lond) 2015;39:1696–1702. doi: 10.1038/ijo.2015.130. [DOI] [PubMed] [Google Scholar]
- 18.Singhal V, Maffazioli GD, Ackerman KE, Lee H, Elia EF, Woolley R, Kolodny G, Cypess AM, Misra M. Effect of Chronic Athletic Activity on Brown Fat in Young Women. PLoS One. 2016;11:e0156353. doi: 10.1371/journal.pone.0156353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dela F, Mikines KJ, von Linstow M, Secher NH, Galbo H. Effect of training on insulin-mediated glucose uptake in human muscle. Am J Physiol. 1992;263:E1134–1143. doi: 10.1152/ajpendo.2006.263.6.E1134. [DOI] [PubMed] [Google Scholar]
- 20.Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol (Oxf) 2008;192:127–135. doi: 10.1111/j.1748-1716.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- 21.Turcotte LP, Fisher JS. Skeletal muscle insulin resistance: roles of fatty acid metabolism and exercise. Phys Ther. 2008;88:1279–1296. doi: 10.2522/ptj.20080018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reichkendler MH, Auerbach P, Rosenkilde M, Christensen AN, Holm S, Petersen MB, Lagerberg A, Larsson HB, Rostrup E, Mosbech TH, Sjodin A, Kjaer A, Ploug T, Hoejgaard L, Stallknecht B. Exercise training favors increased insulin-stimulated glucose uptake in skeletal muscle in contrast to adipose tissue: a randomized study using FDG PET imaging. Am J Physiol Endocrinol Metab. 2013;305:E496–506. doi: 10.1152/ajpendo.00128.2013. [DOI] [PubMed] [Google Scholar]
- 23.van der Lans AA, Wierts R, Vosselman MJ, Schrauwen P, Brans B, van Marken Lichtenbelt WD. Cold-activated brown adipose tissue in human adults: methodological issues. Am J Physiol Regul Integr Comp Physiol. 2014;307:R103–113. doi: 10.1152/ajpregu.00021.2014. [DOI] [PubMed] [Google Scholar]
- 24.Lundstrom E, Strand R, Johansson L, Bergsten P, Ahlstrom H, Kullberg J. Magnetic resonance imaging cooling-reheating protocol indicates decreased fat fraction via lipid consumption in suspected brown adipose tissue. PLoS One. 2015;10:e0126705. doi: 10.1371/journal.pone.0126705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell B, Zito G, Colquhoun R, Martinez N, St Louis C, Johnson M, Buchanan L, Lehn M, Smith Y, Cloer B, Raines K. Inter- and intra-day test-retest reliability of the Cosmed Fitmate ProTM indirect calorimeter for resting metabolic rate. J Int Soc Sports Nutr. 2014;11:1–2. [Google Scholar]
- 26.Nieman DC, Austin MD, Benezra L, Pearce S, McInnis T, Unick J, Gross SJ. Validation of Cosmed’s FitMate in measuring oxygen consumption and estimating resting metabolic rate. Res Sports Med. 2006;14:89–96. doi: 10.1080/15438620600651512. [DOI] [PubMed] [Google Scholar]
- 27.Harris JA, Benedict FG. A Biometric Study of Human Basal Metabolism. Proc Natl Acad Sci US A. 1918;4:370–373. doi: 10.1073/pnas.4.12.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Marken Lichtenbelt WD, Schrauwen P. Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301:R285–296. doi: 10.1152/ajpregu.00652.2010. [DOI] [PubMed] [Google Scholar]
- 29.Mueller PJ. Exercise training and sympathetic nervous system activity: evidence for physical activity dependent neural plasticity. Clin Exp Pharmacol Physiol. 2007;34:377–384. doi: 10.1111/j.1440-1681.2007.04590.x. [DOI] [PubMed] [Google Scholar]


