Abstract
Background:
Lichen planopilaris is an inflammatory cicatricial alopecia, and its management is a challenge for dermatologists. We aimed to compare the efficacy of methotrexate and hydroxychloroquine on refractory lichen planopilaris.
Methods:
In a randomized clinical trial, 29 patients were randomly allocated to receive either 15 mg methotrexate/week or 200 mg hydroxychloroquine twice a day for 6 months. Side effects, symptoms/signs, and laboratory tests were assessed periodically. Lichen Planopilaris Activity Index (LPPAI) was measured before intervention and at 2, 4, and 6 months after. The changes from baseline to the end of the study were analyzed within each group and between the two groups by per-protocol and intention-to-treat analysis.
Results:
After 2 months, mean (standard deviation [SD]) decrease in LPPAI in methotrexate group was significantly more than that in hydroxychloroquine group (1.68 [1.24] vs. 0.8 [0.71], respectively, P = 0.047). Furthermore, after 6 months, mean (SD) decrease in LPPAI in methotrexate group was significantly higher than that in hydroxychloroquine group (3.3 [2.09] vs. 1.51 [0.91], respectively, P = 0.01). The following symptoms/signs showed significant improvements in frequency and/or severity in methotrexate group after intervention: pruritus (P = 0.007), erythema (P = 0.01), perifollicular erythema (P = 0.01), perifollicular scaling (P = 0.08), spreading (P = 0.001), and follicular keratosis (P = 0.04). In hydroxychloroquine group, only erythema (P = 0.004) showed significant improvement.
Conclusions:
Methotrexate was more effective than hydroxychloroquine in treating refractory lichen planopilaris.
Keywords: Hydroxychloroquine, lichen planopilaris, methotrexate
Introduction
Lichen planopilaris, a common form of primary cicatricial alopecia, is mediated by chronic lymphocytic inflammation around the isthmus of the hair follicle leading to the destruction of follicle germ cells.[1,2,3] The main clinical finding is progressive patchy scarring alopecia usually seen in middle-aged women. The clinical symptoms include itching, burning, and pain of the scalp that may impair the quality of life.[3,4,5] Different medications have been applied to treat lichen planopilaris, both topically such as intralesional corticosteroids, and systematically such as hydroxychloroquine.[6,7,8] Hydroxychloroquine may reduce the symptoms and signs in prolonged treatment.[8] Immunosuppressive agents such as mycophenolate mofetil and azathioprine have been administered for more severe cases.[9,10,11] Adalimumab, oral retinoids, thalidomide, and cyclosporine have been used to treat resistant and refractory lichen planopilaris.[12,13,14,15] Methotrexate, with or without topical corticosteroids, has been employed to treat oral,[16] vulvovaginal,[17,18] anogenital, and generalized lichen planus[18,19,20] with acceptable clinical efficacy. Given the persistence inflammation and lymphocytic infiltrations in pathophysiology of lichen planopilaris,[6] strong anti-inflammatory effects of methotrexate may cease the inflammation in this chronic inflammatory diseases.[5] The aim of this study was to evaluate the safety and efficacy of 6-month methotrexate versus hydroxychloroquine in patients with refractory lichen planopilaris.
Methods
Study design and participants
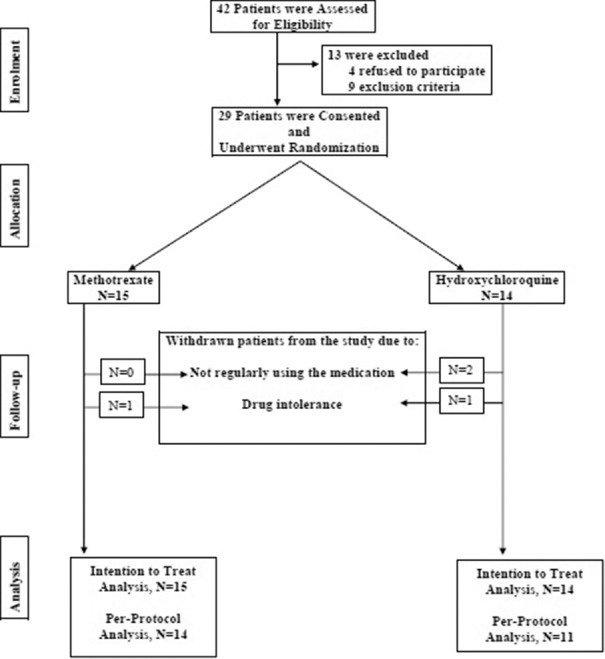
The current study was a randomized clinical trial conducted from February 2015 to December 2016. This study was done in dermatologic clinic affiliated with Isfahan University of Medical Sciences, approved by the Institutional Review Board and Ethics Committee of the university and was registered in Iranian Registry of Clinical Trials (IRCT2015062822959N1). Forty-two patients, ≥18 years, diagnosed with active refractory lichen planopilaris were assessed for eligibility to participate in the study [Figure 1]. The diagnosis was made based on clinical evaluation and was established by histopathological confirmation. Refractory lichen planopilaris defined as no response to treatment with topical agents/systematic medications in the past 6 months. Written informed consent was obtained from all the participants. Four patients refused to participate [Figure 1]. Complete physical examination including ophthalmologic examination and the following laboratory evaluations were carried out for all consented patients: complete blood count, renal function tests, liver function tests, viral markers, and baseline activity of glucose-6-phosphate dehydrogenase measurement. Exclusion criteria were pregnancy, breastfeeding, gastrointestinal disorders, vision problems/retinopathy, porphyria, psoriasis, anemia (Hb <9 mg/dl), leukopenia (white blood cells <4000/dl), thrombocytopenia (platelets <100,000/dl), increased liver enzymes more than three times of the upper normal limit, significant liver disease, positive viral hepatic markers, history of convulsion, and excessive alcohol intake. Nine patients were excluded after application of exclusion criteria [Figure 1]. The following withdrawal criteria were applied: not showing up for follow-up visits, not taking the medication according to the study protocol, receiving other topical or immunosuppressive agents during the study, and nontolerable side effects. Four patients withdrew from the study [Figure 1].
Figure 1.
Consort flow chart of the study
Treatment protocol
All medications were discontinued at least 1 month before the start of the study. Twenty-nine patients were randomly allocated to receive either 15 mg methotrexate per week or 200 mg hydroxychloroquine twice a day for 6 months. History taking including medication side effects, physical examination, and the following laboratory evaluations were carried out periodically: complete blood count, renal function tests, and liver function tests. The patients were instructed to record/report any side effect experienced.
Outcome measures
The activity of lichen planopilaris in each patient was measured using Lichen Planopilaris Activity Index (LPPAI) scoring system and photographic examination. LPPAI was measured before the intervention and at 2, 4, and 6 months after intervention. LPPAI was calculated as (itch + pain + burn)/3 + (scalp erythema + perifollicular erythema + perifollicular scale)/3 + 2.5 (pull test) + 1.5 (spreading/2). Follicular keratosis was also evaluated at baseline and 2, 4, and 6 months after intervention.
Photography was assessed at baseline and at the end of the study by two dermatologists who were blind to group allocation. The most active point was tattooed and surrounded by ink mark in a circle of 0.5 inch radius. A standardized seven-point scale was used to interpret photography. The scale included greatly decreased = −3, moderately decreased = −2, slightly decreased = −1, no change = 0, slightly increased = +1, moderately increased = +2, and greatly increased = +3. There was a “don’t know” option which denoted the photographs were not suitable for proper clinical evaluation.[21] Medication side effects were carefully monitored to evaluate the safety of administered medications.
Statistical analysis
Disease characteristics at baseline and after 2, 4, and 6 months were compared between the two groups using Chi-square and Fisher exact tests for categorical variables, Mann–Whitney U-test for nonparametric variables and Student's t-test for parametric variables. The changes from the baseline to the end of study period within each group were tested using Wilcoxon signed-rank test for nonparametric variables and paired t-test for parametric variables. Spearmen correlation was conducted to evaluate the correlation between the two subspecialty assessments of photography. Per-protocol analysis was carried out on all patients remained in the study. To avoid the bias associated with the nonrandom loss of patients, intention-to-treat (ITT) analysis was conducted on all enrolled patients at the baseline. The worst case scenario was considered for patients who were lost to follow-up. P < 0.05 was considered statistically significant. SPSS version 17 (Chicago, IL, USA) was used for data analysis.
Results
Baseline characteristics
Forty-two patients were assessed, four refused to participate, and nine were excluded [Figure 1]. Fifteen and 14 patients were enrolled in methotrexate and hydroxychloroquine groups, respectively. Thirteen (86.7%) and 8 (57.1%) ones were females in methotrexate and hydroxychloroquine groups, respectively. The difference was not significant (P = 0.1). The mean (standard deviation [SD]) age of participants was 44.9 (11.1) years in methotrexate group and 40.8 (11.7) years in hydroxychloroquine group (P = 0.4). The mean age of disease diagnosis was 42.3 (12.8) and 37.6 (11) years in methotrexate and hydroxychloroquine groups, receptively (P = 0.3). Moreover, disease duration was 3 (2) and 3.14 (4.22) years, respectively (P = 0.9). Furthermore, there was no significant baseline difference between the two groups in terms of family history, organ involvements, clinical findings, and past therapeutic measurements [Table 1]. Similarly, there was no significant difference in frequency of symptoms/signs between the two groups at baseline. The only exception was pull test [Table 2]. The mean LPPAI at baseline in methotrexate group was significantly higher than that in hydroxychloroquine group [Table 3].
Table 1.
Family history, organ involvements, and past therapeutic experiences compared between the two groups
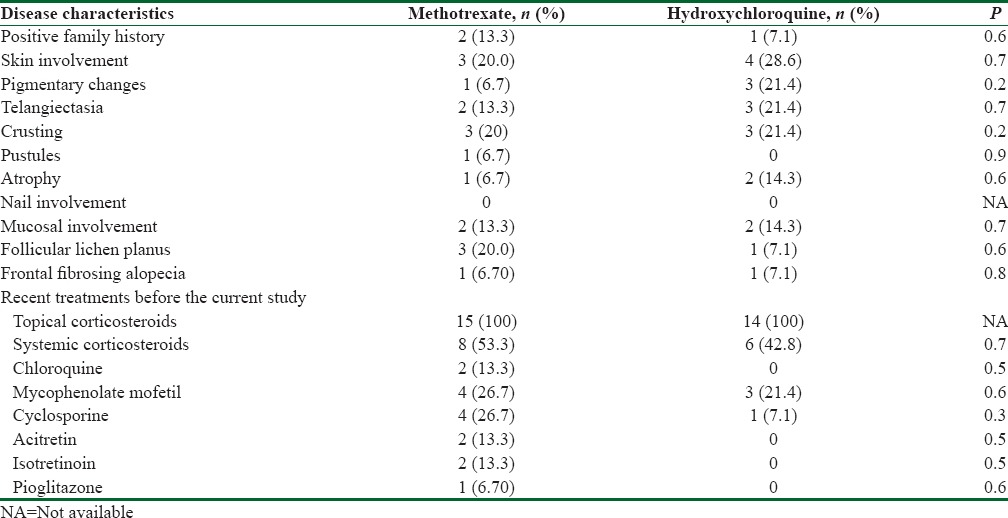
Table 2.
Frequency of symptoms and signs compared between the two groups
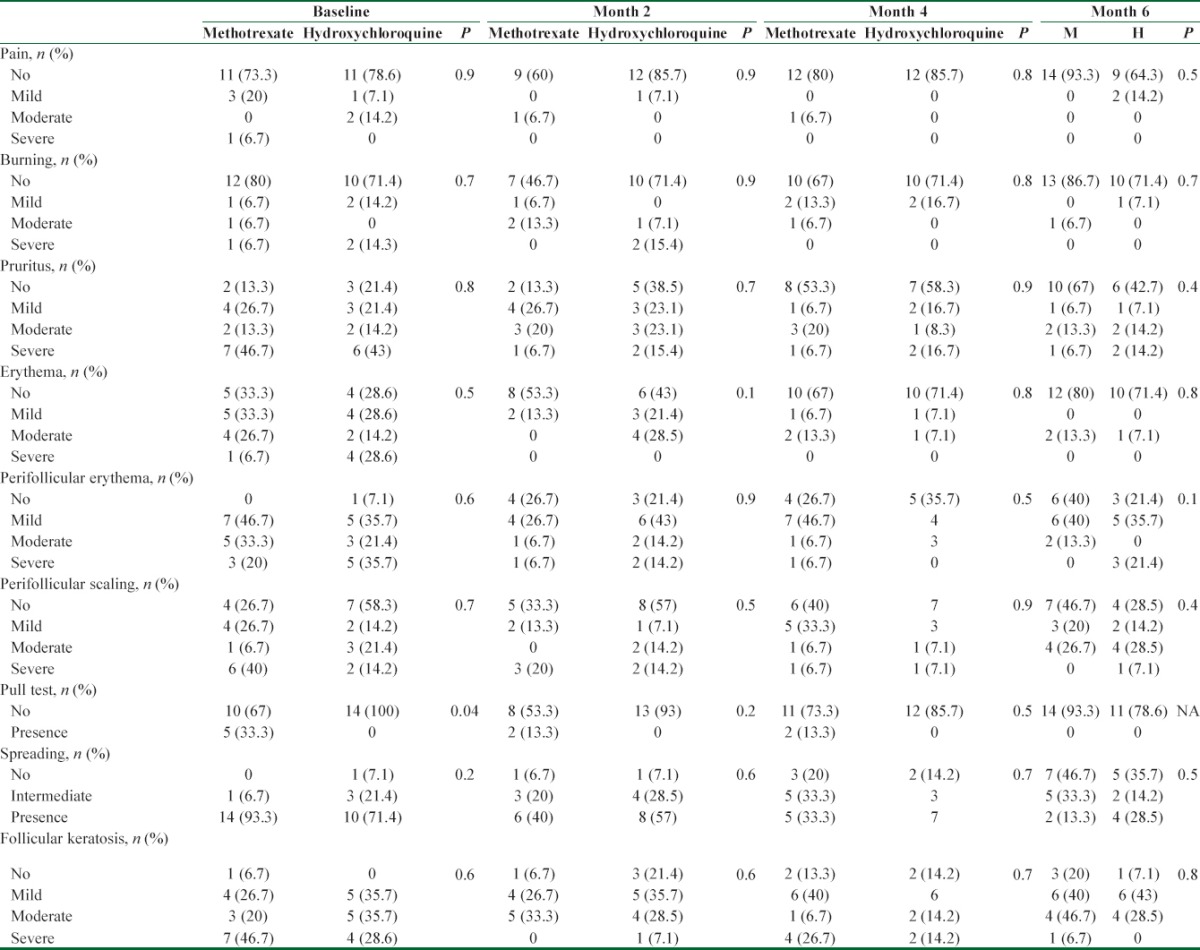
Table 3.
Lichen Planopilaris Activity Index changes compared between the two groups
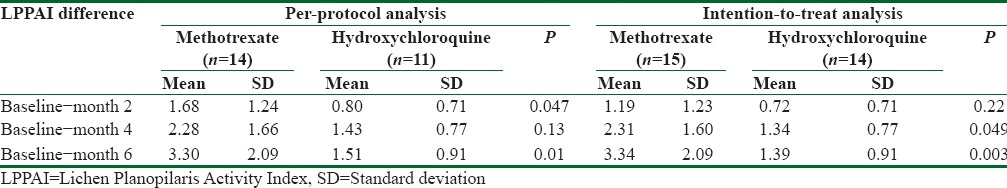
Between groups per-protocol analysis after intervention
Mean (SD) LPPAI in methotrexate group at baseline, 4.63 (1.83), was significantly higher than that in hydroxychloroquine group, 3.46 (0.94), (P = 0.04). Table 3 reveals LPPAI difference between the two groups at baseline and during the study. Mean (SD) decrease in LPPAI in methotrexate group after 2 months was significantly higher than that in hydroxychloroquine group [Table 3]. Similarly, at the end of the study, LPPAI in methotrexate group decreased more significantly than that in hydroxychloroquine group [Table 3]. Figure 2 demonstrates the trend of LPPAI change in both groups during the study. The sharper declines of LPPAI in methotrexate group versus the milder declines in hydroxychloroquine group during the first and the last 2 months of study are noticeable. The decline in LPPAI in methotrexate group is steady and continuous during all study period whereas the decline in LPPAI in hydroxychloroquine group is unremarkable in the last 2 months [Figure 2]. Photographic assessment conducted by two different subspecialists showed also no significant difference between the two groups [Table 4]. Spearmen correlation was conducted to understand the correlation between the two assessments. The results showed substantial (r = 0.50) and significant (P = 0.013) correlation.
Figure 2.
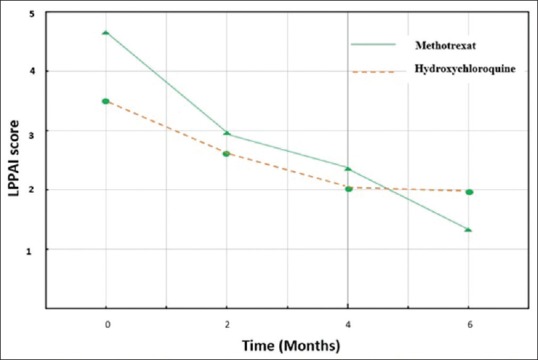
The pattern of decease in Lichen Planopilaris Activity Index in both groups
Table 4.
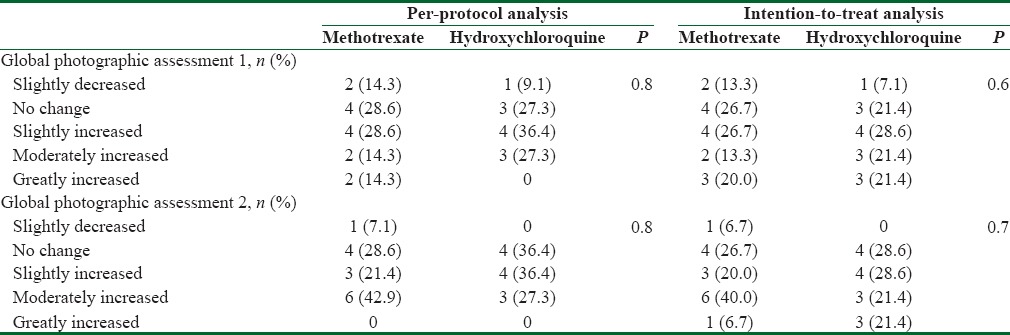
Photographic assessment conducted by two different subspecialists at baseline and at the end of the study. They were compared between the two groups by both per-protocol and intention-to-treat analyses
Within group per-protocol analysis after intervention
Table 2 demonstrates the frequency and severity of nine symptoms/signs at baseline and during the study. Five symptoms/signs showed significant improvement in frequency and/or severity in methotrexate group after 6 months intervention compared to the baseline. The corresponding P values for all nine symptoms/signs were as follow pain (P = 0.06), burning (P = 0.1), pruritus (P = 0.007), erythema (P = 0.01), perifollicular erythema (P = 0.01), perifollicular scaling (P = 0.08), pull test (P = 0.06), spreading (P = 0.001), and follicular keratosis (P = 0.04). On the contrary, only erythema showed a significant improvement in frequency and/or severity in hydroxychloroquine group after 6 months intervention compared to the baseline. The corresponding P values for all symptoms and signs were as follow pain (P = 0.75), burning (P = 0.25), pruritus (P = 0.08), erythema (P = 0.004), perifollicular erythema (P = 0.2), perifollicular scaling (P = 0.8), pull test (P = 0.9), spreading (P = 0.06), and follicular keratosis (P = 0.2).
Table 3 reveals LPPAI difference within both groups at baseline and during the study. Consistent decrease in LPPAI was observed within methotrexate group at month 2 compared to the baseline (P = 0.006), at month 4 compared to the month 2 (P = 0.005), and at month 6 compared to the month 4 (P = 0.03). On the other hand, LPPAI decreased significantly within hydroxychloroquine group at month 2 compared to the baseline (P = 0.002) and at month 4 compared to the month 2 (P = 0.01). No significant decrease in LPPAI at month 6 compared to the month 4 was observed within hydroxychloroquine group (P = 0.8).
Intention to treat analysis
The worst case scenario was considered for lost-to-follow-up patients. After 4 months, mean (SD) decrease in LPPAI in methotrexate group was significantly higher than that in hydroxychloroquine group [Table 3]. Similarly, at the end of the study, mean (SD) decrease in LPPAI in methotrexate group was significantly more than that in hydroxychloroquine group [Table 3]. Consistent decrease in LPPAI was observed within methotrexate group; at month 2 compared to the baseline (P = 0.01), at month 4 compared to the month 2 (P = 0.001), and at month 6 compared to the month 4 (P = 0.03). On the other hand, LPPAI decreased significantly within hydroxychloroquine group, at month 2 compared to the baseline (P = 0.005) and at month 4 compared to the month 2 (P = 0.02). The significance of the difference in photographic assessment was not changed when analyzed by either assessor [Table 4].
Medication side effects
Two patients experienced medication side effects. One (6.7%) in methotrexate group who showed elevated liver enzymes and the other (7.1%) in hydroxychloroquine group who demonstrated drug eruption.
Discussion
The current study demonstrated higher therapeutic effects of methotrexate than hydroxychloroquine on lichen planopilaris in a 6-month randomized clinical trial. Considerable improvements were recorded in frequency and severity of follicular keratosis, pruritus, perifollicular erythema, spreading, and erythema in patients received methotrexate whereas only erythema improved significantly in patients who received hydroxychloroquine. When disease remission occurred, probably, follicular keratosis was the last sign which was improved significantly only in methotrexate group. The significant improvements in symptoms and signs plus more than 70% drop in LPPAI in methotrexate group at the end of study versus <45% drop in final LPPAI in hydroxychloroquine group were the most remarkable findings of the study. In other words, the mean LPPAI at the end of the study was less than one-third of the baseline in patients received methotrexate and more than half of the baseline in patients received hydroxychloroquine. The trend of change was sharp and significant in methotrexate group at all time points during the study whereas it was not significant after month four in hydroxychloroquine group. These findings were observed in both per-protocol and ITT analyses. When ITT analysis was applied, the mean LPPAI difference was increased in both groups at all time points after baseline. However, the increments did not change the significance of findings. Photographic examination might be able to detect improvements in severe cases. Subtle but significant improvements could be easily overlooked in photographic assessment. Both medications were tolerated very well with minimum side effects.
Diverse results have been achieved when different anti-inflammatory medications such as hydroxychloroquine, pioglitazone, tetracycline, mycophenolate mofetil, and cyclosporine were employed to treat lichen planopilaris.[6,7,8,9,10,11,12,13,14] Some reports showed the promising effect of hydroxychloroquine whereas some others showed the reverse.[8,22] Similarly, although cyclosporine demonstrated considerable effects on LPP, its discontinuation might be associated with high relapse rate.[23] Similar therapeutic effects of mycophenolate mofetil and topical clobetasol have been also shown.[9]
It seems the current research is the first clinical randomized trial applied to evaluate the therapeutic effects of methotrexate on lichen planopilaris. This is a cell-mediated autoimmune disorder with activated T-lymphocytes target follicular antigens.[6] Possible pharmacological mechanisms of methotrexate that might modify the path of disease include inhibition of purine and pyrimidine synthesis, suppression of transmethylation reactions with an accumulation of polyamines and reduction of antigen-dependent T-cell proliferation.[24,25] Given the favorable efficacy, reasonable cost, safety profile, and the ease of administration, methotrexate might be a new effective medication in treating lichen planopilaris.
The strengths of our randomized clinical trial were the novel medication applied and the combined assessments of LPPAI, follicular keratosis, and photographic examination. The limitations of the current study were low sample size, relatively short follow-up, and lack of histopathologic evaluations.
Conclusions
Methotrexate was more effective than hydroxychloroquine in treating refractory lichen planopilaris. Further multi-center studies with larger sample sizes and longer follow-ups are warranted.
Financial support and sponsorship
This study was supported by Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Olsen EA. Female pattern hair loss and its relationship to permanent/cicatricial alopecia: A new perspective. J Investig Dermatol Symp Proc. 2005;10:217–21. doi: 10.1111/j.1087-0024.2005.10109.x. [DOI] [PubMed] [Google Scholar]
- 2.Ochoa BE, King LE, Jr, Price VH. Lichen planopilaris: Annual incidence in four hair referral centers in the United States. J Am Acad Dermatol. 2008;58:352–3. doi: 10.1016/j.jaad.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Assouly P, Reygagne P. Lichen planopilaris: Update on diagnosis and treatment. Semin Cutan Med Surg. 2009;28:3–10. doi: 10.1016/j.sder.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Kang H, Alzolibani AA, Otberg N, Shapiro J. Lichen planopilaris. Dermatol Ther. 2008;21:249–56. doi: 10.1111/j.1529-8019.2008.00206.x. [DOI] [PubMed] [Google Scholar]
- 5.Chiang YZ, Bundy C, Griffiths CE, Paus R, Harries MJ. The role of beliefs: Lessons from a pilot study on illness perception, psychological distress and quality of life in patients with primary cicatricial alopecia. Br J Dermatol. 2015;172:130–7. doi: 10.1111/bjd.13259. [DOI] [PubMed] [Google Scholar]
- 6.Manousaridis I, Manousaridis K, Peitsch WK, Schneider SW. Individualizing treatment and choice of medication in lichen planus: A step by step approach. J Dtsch Dermatol Ges. 2013;11:981–91. doi: 10.1111/ddg.12141. [DOI] [PubMed] [Google Scholar]
- 7.Baibergenova A, Donovan J. Lichen planopilaris: Update on pathogenesis and treatment. Skinmed. 2013;11:161–5. [PubMed] [Google Scholar]
- 8.Chiang C, Sah D, Cho BK, Ochoa BE, Price VH. Hydroxychloroquine and lichen planopilaris: Efficacy and introduction of lichen planopilaris activity index scoring system. J Am Acad Dermatol. 2010;62:387–92. doi: 10.1016/j.jaad.2009.08.054. [DOI] [PubMed] [Google Scholar]
- 9.Lajevardi V, Ghodsi SZ, Goodarzi A, Hejazi P, Azizpour A, Beygi S. Comparison of systemic mycophenolate mofetil with topical clobetasol in lichen planopilaris: A parallel-group, assessor-and analyst-blinded, randomized controlled trial. Am J Clin Dermatol. 2015;16:303–11. doi: 10.1007/s40257-015-0122-z. [DOI] [PubMed] [Google Scholar]
- 10.Tursen U, Api H, Kaya T, Ikizoglu G. Treatment of lichen planopilaris with mycophenolate mofetil. Dermatol Online J. 2004;10:24. [PubMed] [Google Scholar]
- 11.Mirmirani P, Willey A, Price VH. Short course of oral cyclosporine in lichen planopilaris. J Am Acad Dermatol. 2003;49:667–71. doi: 10.1067/s0190-9622(03)00873-9. [DOI] [PubMed] [Google Scholar]
- 12.Kreutzer K, Effendy I. Therapy-resistant folliculitis decalvans and lichen planopilaris successfully treated with adalimumab. J Dtsch Dermatol Ges. 2014;12:74–6. doi: 10.1111/ddg.12224. [DOI] [PubMed] [Google Scholar]
- 13.Spano F, Donovan JC. Efficacy of oral retinoids in treatment-resistant lichen planopilaris. J Am Acad Dermatol. 2014;71:1016–8. doi: 10.1016/j.jaad.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 14.George SJ, Hsu S. Lichen planopilaris treated with thalidomide. J Am Acad Dermatol. 2001;45:965–6. doi: 10.1067/mjd.2001.119559. [DOI] [PubMed] [Google Scholar]
- 15.Harries MJ, Messenger A. Treatment of frontal fibrosing alopecia and lichen planopilaris. J Eur Acad Dermatol Venereol. 2014;28:1404–5. doi: 10.1111/jdv.12362. [DOI] [PubMed] [Google Scholar]
- 16.Lajevardi V, Ghodsi SZ, Hallaji Z, Shafiei Z, Aghazadeh N, Akbari Z. Treatment of erosive oral lichen planus with methotrexate. J Dtsch Dermatol Ges. 2016;14:286–93. doi: 10.1111/ddg.12636. [DOI] [PubMed] [Google Scholar]
- 17.Jang N, Fischer G. Treatment of erosive vulvovaginal lichen planus with methotrexate. Australas J Dermatol. 2008;49:216–9. doi: 10.1111/j.1440-0960.2008.00472.x. [DOI] [PubMed] [Google Scholar]
- 18.Nylander Lundqvist E, Wahlin YB, Hofer PA. Methotrexate supplemented with steroid ointments for the treatment of severe erosive lichen ruber. Acta Derm Venereol. 2002;82:63–4. doi: 10.1080/000155502753600966. [DOI] [PubMed] [Google Scholar]
- 19.Hazra SC, Choudhury AM, Khondker L, Khan SI. Comparative efficacy of methotrexate and mini pulse betamethasone in the treatment of lichen planus. Mymensingh Med J. 2013;22:787–97. [PubMed] [Google Scholar]
- 20.Kanwar AJ, De D. Methotrexate for treatment of lichen planus: Old drug, new indication. J Eur Acad Dermatol Venereol. 2013;27:e410–3. doi: 10.1111/j.1468-3083.2012.04654.x. [DOI] [PubMed] [Google Scholar]
- 21.Olsen EA, Whiting DA, Savin R, Rodgers A, Johnson-Levonas AO, Round E, et al. Global photographic assessment of men aged 18 to 60 years with male pattern hair loss receiving finasteride 1 mg or placebo. J Am Acad Dermatol. 2012;67:379–86. doi: 10.1016/j.jaad.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 22.Donati A, Assouly P, Matard B, Jouanique C, Reygagne P. Clinical and photographic assessment of lichen planopilaris treatment efficacy. J Am Acad Dermatol. 2011;64:597–8. doi: 10.1016/j.jaad.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 23.Rácz E, Gho C, Moorman PW, Noordhoek Hegt V, Neumann HA. Treatment of frontal fibrosing alopecia and lichen planopilaris: A systematic review. J Eur Acad Dermatol Venereol. 2013;27:1461–70. doi: 10.1111/jdv.12139. [DOI] [PubMed] [Google Scholar]
- 24.Tian H, Cronstein BN. Understanding the mechanisms of action of methotrexate: Implications for the treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2007;65:168–73. [PubMed] [Google Scholar]
- 25.Brown PM, Pratt AG, Isaacs JD. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat Rev Rheumatol. 2016;12:731–42. doi: 10.1038/nrrheum.2016.175. [DOI] [PubMed] [Google Scholar]