Abstract
Humoral alloreactivity has been recognized as a common cause of kidney transplant dysfunction. B-cell activation, differentiation, and antibody production are dependent on IL-21+CXCR5+follicular T-helper (Tfh) cells. Here, we studied whether belatacept, an inhibitor of the costimulatory CD28-CD80/86-pathway, interrupts the crosstalk between Tfh- and B-cells more efficiently than the calcineurin inhibitor tacrolimus. The suppressive effects of belatacept and tacrolimus on donor antigen-driven Tfh–B-cell interaction were functionally studied in peripheral blood mononuclear cells from 40 kidney transplant patients randomized to a belatacept- or tacrolimus-based immunosuppressive regimen. No significant differences in uncultured cells or donor antigen-stimulated cells were found between belatacept- and tacrolimus-treated patients in the CXCR5+Tfh cell generation and activation (upregulation of PD-1). Belatacept and tacrolimus in vitro minimally inhibited Tfh-cell generation (by ~6–7%) and partially prevented Tfh-cell activation (by ~30–50%). The proportion of IL-21+-activated Tfh-cells was partially decreased by in vitro addition of belatacept or tacrolimus (by ~60%). Baseline expressions and proportions of activated CD86+ B-cells, plasmablasts, and transitional B-cells after donor antigen stimulation did not differ between belatacept- and tacrolimus-treated patients. Donor antigen-driven CD86 upregulation on memory B-cells was not fully prevented by adding belatacept in vitro (~35%), even in supratherapeutic doses. In contrast to tacrolimus, belatacept failed to inhibit donor antigen-driven plasmablast formation (~50% inhibition vs. no inhibition, respectively, p < 0.0001). In summary, donor antigen-driven Tfh-B-cell crosstalk is similar in cells obtained from belatacept- and tacrolimus-treated patients. Belatacept is, however, less potent in vitro than tacrolimus in inhibiting Tfh-cell-dependent plasmablast formation.
Keywords: belatacept, costimulatory blockade, follicular T-helper cells, immunoglobulins, plasmablasts, tacrolimus, transitional B-cells
Introduction
B-cells and antibodies against the allograft are increasingly recognized to contribute to alloreactivity and subsequent graft failure after kidney transplantation under the currently used calcineurin inhibitor (CNI)-based immunosuppressive regimen (1–7). CD4+CXCR5+follicular T-helper (Tfh) cells are key mediators in B-cell activation, differentiation, and antibody production (8–12). Moreover, these cells infiltrate the allograft and colocalize with B-cells during acute rejection after kidney transplantation (13, 14). In alloreactivity, both Tfh- and B-cells are activated by the same antigen via their T- and B-cell receptor, respectively (15). The CD40-40L, CD28-CD80/86, and ICOS-ICOSL costimulatory pathways and the cytokines IL-6 and IL-21 are important in this Tfh–B-cell interaction and for B-cell differentiation into immunoglobulin-producing plasma cells (16–21).
Belatacept is a selective inhibitor of the CD28-CD80/86 pathway and subsequently interrupts Tfh–B-cell interaction (21, 22). In animal transplant models, belatacept, or the lower affinity version abatacept (CTLA4 Immunoglobulin), inhibited germinal center formation, clonal B-cell expansion, IL-21 production, and the development of donor-specific anti-human leukocyte antigen antibodies (DSA) (14, 23). These findings were in line with observations from a large randomized, controlled trial in kidney transplant patients where the belatacept-based regimen resulted in a significantly lower prevalence of DSA than the cyclosporine A (CsA)-based regimen at 7 years after transplantation: 4.6 vs. 17.8%, respectively (24). However, in all these clinical studies, belatacept was combined with other immunosuppressive drugs: in the BENEFIT and BENEFIT-EXT trials belatacept was combined with mycophenolate mofetil (MMF) and prednisone, and in the animal studies, belatacept was combined with either sirolimus or T-cell-depleting antibodies (14, 23–25).
Contradictory effects of tacrolimus on B-cell activation, proliferation, and differentiation have been reported (26–28) because tacrolimus only inhibits calcium-influx dependent and not calcium-independent, B- and T-cell activation (27, 29). This calcineurin-mediated activation is dependent on the type of stimulus (26, 28, 29). B-cell activation can thus be prevented by calcineurin-inhibition in an antigen-dependent manner. The effect of tacrolimus on donor antigen-stimulated Tfh–B-cell interaction is unknown in kidney transplantation.
In addition to the in vivo animal studies and clinical data that suggest belatacept effectively inhibits the humoral immune response specific for donor antigen (14, 23, 24), this class of immunosuppressive agents may also favor a more regulatory rather than effector alloreactive B-cell activity by enhancing the survival of transitional B-cells over memory B-cells in the long term (30). Theoretically, this may reduce rejection risk (15, 30–34).
So far no studies have been conducted which compared the effects of belatacept to tacrolimus, on Tfh–B-cell interaction in kidney transplantation. We hypothesized that belatacept more efficiently interrupts Tfh-B-cell crosstalk than tacrolimus. Therefore, we compared (i) the frequencies of Tfh and B-cell subsets between belatacept- and tacrolimus-treated patients; (ii) the in vitro donor antigen-driven Tfh–B-cell interaction in peripheral blood mononuclear cells (PBMCs) obtained from belatacept- and tacrolimus-treated kidney transplant patients; and (iii) the isolated the effects of additional belatacept and tacrolimus in vitro on donor antigen-driven Tfh–B-cell interaction in PBMCs obtained from the same patients.
Materials and Methods
Study Population and Materials
Materials were collected from 40 kidney transplant patients and their donors who participated in a prospective, randomized-controlled trial (approved by the Medical Ethical Committee of the Erasmus MC, University Medical Centre Rotterdam; MEC-2012-42, EUDRACT CT # 2012-003169-16). After written informed consent, patients were included and randomized to a tacrolimus-based (control) or belatacept-based (experimental) immunosuppressive regimen. For in- and exclusion criteria, refer to Table S1 in Supplementary Material. All procedures were in accordance with the ethical standards of the Declaration of Istanbul (35). In short, both groups received basiliximab induction therapy (Simulect®, Novartis, Basel, Switzerland), followed by maintenance therapy with MMF and prednisolone, which was tapered to 5 mg by month 3 after transplantation. Maintenance therapy with tacrolimus (Prograf®, Astellas Pharma, Tokyo, Japan) was adjusted to predose levels of 5–10 ng/mL, while belatacept (Nulojix®, Bristol-Meyers Squibb, NYC, NY, USA) was dosed according to bodyweight (Less-Intensive regimen of the BENEFIT trials) (36).
Lithium heparin blood was collected from patients 1 day before transplantation and 3 months after transplantation or during clinically suspected acute rejection before any additional anti-rejection therapy was given. All samples were processed within 24 h of withdrawal. If patients had a biopsy-proven acute rejection (BPAR) (2) materials of that time point were used instead of their materials of 3 months after transplantation. Lithium heparinized blood from donors was collected 1 day before transplantation. PBMCs were isolated from blood using the Ficoll density isolation method.
Mixed Lymphocyte Reactions (MLRs)
Patients PBMCs obtained after transplantation were thawed and used in MLRs. PBMCs were obtained 3 months after transplantation in stable, non-rejecting patients or before additional antirejection therapy was given in rejecting patients. Live cells were counted under a light microscope and distinguished from dead cells with Trypan Blue. Per patient ~5 × 105 uncultured PBMCs were stained for phenotypical analyses. A total of 5 × 104 patients’ PBMCs/well (in a 96-wells plate) were stimulated for 7 days at 37°C with 5 × 104 carboxyfluorescein succinimidyl ester (CFSE)-labeled, irradiated donor PBMCs (40 Gy) in RPMI 1640 + 10% heat-inactivated fetal bovine serum. Half of patients’ PBMCs were incubated for 1 h with clinically therapeutic concentrations of belatacept (10 μg/mL) (37) or tacrolimus (10 ng/mL), dependent on the randomization group, before donor antigen was added. After the donor antigen was added, these amounts of immunosuppressive drugs remained in the culture for the whole period of 7 days. At the end of day 6, 100 µL supernatant per well was harvested and stored at −20°C. Subsequently, Monensin and Brefeldin (GolgiStop and GolgiPlug, BD Biosciences, Franklin Lakes, NJ, USA) were added for 16 h over night in a concentration of 1:1,500 and 1:1,000, respectively, to allow the measurement of intracellularly accumulated cytokines in PBMCs.
Refer to Figure 1 for the different comparisons made in our study. Proportions of studied cell populations (see Flow Cytometry) were compared between:
-
(i)
belatacept- and tacrolimus-treated patients in uncultured, unstimulated PBMCs;
-
(ii)
belatacept- and tacrolimus-treated patients in 7-day donor antigen-stimulated PBMCs;
-
(iii)
uncultured, unstimulated PBMCs and 7-day donor antigen-stimulated PBMCs in belatacept-treated patients;
-
(iv)
uncultured, unstimulated PBMCs and 7-day donor antigen-stimulated PBMCs in tacrolimus-treated patients.
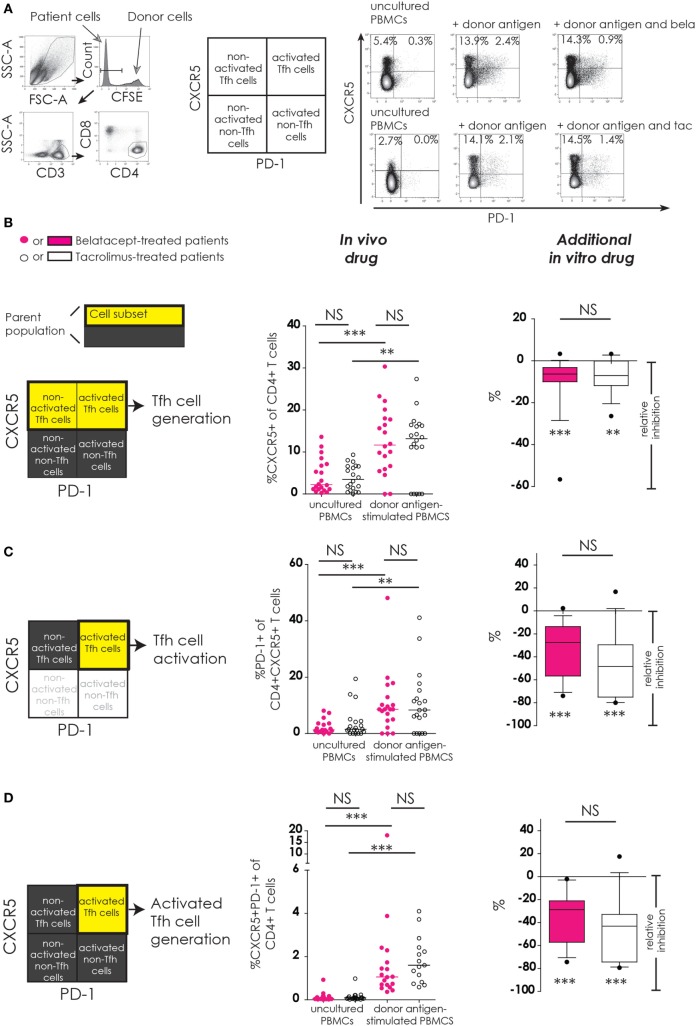
Figure 1.
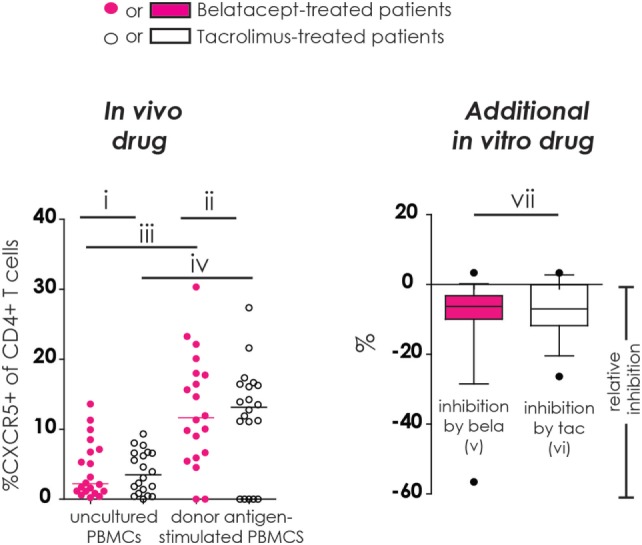
Different comparisons made in conducted studies (example figure). All figures in this manuscript comprise seven different comparisons. In this example, the proportion of CXCR5+ cells within CD4+ T-cells was used. In the left column (“in vivo drug”), the proportions of studied cell populations (see Flow Cytometry and Materials and Methods) were compared between (i) belatacept- and tacrolimus-treated patients in uncultured, unstimulated peripheral blood mononuclear cells (PBMCs); (ii) belatacept- and tacrolimus-treated patients in 7-day donor antigen-stimulated PBMCs; (iii) uncultured, unstimulated PBMCs and 7-day donor antigen-stimulated PBMCs in belatacept-treated patients; and (iv) uncultured, unstimulated PBMCs and 7-day donor antigen-stimulated PBMCs in tacrolimus-treated patients. In the right column (“Additional in vitro drug”), the relative inhibition by additional in vitro belatacept or tacrolimus is depicted by “v” and “vi”, respectively, in this example figure. If the median relative inhibition is significantly smaller than 0, the in vitro drug significantly decreases the proportion of the studied cell type. Per cell type, the relative inhibitions were compared between belatacept and tacrolimus in vitro. This is depicted by “vii” in this example figure. Refer to Figure 2B for the population used in this example (proportion of CXCR5+ within CD4+ T cells).
Patient PBMCs obtained 1 day before transplantation were also cultured with donor antigen in the same way to investigate whether PBMCs obtained from an immunosuppressed environment reacted differently on donor antigen compared to PBMCs before any immunosuppression was given.
Post-transplant PBMCs obtained from three belatacept-treated and three tacrolimus-treated patients were used in MLRs to study the CD40-CD40L, PD1-PDL1, and ICOS-ICOSL interaction during costimulation blockade by belatacept 10 µg/mL or calcineurin-inhibition by tacrolimus 10 ng/mL. MLRs were conducted as described above, only with 5 × 104 CD3 and CD19-depleted irradiated donor PBMCs instead of CFSE-labeled donor PBMCs. The same methods were used in six independent MLRs of healthy controls’ PBMCs to determine free CD80/86 expression after allo-antigen stimulation in the presence of various concentrations of belatacept (0–1,000 µg/mL) or tacrolimus (0–100 ng/mL).
CFSE Labeling of PBMCs
To distinguish between patient and donor PBMCs in the MLRs, donor PBMCs were labeled with the cell-permeable, intracellular linker CFSE (Thermo Fisher Scientific, Waltham, MA, USA), according to manufacturer’s manual. CFSE-labeled donors’ PBMCs expressed as median fluorescence intensity (MFI) >104 on the FITC channel.
Cocultures of Isolated Follicular T-Helper (Tfh) Cells and Memory B-Cells (13, 38)
CD3+CD4+CXCR5+ T-cells and CD19+CD27+ B-cells from three healthy controls and three patients before transplantation were isolated using a FACSAria II 4L SORP™ (BD Biosciences). From both populations 2 × 104 cells/well were cocultured for 7 days at 37°C with 4 × 104 40 Gy irradiated CD3/CD19-depleted, allogeneic PBMCs. Half of the wells were spiked with belatacept 10 µg/mL or tacrolimus 10 ng/mL. After 7 days coculture, supernatants were collected and stored at −20°C until analysis, and the proportion of memory B-cells that differentiated into CD27+CD38++ plasmablasts was measured.
Flow Cytometry
For a complete overview of the monoclonal antibodies used, see Table S2 in Supplementary Material. Follicular T-helper (Tfh) cells were defined as CD3+CD4+CXCR5+ T lymphocytes and classified as activated or resting by their expression of the activation marker and coinhibitor PD-1 (39, 40). Tfh-cell generation was defined as an increase in the proportion of CXCR5+ within CD4+ T-cells; Tfh-cell activation comprised the increase in the proportion of PD-1+ within CD4+CXCR5+ T-cells; and the generation of activated Tfh-cells was equivalent to an increase in the proportion of CXCR5+PD-1+ within CD4+ T-cells. The characteristic Tfh-cell cytokine IL-21 was determined in donor antigen-stimulated PBMCs in the presence or absence of belatacept or tacrolimus.
Within CD19+ B-cells, we distinguished CD27− naïve B-cells, CD27+ memory B-cells, CD24+CD38++ transitional B-cells, and CD27+CD38++ plasmablasts. Free CD86 expression on B-cells was measured on donor antigen-stimulated PBMCs by using an antibody that is competitive with belatacept for CD86 but binds with lower affinity (41). Expressions of the immune regulatory cytokine IL-10 in transitional B-cells and of the aggressive effector cytokine TNFα in plasmablasts were also assessed after 7 days of donor antigen stimulation.
ELISA for IgM and IgG3 Measurements
IgM concentrations in supernatants from all cell cultures were determined by ELISA. A calibration curve using human IgM 1.6–100 ng/mL (Sigma-Aldrich, St. Louis, MO, USA) was used to quantify results. All experiments were performed in duplo (medians were used for end result). Supernatants were diluted, if necessary, to fit within the measurements of the calibration curve. Measurements <1.6 ng/mL were considered negative. IgG3 concentrations were measured in the same way using an ELISA-kit with a calibration curve of 4.4–200 ng/mL (Affymetrix/eBioscience, Santa Clara, CA, USA).
Single Bead Luminex Assay
DSA were measured in (14–150×) concentrated culture supernatants using the Single Antigen beads mix from the LABScreen Single Antigen class II kit (Thermo Fisher, Waltham, MA, USA) (13). Microbeads were analyzed with a Luminex LabscanTM 100 analyzer using the Luminex 100IS software and analyzed using the HLA Fusion 3.0 software. All samples fulfilled the quality criteria for reactivity of the control beads.
Calculation of the Relative Inhibition
The relative inhibition was used to account for inter-patient variability in the response to donor antigen (Figure 1, “additional in vitro” column). The relative inhibition by additional in vitro belatacept or tacrolimus was calculated for the donor antigen-driven Tfh-cell generation, Tfh-cell activation, and the generation of activated Tfh-cells as well as for the donor antigen-driven intracellular IL-21 by activated Tfh-cells and the formation of IL21+ activated Tfh-cells. The relative inhibition by the in vitro drugs was also assessed for the upregulation of CD86 on naïve and memory B-cells, the formation of plasmablasts and their IgM production, and the transitional B-cell survival. For these calculations, the proportions of aforementioned cell subsets after donor antigen stimulation were set to 0 by using the following equation:
If the median relative inhibition is significantly smaller than 0, the in vitro drug significantly decreases the proportion of the studied cell type (Figure 1, comparison “v” and “vi”). Per cell type, the relative inhibitions were compared between belatacept and tacrolimus in vitro (Figure 1, comparison “vii”).
Statistical Analyses
Proportions of cell subsets in uncultured or donor antigen-stimulated PBMCs were compared between the belatacept and tacrolimus group using the Mann–Whitney U test (Figure 1, comparisons “i” and “ii”) as well as baseline characteristics that were continuous variables. Baseline characteristics that were categorical variables were compared with the Fisher’s exact test. Proportions of cell subsets between uncultured and donor antigen-stimulated PBMCs were compared using the Wilcoxon signed rank test (Figure 1, comparisons “iii” and “iv”). The median relative inhibition was compared to a theoretical mean of 0 (=no inhibition) with the Wilcoxon signed rank test to determine if the inhibition by the in vitro drug was statistically significant (Figure 1, comparisons “v” and “vi”). The relative inhibitions by in vitro belatacept and in vitro tacrolimus were compared using the Mann–Whitney U test (Figure 1, comparison “vii”).
Multivariable linear regressions were used to examine the in vitro effects of belatacept compared to tacrolimus on donor antigen-activated Tfh and B-cell subsets, adjusted for confounders [presence of in vitro added drugs (present vs. absent), time point (after vs. before transplantation), and BPAR (PBMCs obtained during rejection vs. 3 months after transplantation)]. To avoid multiple testing errors, only cell subsets in which the relative inhibition significantly differed between belatacept and tacrolimus in vitro were included for these analyses.
SPSS Statistics 21.0 (IBM, Armonk, NY, USA) was used for statistical analyses. Unless mentioned otherwise, medians (+range) are given for continuous variables, and numbers (+proportions) are given for categorical variables. p-Values with a two-sided α of <0.05 were considered statistically significant.
Results
Study Population
No significant differences were observed with regard to baseline characteristics between the two treatment groups (Table S3 in Supplementary Material).
The Effects of Belatacept and Tacrolimus on Follicular T-Helper (Tfh) cells
Tfh-Cell Generation and Activation (CXCR5 and PD-1 Upregulation)
Refer to Figure 1 (example figure using the data from Figure 2B) for the different comparisons made in our study for the different cell subsets (more details in Section “Materials and Methods”). Results start from Figure 2. The surface expression of the Tfh marker CXCR5 and the activation marker PD-1 were determined on CD4+ Tfh cells (Figure 2).
Figure 2.
No differences between belatacept and tacrolimus in vivo or in vitro on donor antigen-driven follicular T-helper (Tfh) cell formation and activation in cultured peripheral blood mononuclear cells (PBMCs). Two typical examples are depicted for CXCR5 and PD-1 expression on Tfh cells in uncultured PBMCs and after 7 days of donor antigen stimulation, in the presence or absence of belatacept and tacrolimus (A). Follicular T-helper (Tfh) cells were distinguished from non-Tfh-cells by surface CXCR5 expression, while activated cells were defined by surface PD-1 expression. Donor PBMCs were discriminated by carboxyfluorescein succinimidyl ester labeling them prior to the mixed lymphocyte reaction and gating them out after. The proportions are depicted of CXCR5+ within CD4+ T-cells (B); PD-1+ within CD4+CXCR5+ T-cells (C); and CXCR5+PD-1+ within CD4+ T-cells (D). In the graphs in the “In vivo drug” column, proportions of aforementioned cell populations were compared (i) between belatacept- and tacrolimus-treated patients in uncultured, unstimulated PBMCs; (ii) between belatacept- and tacrolimus-treated patients in 7-day donor antigen stimulated PBMCs; (iii) between uncultured, unstimulated PBMCs and 7-day donor antigen stimulated PBMCs in belatacept-treated patients; and (iv) between uncultured, unstimulated PBMCs and 7-day donor antigen stimulated PBMCs in tacrolimus-treated patients. Every dot represents PBMCs of a single patient. In the graphs in the “additional in vitro drug” column the relative inhibitions by additional in vitro belatacept and tacrolimus are depicted for aforementioned cell populations in the same belatacept- and tacrolimus-treated patients. The proportions of these cell populations after donor antigen stimulation in the absence of in vitro drugs are set to 0. The median relative inhibitions by belatacept and tacrolimus were tested against a theoretical median of 0. Asterisks below the boxes depict the p-values of these tests. The relative inhibitions were compared between belatacept and tacrolimus in vitro. Lines in boxes represent medians, borders of boxes represent 25th and 75th percentiles, and error bars present 10th and 90th percentiles. Every box represents cultures of PBMCs obtained from n = 20 belatacept-treated or n = 20 tacrolimus-treated patients. **p < 0.01, ***p < 0.001, NS, not significant.
Baseline expression of CXCR5 on CD4+ T-cells in uncultured PBMCs was comparable between belatacept- and tacrolimus-treated patients (Figure 2B, “in vivo drug” column). Following donor antigen stimulation, Tfh-cell generation, defined by the expression of CXCR5 on CD4+ T-cells, increased~3- to 4-fold in PBMCs obtained from belatacept- and tacrolimus-treated patients, p < 0.001 and p < 0.01, respectively (Figure 2B, “in vivo drug” column). This process was inhibited when the samples were spiked in vitro by adding tacrolimus and belatacept. The relative inhibition of Tfh-cell generation, however, was similar between belatacept and tacrolimus: −6.3% (−56.6 to +3.3%), p < 0.001, by belatacept and −7.0% (−26.4 to +3.3%), p < 0.01, by tacrolimus (Figure 2B, “additional in vitro drug” column).
The expression of PD-1 on CD4+CXCR5+ T-cells in uncultured PBMCs was similarly low in belatacept- and tacrolimus-treated patients (medians 1.3 and 1.5%, respectively; Figure 2C, “in vivo drug” column). Tfh-cells of belatacept- and tacrolimus-treated patients were significantly activated after CD4+ T-cells donor antigen stimulation, i.e., a significant increase of PD-1 expression on CXCR5+ was observed. The relative inhibition of Tfh-cell activation was −27.5% (−74.0 to +2.3%), p < 0.001, by belatacept and −48.4% (−80.0 to 16.7%), p < 0.001, by tacrolimus, inhibition by belatacept vs. tacrolimus; p = 0.13 (Figure 2C, “additional in vitro drug” column).
The proportion of CXCR5+PD-1+ double-positive CD4+ T-cells was negligible in uncultured PBMCs from belatacept- and tacrolimus-treated patients (Figure 2D, “in vivo drug” column). The generation of activated Tfh-cells (defined by an increase of the proportion of CXCR5+PD-1+ double-positive CD4+ T-cells) was 1.1% (0.4–18.1%) in donor antigen-stimulated PBMCs from belatacept-treated patients and 1.6% (0.6–4.1%) in those from tacrolimus-treated patients. These proportions were not significantly different. The generation of activated Tfh-cells was inhibited by both belatacept and tacrolimus in vitro (Figure 2D, “additional in vitro drug” column): the relative inhibition was −28.8% (−74.3 to −2.1%), p < 0.001 by belatacept, and −32.9% (−79.4 to +17.5%) by tacrolimus, p < 0.001.
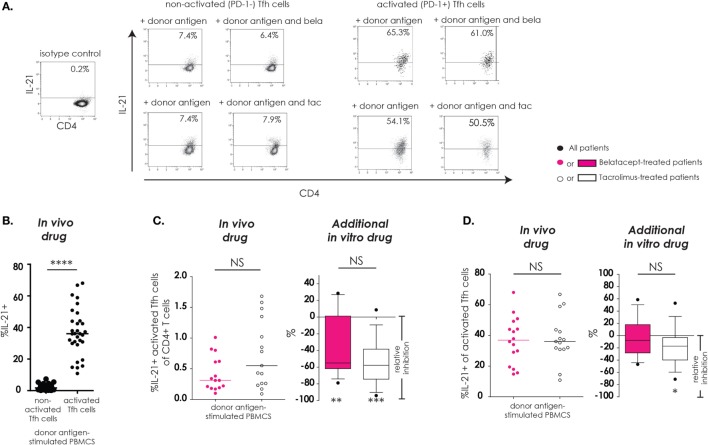
Tfh Cell Function (Intracellular IL-21 Production)
As described previously, “activated Tfh-cells” were defined as Tfh-cells that upregulated PD-1 after donor antigen stimulation and “non-activated Tfh-cells” were defined as Tfh-cells that failed to upregulate PD-1 after donor antigen stimulation. IL-21, a key cytokine in Tfh–B-cell interaction, and subsequent B-cell differentiation into immunoglobulin-producing plasma cells, was assessed in Tfh-cells (Figure 3). The donor antigen-stimulated IL-21 production was highest in activated Tfh-cells (Figure 3B). The proportions of IL21+-activated Tfh-cells within CD4+ T-cells were similar between donor antigen-stimulated PBMCs from the belatacept and tacrolimus groups (Figure 3C, “in vivo drug” columns). The total proportion of IL21+-activated Tfh-cells was partially decreased by belatacept and tacrolimus in vitro. The relative inhibition was −55.0% (−79.0 to +28.6%), p < 0.01, in the presence of belatacept and −57.7% (−94.1 to +8.7%), p < 0.001, in the presence of tacrolimus (Figure 3C, “in vivo drug” columns). No differences between the inhibition by belatacept and tacrolimus were observed (Figure 3C, “additional in vitro drug” column). When we focused on the remaining activated Tfh-cells in the presence of in vitro drugs, a substantial proportion could still produce IL-21. Even though the relative inhibitions of intracellular IL-21 production were not significantly different between belatacept and tacrolimus in vitro, only the latter (minimally) inhibited IL-21 production by activated Tfh-cells: relative inhibition −17.3% [−71.2 to + 52.9%, p < 0.05 (Figure 3D, “additional in vitro drug” column)].
Figure 3.
IL-21 production by remaining activated follicular T-helper (Tfh) cells was not inhibited by belatacept in vitro. A typical example is depicted for the intracellular IL-21 production after donor antigen stimulation in non-activated and activated Tfh-cells (CXCR5+PD-1− and CXCR5+PD-1+ CD4+ T-cells, respectively) in the presence and absence of belatacept (A). The proportions of IL-21+ cells within non-activated and activated Tfh-cells were compared after 7 days of donor antigen stimulation of peripheral blood mononuclear cells (PBMCs) obtained from both belatacept- and tacrolimus-treated patients (B). The proportions of IL21+-activated Tfh-cells within CD4+ T-cells (C) and the proportions of IL-21+ cells within activated Tfh-cells (D) were compared between 7-day donor antigen-stimulated PBMCs obtained from the belatacept and tacrolimus group (“in vivo” column), as well as the relative inhibitions by in vitro addition of belatacept or tacrolimus (“additional in vitro” column). In the graphs in the “In vivo drug” columns, every dot represents PBMCs of a single patient. In the graphs in the “additional in vitro drug” columns, the relative inhibitions by additional in vitro belatacept and tacrolimus are depicted for aforementioned cell populations in the same belatacept- and tacrolimus-treated patients. The proportions of these cell populations after donor antigen stimulation in the absence of in vitro drugs are set to 0. The median relative inhibitions by belatacept and tacrolimus were tested against a theoretical median of 0. Asterisks below the boxes depict the p-values of these tests. The relative inhibitions were compared between belatacept and tacrolimus in vitro. Lines in boxes represent medians, borders of boxes represent 25th and 75th percentiles, error bars present 10th and 90th percentiles. Every box represents cultures of PBMCs obtained from n = 20 belatacept-treated or n = 20 tacrolimus-treated patients. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, NS, not significant.
Summary of the Effects of Belatacept and Tacrolimus on Tfh-Cells
Belatacept and tacrolimus minimally inhibited Tfh-cell generation and partially prevented Tfh-cell activation. The proportion of IL-21+-activated Tfh-cells was not completely diminished by in vitro addition of belatacept or tacrolimus. Thus, the remaining activated Tfh-cells have the potential capacity to provide B-cell help. Next, we tested the immunosuppressive effects of both agents on B-cell activation and functional Tfh-B-cell crosstalk.
The Effects of Belatacept and Tacrolimus on B-Cells
B-Cell Activation (CD86 Upregulation)
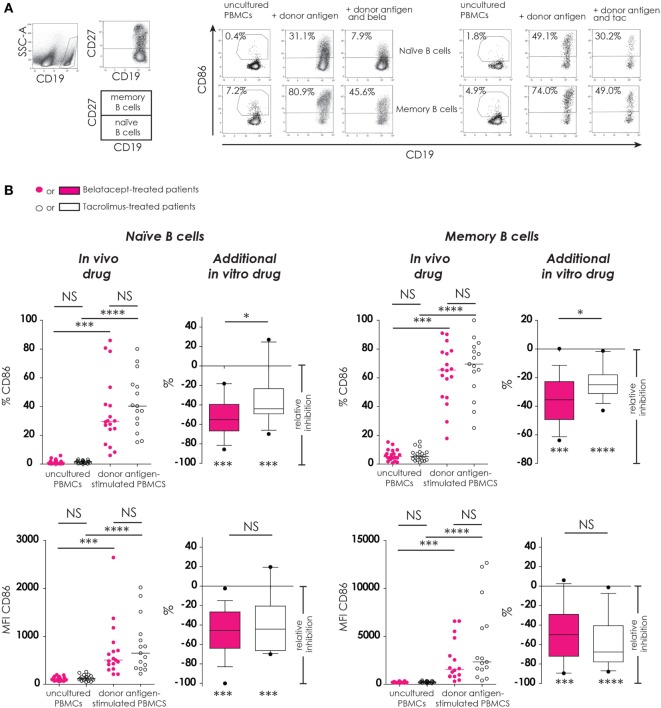
Part of the activation of B-cells and their ability to proliferate, differentiate, and function as antigen-presenting cells is reflected not only by their (free) CD86-expression but also by the expression of CD40 and ICOS-L. Here, the efficacy of belatacept was determined by means of B-cell activation, i.e., the free expression of CD86, which was measured on naïve CD19 + CD27− and memory CD19+CD27+ B-cells (in proportions and MFIs), using tacrolimus as control (Figure 4). The expression of CD40 and ICOS-L on B-cells in the presence of belatacept is described in section “The Effect of Belatacept and Tacrolimus on Redundant Co-Stimulatory Pathways.”
Figure 4.
Donor antigen-stimulated CD86 upregulation on B-cells is only partially blocked by belatacept in vitro. Two typical examples are depicted for the free CD86 expression after 7 days of donor antigen stimulation on naïve (CD27−) and memory (CD27+) CD19+ B-cells, in the presence and absence of belatacept or tacrolimus (A). The proportions are depicted of CD86+cells within naïve and memory B-cells as well as the median fluorescence intensities (MFIs) of CD86 within naïve and memory B-cells (B). In the graphs in the “In vivo drug” columns, proportions and MFIs of aforementioned cell populations were compared (i) between belatacept- and tacrolimus-treated patients in uncultured, unstimulated PBMCs; (ii) between belatacept- and tacrolimus-treated patients in 7-day donor antigen stimulated PBMCs; (iii) between uncultured, unstimulated PBMCs and 7-day donor antigen stimulated PBMCs in belatacept-treated patients; and (iv) between uncultured, unstimulated PBMCs and 7-day donor antigen stimulated PBMCs in tacrolimus-treated patients. Every dot represents a single culture of PBMCs. In the graphs in the “additional in vitro drug” columns, the relative inhibitions by additional in vitro belatacept and tacrolimus are depicted for aforementioned cell populations in the same belatacept- and tacrolimus-treated patients. The proportions or MFIs of these cell populations after donor antigen stimulation in the absence of in vitro drugs are set to 0. The median relative inhibitions by belatacept and tacrolimus were tested against a theoretical median of 0. Asterisks below the boxes depict the p-values of these tests. The relative inhibitions were compared between belatacept and tacrolimus in vitro. Lines in boxes represent medians, borders of boxes represent 25th and 75th percentiles, error bars present 10th and 90th percentiles. Every box represents cultures of PBMCs obtained from n = 20 belatacept-treated or n = 20 tacrolimus-treated patients. *p < 0.05, ***p < 0.001, ****p < 0.0001, NS, not significant.
CD86 expression was almost absent on naïve B-cells and low on memory B-cells in unstimulated uncultured PBMCs (Figure 4B, “in vivo” columns). No differences were observed between belatacept- or tacrolimus-treated patients. After donor antigen stimulation, both the proportions of CD86+ B-cells, as well as the expression of CD86 (MFIs) significantly increased on both naïve and memory B-cells (Figure 4B, “in vivo” columns). These were not different between the belatacept and tacrolimus group.
Despite the selective binding of belatacept to CD86 (22), the upregulation of CD86 was not completely blocked by the in vitro addition of belatacept. The relative inhibition of CD86 upregulation (proportion) by belatacept was −55.2% (−85.7 to −18.8%), p < 0.001, on naïve B-cells and −35.5% (−63.9 to +0.2%), p < 0.001, on memory B-cells (Figure 4B, “additional in vitro drug”). The relative inhibition of CD86 upregulation on naïve and memory B-cells was significantly more by belatacept than by tacrolimus in vitro, p < 0.05. MFIs of CD86 on naïve and memory B-cells were significantly decreased by both belatacept and tacrolimus in vitro (Figure 4B, “additional in vitro drug”). The relative inhibitions of CD86 MFIs were comparable between belatacept and tacrolimus.
To determine if the residual B-cell activation in the presence of immunosuppressive drugs was dose-dependent, the relative inhibitions by belatacept and tacrolimus were measured in the presence of supratherapeutic concentrations. Even in the presence of supratherapeutic concentrations of belatacept, membrane CD86 expression on allo-antigen-stimulated B-cells was still detectable (Figure S1 in Supplementary Material): the relative inhibition by 1,000 µg/mL belatacept (100× higher than the therapeutic concentration) was −72.4% (−86.5 to −19.7%), p < 0.05, in naïve B-cells and −43.2% (−53.9 to −7.4%), p < 0.05, in memory B-cells.
Since belatacept binds CD80 with much higher affinity than CD86 (22, 41), the residual surface expression of CD80 was low on activated naïve and memory B-cells in the presence of the different doses of belatacept (Figure S1 in Supplementary Material): The relative inhibition by 1,000 µg/mL belatacept was −90.2% (−97.5 to −75.4%), p < 0.05, in naïve B-cells and −85.0% (−95.1 to −57.3%), p < 0.05, in memory B-cells. CD80 expression on B-cells was not significantly decreased in the presence of tacrolimus.
B-Cell Differentiation (Plasmablast Formation)
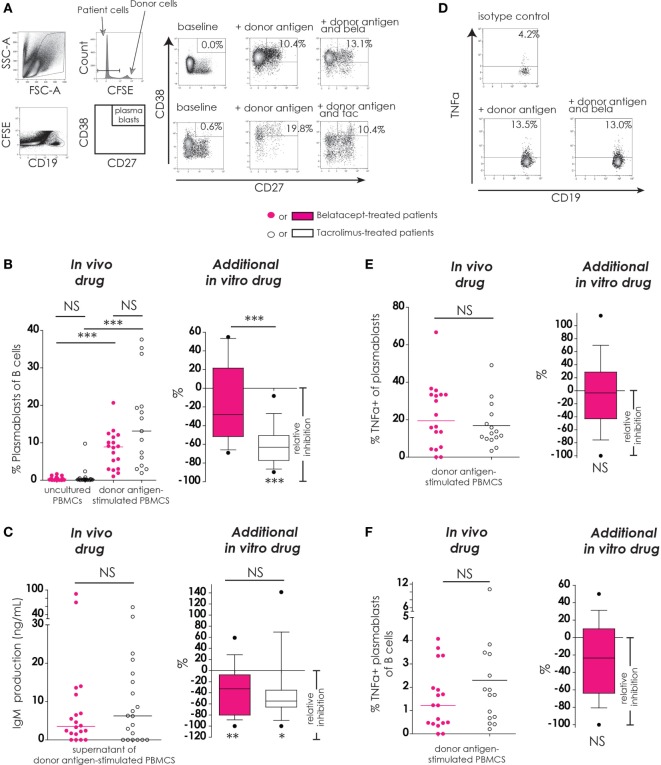
To study the effect of belatacept and tacrolimus on the antigen-dependent Tfh–B-cell interaction, differentiation of B-cells into plasmablasts was measured in donor antigen-activated PBMCs obtained after transplantation (Figure 5).
Figure 5.
Belatacept in vitro did not inhibit donor antigen-driven plasmablast formation or TNFα production in a peripheral blood mononuclear cell (PBMC)-based assay, but suppressed IgM production. The gating strategy is depicted for plasmablasts (CD19+CD27+CD38++) after 7 days of donor antigen stimulation, in the presence or absence of belatacept and tacrolimus (A). Donor PBMCs were discriminated by carboxyfluorescein succinimidyl ester labeling them prior to the mixed lymphocyte reaction and gating them out after. The proportions of plasmablasts are shown for 7-day donor antigen-stimulated PBMCs obtained from the belatacept or tacrolimus group (“in vivo” column), as well as the relative inhibitions by in vitro addition of belatacept or tacrolimus (“additional in vitro” column) (B). The IgM concentrations in the supernatants are shown for the same cultures as previously mentioned (“in vivo” column), as well as the relative inhibitions by in vitro addition of belatacept or tacrolimus (“additional in vitro” column) (C). A typical example is depicted for intracellular TNFα-production by plasmablasts after 7 days of donor antigen stimulation, in the presence or absence of belatacept (D). The proportions of TNFα+ cells within plasmablasts (E) and the proportions of TNFα+ plasmablasts within B-cells (F) are shown for 7-day donor antigen-stimulated PBMCs obtained from the belatacept or tacrolimus group (“in vivo” column), as well as the relative inhibitions by in vitro addition of belatacept (“additional in vitro” column). The proportions of TNFα+ plasmablasts could not be reliably determined in the presence of tacrolimus in vitro, because of the strong inhibition of plasmablast formation by tacrolimus. In the graphs in the “In vivo drug” columns, every dot represents PBMCs of a single patient. In the graphs in the “additional in vitro drug” columns, the relative inhibitions by additional in vitro belatacept and tacrolimus are depicted for aforementioned cell populations in the same belatacept- and tacrolimus-treated patients. The proportions of these cell populations after donor antigen stimulation in the absence of in vitro drugs are set to 0. The median relative inhibitions by belatacept and tacrolimus were tested against a theoretical median of 0. Asterisks below boxes depict the p-values of these tests. The relative inhibitions were compared between in vitro belatacept and tacrolimus. Lines in boxes represent medians, borders of boxes represent 25th and 75th percentiles, and error bars present 10th and 90th percentiles. Every box represents cultures of PBMCs obtained from n = 20 belatacept-treated or n = 20 tacrolimus-treated patients. *p < 0.05, **p < 0.01, ***p < 0.001, NS, not significant. N.B.: The median fluorescence intensity slightly decreases in (antigen-) stimulated cells compared to unstimulated cells, partly because of the intracellular staining protocol that was used to determine intracellular cytokine expression. Therefore, the gates in the unstimulated and stimulated cells are not exactly the same.
The proportions of plasmablasts were equally low in PBMCs from belatacept- and tacrolimus-treated patients (Figure 4B, “in vivo drug” column). Plasmablast formation was significant after donor antigen stimulation in PBMCs from the belatacept group [8.8% (1.0–20.7%), p < 0.001] and from the tacrolimus group [13.1% (1.9–37.6%), p < 0.001], belatacept vs. tacrolimus group, p = 0.10. Only tacrolimus significantly inhibited plasmablast formation with a relative inhibition of −50.5% (−89.7 to −8.2%), p < 0.0001 (Figure 5B, “additional in vitro drug” column). Belatacept failed to inhibit this alloreactive process in PBMCs, and its relative inhibition [−28.1% (−69.1 to +54.8%)] was significantly less than the inhibition by tacrolimus, p < 0.001.
Plasmablast Function (IgM Production and Intracellular TNFα Production)
IgM production by donor antigen-stimulated PBMCs was not significantly different in PBMCs obtained from the belatacept-treated patients compared to the tacrolimus-treated patients (Figure 5C, “in vivo drug” column). The relative inhibitions of IgM production by belatacept and tacrolimus in vitro were −32.9% (−100.0 to +59.1%), p < 0.01, and −54.9% (−100.0 to +141.4%), p < 0.05, respectively (Figures 5C, “additional in vitro drug” column). Even though tacrolimus more efficiently inhibited plasmablast formation than belatacept, the inhibition of IgM production did not significantly differ between these two drugs.
Since belatacept is a fusion protein consisting of the Fc-fragment of IgG1 (22), total human IgG could not be determined by ELISA. No IgG DSA were detected by Luminex in supernatants of the MLRs. IgG3 could be detected in eight cultures with donor-antigen stimulated PBMCs [median 6.6 (4.8–4.3) ng/mL; 5× from tacrolimus-treated and 3× from belatacept-treated patients] and was −12.3% (−79.3 to + 33.5%) inhibited in these samples by tacrolimus or belatacept, p = 0.01 (Figure S2 in Supplementary Material). Because of the limited amount of IgG3+ supernatants no subgroup analysis per treatment arm was performed.
Of the B-cells that differentiated into plasmablasts 19.4% (0.0–66.7%) expressed intracellular TNFα in the PBMCs obtained from belatacept-treated patients and 12.7% (3.4–49.1%) in those from the tacrolimus-treated patients, p = 0.34 (Figure 5E, “in vivo” column). The proportions of TNFα+ plasmablasts within total B-cells were also similar in PBMCs from belatacept- and tacrolimus-treated patients: 1.2% (0.0–4.1%) and 1.7% (0.2–10.1%), respectively, p = 0.34 (Figure 5F, “in vivo” column). Belatacept did not affect the proportion of TNFα+ within plasmablasts nor the proportions of TNFα+ plasmablasts within total B-cells (Figures 5E,F, “additional in vitro” column). The proportions of TNFα+ plasmablasts could not be reliably determined in the presence of tacrolimus, because of the strong inhibition of plasmablast formation by tacrolimus in vitro.
B-Cell Differentiation and Plasmablast Function in an Isolated Coculture System
To eliminate the effects of other cell types and cytokines present in the PBMC-based assay, we tested the effects of belatacept and tacrolimus in an isolated system of antigen-activated CXCR5+ Tfh and CD19+CD27+ memory B-cells (Figure S3 in Supplementary Material). The differentiation of memory B-cells into IgM producing plasmablasts was used as read out. Plasmablast formation of 13.2% (2.1–44.9%) was decreased by the addition of belatacept to 1.7% (1.3–4.2%), and by tacrolimus to 0.5% (0.1–0.9%), both p < 0.05 (Figure S3D in Supplementary Material). Tacrolimus more potently inhibited the plasmablast formation than belatacept, p < 0.05. The same pattern was seen in the IgM production of 253.8 ng/mL (86.5–541.5 ng/mL) (Figure S3E in Supplementary Material). Belatacept decreased IgM production to 18.1 ng/mL (13.7–68.4 ng/mL), and tacrolimus to 6.2 ng/mL (2.2–8.6 ng/mL) (both p < 0.05; tacrolimus vs. belatacept p < 0.05).
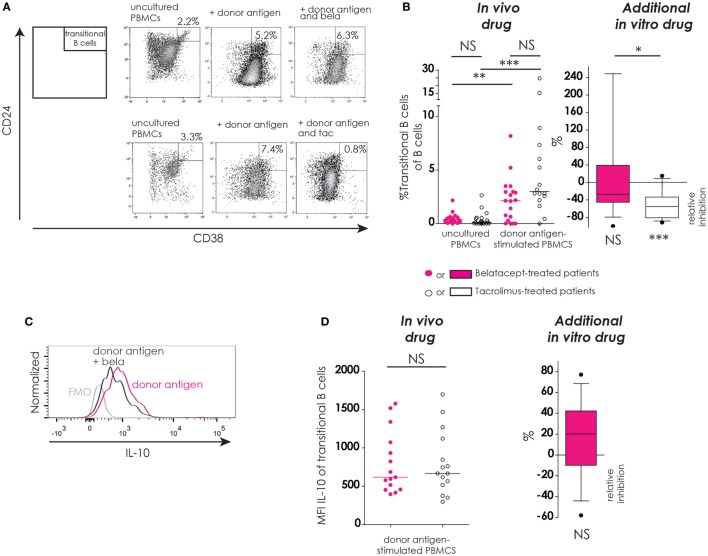
Immune Regulatory Phenotype (IL-10+ Transitional B-Cell Survival)
The presence of B-cells with a regulatory phenotype, i.e., IL10+ transitional B-cells, was assessed after donor antigen stimulation in the presence or absence of belatacept or tacrolimus (Figure 6).
Figure 6.
Transitional B-cells and their donor antigen-driven IL-10 production were conserved by belatacept in vitro but inhibited by tacrolimus. The gating strategy is depicted for transitional B-cells (CD24+CD38++) after donor antigen stimulation (A). Cells were gated from CD19+ B-cells like depicted in Figure 4A. The proportions of transitional B-cells are shown for 7-day donor antigen-stimulated peripheral blood mononuclear cells (PBMCs) obtained from the belatacept or tacrolimus group (“in vivo” column), as well as the relative inhibitions by in vitro addition of belatacept or tacrolimus (“additional in vitro” column) (B). A typical example is depicted for intracellular IL-10 expression [median fluorescence intensity (MFI)] by transitional B-cells after 7 days of donor antigen stimulation, in the presence or absence of belatacept, including a Fluorescence-Minus-One control (FMO) (C). The MFIs of IL-10 within transitional B-cells are shown for 7-day donor antigen-stimulated PBMCs obtained from the belatacept or tacrolimus group (“in vivo” column), as well as the relative inhibitions by in vitro addition of belatacept (“additional in vitro” column) (D). The MFI of IL-10 within transitional B-cells could not be reliably determined in the presence of tacrolimus in vitro, because of the decreased transitional B-cells survival in the presence of tacrolimus. In the graph in the “In vivo drug” column in (B), proportions of transitional B-cell populations were compared (i) between belatacept- and tacrolimus-treated patients in uncultured, unstimulated PBMCs; (ii) between belatacept- and tacrolimus-treated patients in 7-day donor antigen stimulated PBMCs; (iii) between uncultured, unstimulated PBMCs and 7-day donor antigen stimulated PBMCs in belatacept-treated patients; and (iv) between uncultured, unstimulated PBMCs and 7-day donor antigen stimulated PBMCs in tacrolimus-treated patients. Every dot represents PBMCs of a single patient. In the graphs in the “Additional in vitro drug” column the relative inhibitions by additional in vitro belatacept and tacrolimus are depicted for aforementioned cell populations in the same belatacept- and tacrolimus-treated patients. The proportions of these cell populations after donor antigen stimulation in the absence of in vitro drugs are set to 0. The median relative inhibitions by belatacept and tacrolimus were tested against a theoretical median of 0. Asterisks below boxes depict the p-values of these tests. The relative inhibitions were compared between in vitro belatacept and tacrolimus. Lines in boxes represent medians, borders of boxes represent 25th and 75th percentiles, error bars present 10th and 90th percentiles. Every box represents cultures of PBMCs obtained from n = 20 belatacept-treated or n = 20 tacrolimus-treated patients. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, NS, not significant. N.B.: The MFI slightly decreases in antigen-stimulated cells compared to unstimulated cells, partly because of the intracellular staining protocol that was used to determine intracellular cytokine expression. Therefore, the gates in the unstimulated and stimulated cells are not exactly the same.
In unstimulated, uncultured PBMCs the proportions of transitional B-cells were below 3% in both treatment groups (Figure 5B, “in vivo drug” column). After donor antigen stimulation, an increase in the proportion of transitional B-cells was observed in PBMCs from belatacept- and tacrolimus-treated patients, to 2.1% (0.0–8.2%), p < 0.01, and 3.0% (0.0−24.7%), p < 0.001, respectively (Figure 5B, “in vivo drug” column). The survival of these transitional B-cells was not different between the belatacept and tacrolimus groups. The in vitro addition of tacrolimus, however, diminished transitional B-cell survival [relative inhibition: −55.4% (−91.4 to −15.1%), p < 0.001], while belatacept did not affect the survival of these potentially regulatory B-cells [relative inhibition: −27.2% (−100.0 to +247.4%), p = 0.54], tacrolimus vs. belatacept, p < 0.05.
Both the transitional B-cells in the PBMCs obtained from belatacept-treated patients as those from tacrolimus-treated patients expressed IL-10 after donor antigen stimulation: MFI 617 (397 to 1577) and MFI 666 (297 to 1697), respectively, p = 1.00 (Figure 6D, “in vivo” column). Belatacept in vitro did not decrease IL-10 production in transitional B-cells (Figure 6D, “additional in vitro” column). The MFI of IL-10 within transitional B-cells could not be reliably determined in the presence of tacrolimus, because of the decreased transitional B-cells survival in the presence of tacrolimus in vitro.
Summary of the Effects of Belatacept and Tacrolimus on B-Cells
Donorantigen-driven CD86 upregulation was not fully inhibited by belatacept, especially on memory B-cells, even by supratherapeutic doses of belatacept. In contrast to tacrolimus, belatacept could not inhibit donorantigen-driven plasmablast formation in a PBMC-based assay but only in an isolated Tfh-B-cell coculture. Also in the latter, belatacept was less effective than tacrolimus. The survival of the potentially immune regulatory IL-10+ transitional B-cells was, however, not affected by belatacept, while this was diminished by tacrolimus.
Redundancy of the Immune System
The Effect of Belatacept and Tacrolimus on Redundant Co-Stimulatory Pathways
To explain why belatacept did not inhibit plasmablast formation in our PBMC studies, and because patients’ cellular interactions are influenced by redundant and pleiotropic mechanisms of immune cells, surface receptors of other costimulatory pathways were measured (Figure S4 in Supplementary Material).
In PBMCs from three belatacept-treated and three tacrolimus-treated patients, the ICOS-ICOSL, PD-1-PD-L1, and the CD40L-CD40 pathways were studied after donor antigen stimulation Expressions of all surface molecules, except for CD28 and ICOSL, were increased on Tfh and B-cells after donor antigen stimulation (Figures S4A,C in Supplementary Material). The upregulation of the costimulatory molecules on Tfh-cells was not fully suppressed by belatacept and to a lesser extent than by tacrolimus (Figure S4 in Supplementary Material).
Multivariable Regression Analyses
The Effect of Belatacept and Tacrolimus in Vitro on Tfh- and B-Cells
The effect of belatacept in vitro was compared to the effect of tacrolimus in vitro in multivariable regression analyses for proportions of CD86+ naïve and memory B-cells, plasmablast formation, and transitional B-cell survival (adjusted for the variables as stated in Table 1). These cell subsets were chosen, because they significantly differed when belatacept was added compared to tacrolimus in vitro (Figures 4–6). Multivariable analyses confirmed that belatacept and tacrolimus differed in inhibition of plasmablast formation: plasmablast formation was 4.5% (SE 1.3) higher in the presence of belatacept than in the presence of tacrolimus in vitro, p = 0.001 (Table 1). In the multivariable analysis, transitional B-cell survival (defined as the proportion of transitional B-cells of total B-cells) was not significantly higher in the presence of belatacept compared to in vitro addition of tacrolimus (p = 0.91). Finally, the free CD86 expression on CD27+ memory B-cells was 9.3% (SE 2.8) lower when the donor antigen-stimulated PBMCs were spiked with belatacept in vitro compared to tacrolimus in vitro, p = 0.001, but no significant difference was found for free CD86-expression on the surface of naïve B-cells (p = 0.12).
Table 1.
The effect of belatacept in vitro on free CD86 expression on B-cells, plasmablast formation, and transitional B-cell survival.
| Dependent variable | Independent variables | Beta | SE | p |
|---|---|---|---|---|
| CD86 expressing naïve B-cells (% CD86+ of CD19+CD27− B-cells) | Belatacept added in vitro (vs. tacrolimus added in vitro) | −4.53 | 2.85 | 0.12 |
| PBMCs obtained after transplantation (vs. before transplantation) | 1.76 | 2.50 | 0.48 | |
| PBMCs obtained from rejector (vs. non-rejector) | −0.09 | 3.02 | 0.98 | |
| Proportion of cell subset without in vitro drugs added | 0.55 | 0.06 | 0.00 | |
| CD86 expressing memory B-cells (% CD86+ of CD19+CD27+ B-cells) | Belatacept added in vitro (vs. tacrolimus added in vitro) | −9.34 | 2.84 | 0.02 |
| PBMCs obtained after transplantation (vs. before transplantation) | −1.15 | 2.49 | 0.65 | |
| PBMCs obtained from rejector (vs. non-rejector) | 2.10 | 3.01 | 0.49 | |
| Proportion of cell subset without in vitro drugs added | 0.61 | 0.07 | 0.00 | |
| Plasmablasts (% CD27+CD38++ of CD19+ B-cells) | Belatacept added in vitro (vs. tacrolimus added in vitro) | 4.45 | 1.28 | 0.00 |
| PBMCs obtained after transplantation (vs. before transplantation) | −1.57 | 1.08 | 0.15 | |
| PBMCs obtained from rejector (vs. non-rejector) | 2.34 | 1.32 | 0.08 | |
| Proportion of cell subset without in vitro drugs added | 0.66 | 0.07 | 0.00 | |
| Transitional B-cells (% of CD19+ B-cells) | Belatacept added in vitro (vs. tacrolimus added in vitro) | −0.09 | −0.01 | 0.91 |
| PBMCs obtained after transplantation (vs. before transplantation) | −0.38 | 0.71 | 0.59 | |
| PBMCs obtained from rejector (vs. non-rejector) | 0.40 | 0.86 | 0.65 | |
| Proportion of cell subset without in vitro drugs added | 0.24 | 0.07 | 0.00 | |
A multivariable regression analysis was performed per cell subset with as dependent variable the value after donor antigen stimulation plus in vitro drugs (belatacept or tacrolimus) and as independent variables addition of in vitro belatacept (vs. in vitro tacrolimus), the time point PBMCs were obtained (after vs. before transplantation), PBMCs obtained during rejection (rejector vs. non-rejector) and the value after donor antigen stimulation without in vitro drugs added. Statistically significant p-values (<0.05) are underscored.
Proportions of the different donor antigen-stimulated subsets without in vitro drugs added were most predictive for the proportions in the presence of in vitro drugs. In 11 belatacept-treated patients and 2 tacrolimus-treated patients the PBMCs were obtained during acute rejection, before additional anti-rejection therapy was given. Obtaining PBMCs from patients who rejected or time point the PBMCs were obtained (before or after transplantation) were not predictive for the proportions of these cells.
PBMCs, peripheral blood mononuclear cells; Rejector vs. non-rejector, patients who rejected vs. patients who did not reject within 12 months after transplantation (biopsy-proven); SE, standard error of beta.
Peripheral blood mononuclear cells were obtained 3 months after transplantation in non-rejectors, and during rejection before additional anti-rejection therapy was given in rejectors. Eleven of twenty belatacept-treated patients and two of twenty tacrolimus-treated patients developed a BPAR. Obtaining PBMCs during acute rejection did not alter the in vitro reaction to donor antigen or drug (Table 1).
The effects of belatacept and tacrolimus in vitro on the different Tfh- and B-cell subsets are summarized in Table 2.
Table 2.
Effects of belatacept and tacrolimus in vitro on donor antigen-activated follicular T-helper (Tfh) and B-cells.
| Immunological reaction | Defined by | Effect by belatacept in vitro | Effect by tacrolimus in vitro | Comparison belatacept vs. tacrolimusa |
|---|---|---|---|---|
| Tfh-cell generation | CXCR5 expression ↑ on CD4+ T-cells | Inhibition (minimal) | Inhibition (minimal) | bela = tac |
| Tfh-cell activation | PD-1 expression ↑ on CD4+CXCR5+ T-cells | Inhibition (partial) | Inhibition (partial) | bela = tac |
| Activated Tfh-cell generation | CXCR5+PD-1+ double expression ↑ on CD4+ T-cells | Inhibition (partial) | Inhibition (partial) | bela = tac |
| IL-21+-activated Tfh-cell generation | The proportion of IL-21+ CXCR5+PD-1+ ↑ within CD4+ T-cells | Inhibition (partial) | Inhibition (partial) | bela = tac |
| IL-21 production by activated Tfh-cells | Intracellular IL-21 expression of CXCR5+PD-1+ CD4+ T-cells | None | Inhibition (minimal) | bela = tac |
| B-cell activation | CD86 expression ↑ on B-cells | Inhibition (partial) | Inhibition (partial) | bela is more efficient than tacb |
| Transitional B-cell survival | The proportion of CD24+CD38++ B-cells | None | Inhibition (partial) | bela is more benificial than tacc |
| Plasmablast formation | Proportion of B-cells differentiated into CD27+CD38++ B-cells | In PBMCs: none | In PBMCs: inhibition (partial) | bela is less efficient than tacd |
| In isolated system with Tfh- and B-cells: inhibition (almost completely) | In isolated system with Tfh- and B-cells: inhibition (completely) | bela is slightly less efficient than tac | ||
| IgM production | Total IgM in supernatant of cocultures | In PBMCs: inhibition (partial) | In PBMCs: inhibition (partial) | bela = tac |
| In isolated system with Tfh- and B-cells: inhibition (almost completely) | In isolated system with Tfh- and B-cells: inhibition (completely) | bela is slightly less efficient than tac |
bela, belatacept; IgM, immunoglobulin M; PBMCs, peripheral blood mononuclear cells; tac, tacrolimus.
aComparison of the relative inhibition by belatacept in vitro and tacrolimus in vitro.
bThese observations were confirmed in a multivariable regression analysis for memory B-cells, but not for naïve B-cells (Table 1).
cThese observations were not confirmed in a multivariable regression analysis (Table 1).
dThese observations were confirmed in a multivariable regression analysis (Table 1).
Discussion
In this study, the effects of belatacept on Tfh–B-cell interaction were compared to those of tacrolimus for the first time in kidney transplant patients. No differences were observed in unstimulated uncultured PBMCs or donor antigen-stimulated PBMCs obtained from belatacept- or tacrolimus-treated patients, which may be explained by the predominant effects by MMF and prednisone in both regimens. Therefore, the isolated effects of in vitro belatacept and tacrolimus were compared. In vitro addition of both drugs only minimally inhibited Tfh-cell generation and partially decreased activation of Tfh-cells (defined by PD-1 upregulation). Activated Tfh-cells produced the highest levels of IL-21, and the total proportion of IL-21+ activated Tfh-cells in the presence of in vitro immunosuppression was also partially reduced. Still, IL-21 production and B-cell help by remaining Tfh-cells were sufficient in the presence of in vitro belatacept, because the donor antigen-driven formation of plasmablasts in our MLR-based PBMC assay was not inhibited by the costimulation blocker, in contrast to in vivo observations in animal studies (14, 23). These newly formed TNFα+ plasmablasts, that have been associated with aggressive reactivity in autoimmunity (42), were suppressed in the presence of tacrolimus.
A first explanation for these findings is the differences between our study and previous work (14, 23, 24). Belatacept has always been compared to CsA and not with the more potent tacrolimus and used in combination with other types of immunosuppressive agents, such as T-cell depleting therapy or mTOR inhibition in the animal studies (14, 23) or MMF and prednisone in the BENEFIT trial (24). The study presented here reports on the isolated effects of belatacept and tacrolimus on the functional interaction of patient-derived Tfh- and B-cells. These differences might have led to an overestimation of the inhibition of Tfh–B-cell interaction by belatacept, not taking into account the effects of other immunosuppressive agents.
A second reason could be the significant residual expression of CD86 on donor antigen-activated B-cells, even in the presence of supratherapeutic concentrations of belatacept. This might be explained by (1) a lower affinity of belatacept for donor antigen-activated CD86 molecules on B-cells, (2) a higher turnover of CD86 by B-cells, or (3) degradation of belatacept during the 7-day cultures. The latter is unlikely, since CD80 was efficiently blocked by belatacept. Until the study presented here, the efficacy of belatacept on occupying CD86 had only been studied on monocyte-derived dendritic cells (DCs) and not on B-cells (41). As a result of the incomplete blockade of CD86 on B-cells, activation and consequently differentiation of B-cells were not prevented by costimulation blockade. The production of IgM was, however, inhibited by belatacept (median ~50%), possibly because CD80 blockade or partial CD86 blockade also leads to impaired immunoglobulin responses (43, 44). Nonetheless, belatacept was not more efficient than tacrolimus in preventing IgM production and even slightly less efficient in an isolated system. The lower percentage of DSA-positive patients in the belatacept than in the CsA group in the BENEFIT trial (24) could be (1) a reflection of better compliance in the first group (45, 46), (2) the lower potency of CsA compared to tacrolimus (25), and (3) higher concentrations of mycophenolate acid in the first group (47, 48).
A third answer can be found in redundant costimulatory pathways taking over during costimulation blockade of the CD28-CD80/86 pathway. Because belatacept affects only this pathway (22), other costimulatory pathways, such as CD40-CD40L and ICOS-ICOSL, may “bypass” blockade of CD28-CD80/86. In our small cohort study of n = 6 independent experiments, the upregulation of CD40L and ICOS on Tfh-cells were less reduced by belatacept than by tacrolimus, making these cells more capable of helping B-cells. Since tacrolimus has a direct effect on T- and B-cells by inhibiting calcineurin downstream the surface receptors (27, 29, 49), its effect is not dependent on costimulation blockade. Further studies that test the combination of belatacept with CD40- or ICOS-blockade could confirm this hypothesis but were beyond the scope of the study presented here.
A final possibility is that belatacept less effectively inhibits DCs and their interaction with Tfh- and B-cells than the interaction between Tfh- and B-cells, especially in an in vitro setting in the absence of a germinal center (50–52). Unlike in donor antigen-stimulated PBMCs, in an isolated system of pure CXCR5+ Tfh and memory B-cells, belatacept successfully inhibited plasmablast formation. A big difference between PBMCs and isolated Tfh and memory B-cells is the absence of patient DCs and their antigen-presenting function in the isolated system, i.e., the absence of the indirect and semidirect pathways of antigen presentation (53). The effect of belatacept on human DCs is not yet studied and a lack hereof could explain the less efficient inhibition by belatacept on Tfh–B-cell interaction. Nevertheless, donor DCs, facilitating the direct pathway for antigen presentation, were still present in the isolated system. This suggests belatacept effectively inhibits the direct, but not the indirect or semidirect pathways of antigen presentation. Absence of other cells, like natural killer cells, could also be an explanation for the successful inhibition by belatacept in the isolated system. We believe a PBMC-based assay is more similar to the milieu in patients than an isolated cell assay, because in the first system, multiple cell types and redundant pathways are of importance.
It should be noted almost no IgG3 by ELISA and no anti-HLA IgGby Luminex were detected in the culture supernatants, possibly because only materials of immunologically low-risk patients were used. Since belatacept is an IgG1, it cannot be ruled out that IgG1 antibodies were present in the cocultures’ supernatants. Another limitation of this study is that anti-CD86 monoclonal antibodies that are non-competitive to belatacept and bind to another epitope than belatacept are not commercially available (41). The total CD86 expression, irrespective of saturated CD86 by belatacept in the cocultures, could therefore not be determined.
A possible beneficial consequence of the incomplete inhibition of B-cells by costimulation blockade is that belatacept favored the (potentially regulatory) IL-10+ transitional B-cell survival, while this was diminished by tacrolimus (30–32). However, these findings were not confirmed by multivariable regression analyses. Therefore, the clinical relevance of these observations needs to be validated in a larger population. Even more, since (1) most rejections occur within the first months after transplantation, when glucocorticoids are still used and negatively influence transitional B-cell survival (30) and (2) the regulatory capacities of antigen-specific transitional B-cells have not yet been confirmed in functional studies in humans. Another favorable outcome of incomplete Tfh-B-cell inhibition by belatacept could be a lower infection risk and more potent vaccine responses in belatacept-treated patients than in tacrolimus-treated patients. So far no evidence for this emerged from previous studies nor has it been confirmed in randomized-controlled trials comparing belatacept and CNI-treated patients (24, 54–56).
In this functional study, belatacept was less potent than tacrolimus in inhibiting donor antigen-driven plasmablast formation.
Ethics Statement
Materials were collected from 40 kidney transplant patients and their donors who participated in a prospective, randomized-controlled trial (approved by the Medical Ethical Committee of the Erasmus MC, University Medical Centre Rotterdam; MEC-2012-42, EUDRACT CT # 2012-003169-16). After written informed consent, patients were included and randomized to a tacrolimus-based (control) or belatacept-based (experimental) immunosuppressive regimen. All procedures were in accordance with the ethical standards of the Declaration of Istanbul (International Summit on Transplant and Organ, 2008).
Author Contributions
GG designed, conducted and analyzed experiments, and wrote the manuscript; DH, WW, and CC designed experiments and edited the manuscript; MD, RK, WV, and NL designed, analyzed, and/or conducted experiments; and DR and AS interpreted data and edited the manuscript.
Conflict of Interest Statement
Despite funding from pharmaceutical companies (see “Funding”), the authors declare that this research was conducted and presented in the absence of any conflict of interest.
Acknowledgments
The authors would like to thank Mrs. M. Cadogan for the management and preparation of blood withdrawals and Dr. J. van Rosmalen for statistical consultation.
Footnotes
Funding. DH has received grant support from Bristol-Myers Squibb and Astellas Pharma and has received lecture and consulting fees from Astellas Pharma and Chiesi Pharmaceuticals. WW and CB received a research grant from Bristol-Myers Squibb. The use of the commercial funders Bristol-Myers Squibb and Astellas Pharma did not alter the authors’ adherence to Frontiers in Immunology’s policies on sharing data and materials.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2017.00641/full#supplementary-material.
References
- 1.Willicombe M, Roufosse C, Brookes P, Mclean AG, Galliford J, Cairns T, et al. Acute cellular rejection: impact of donor-specific antibodies and C4d. Transplantation (2014) 97:433–9. 10.1097/01.TP.0000437431.97108.8f [DOI] [PubMed] [Google Scholar]
- 2.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. Banff 2013 meeting report: inclusion of C4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant (2014) 14:272–83. 10.1111/ajt.12590 [DOI] [PubMed] [Google Scholar]
- 3.Shiu KY, Mclaughlin L, Rebollo-Mesa I, Zhao J, Semik V, Cook HT, et al. B-lymphocytes support and regulate indirect T-cell alloreactivity in individual patients with chronic antibody-mediated rejection. Kidney Int (2015) 88:560–8. 10.1038/ki.2015.100 [DOI] [PubMed] [Google Scholar]
- 4.Zarkhin V, Kambham N, Li L, Kwok S, Hsieh SC, Salvatierra O, et al. Characterization of intra-graft B cells during renal allograft rejection. Kidney Int (2008) 74:664–73. 10.1038/ki.2008.249 [DOI] [PubMed] [Google Scholar]
- 5.Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M, et al. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med (2003) 349:125–38. 10.1056/NEJMoa035588 [DOI] [PubMed] [Google Scholar]
- 6.Hippen BE, Demattos A, Cook WJ, Kew CE, II, Gaston RS. Association of CD20+ infiltrates with poorer clinical outcomes in acute cellular rejection of renal allografts. Am J Transplant (2005) 5:2248–52. 10.1111/j.1600-6143.2005.01009.x [DOI] [PubMed] [Google Scholar]
- 7.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong Van Huyen JP, Mooney N, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med (2013) 369:1215–26. 10.1056/NEJMoa1302506 [DOI] [PubMed] [Google Scholar]
- 8.Xu H, Liu J, Cui X, Zuo Y, Zhang Z, Li Y, et al. Increased frequency of circulating follicular helper T cells in lupus patients is associated with autoantibody production in a CD40L-dependent manner. Cell Immunol (2015) 295:46–51. 10.1016/j.cellimm.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Zhao P, Ma L, Shan Y, Jiang Z, Wang J, et al. Increased interleukin 21 and follicular helper T-like cells and reduced interleukin 10+ B cells in patients with new-onset systemic lupus erythematosus. J Rheumatol (2014) 41:1781–92. 10.3899/jrheum.131025 [DOI] [PubMed] [Google Scholar]
- 10.Yang JA, Tubo NJ, Gearhart MD, Bardwell VJ, Jenkins MK. Cutting edge: Bcl6-interacting corepressor contributes to germinal center T follicular helper cell formation and B cell helper function. J Immunol (2015) 194:5604–8. 10.4049/jimmunol.1500201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos-Amaya A, Rodriguez-Bayona B, Lopez-Blanco R, Andujar E, Perez-Alegre M, Campos-Caro A, et al. Survival of human circulating antigen-induced plasma cells is supported by plasma cell-niche cytokines and T follicular helper lymphocytes. J Immunol (2015) 194:1031–8. 10.4049/jimmunol.1402231 [DOI] [PubMed] [Google Scholar]
- 12.Walters GD, Vinuesa CG. T follicular helper cells in transplantation. Transplantation (2016) 100:1650–5. 10.1097/TP.0000000000001217 [DOI] [PubMed] [Google Scholar]
- 13.de Graav GN, Dieterich M, Hesselink DA, Boer K, Clahsen-Van Groningen MC, Kraaijeveld R, et al. Follicular T helper cells and humoral reactivity in kidney transplant patients. Clin Exp Immunol (2015) 180:329–40. 10.1111/cei.12576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim EJ, Kwun J, Gibby AC, Hong JJ, Farris AB, III, Iwakoshi NN, et al. Costimulation blockade alters germinal center responses and prevents antibody-mediated rejection. Am J Transplant (2014) 14:59–69. 10.1111/ajt.12526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman W, Lakkis FG, Chalasani G. B cells, antibodies, and more. Clin J Am Soc Nephrol (2016) 11:137–54. 10.2215/CJN.09430915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu D, Xu H, Shih C, Wan Z, Ma X, Ma W, et al. T-B-cell entanglement and ICOSL-driven feed-forward regulation of germinal centre reaction. Nature (2015) 517:214–8. 10.1038/nature13803 [DOI] [PubMed] [Google Scholar]
- 17.King C. New insights into the differentiation and function of T follicular helper cells. Nat Rev Immunol (2009) 9:757–66. 10.1038/nri2644 [DOI] [PubMed] [Google Scholar]
- 18.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol (2005) 23:515–48. 10.1146/annurev.immunol.23.021704.115611 [DOI] [PubMed] [Google Scholar]
- 19.Weinstein JS, Bertino SA, Hernandez SG, Poholek AC, Teplitzky TB, Nowyhed HN, et al. B cells in T follicular helper cell development and function: separable roles in delivery of ICOS ligand and antigen. J Immunol (2014) 192:3166–79. 10.4049/jimmunol.1302617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bryant VL, Ma CS, Avery DT, Li Y, Good KL, Corcoran LM, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol (2007) 179:8180–90. 10.4049/jimmunol.179.12.8180 [DOI] [PubMed] [Google Scholar]
- 21.Salek-Ardakani S, Choi YS, Rafii-El-Idrissi Benhnia M, Flynn R, Arens R, Shoenberger S, et al. B cell-specific expression of B7-2 is required for follicular Th cell function in response to vaccinia virus. J Immunol (2011) 186:5294–303. 10.4049/jimmunol.1100406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen CP, Pearson TC, Adams AB, Tso P, Shirasugi N, Strobert E, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant (2005) 5:443–53. 10.1111/j.1600-6143.2005.00749.x [DOI] [PubMed] [Google Scholar]
- 23.Badell IR, Russell MC, Cardona K, Shaffer VO, Turner AP, Avila JG, et al. CTLA4Ig prevents alloantibody formation following nonhuman primate islet transplantation using the CD40-specific antibody 3A8. Am J Transplant (2012) 12:1918–23. 10.1111/j.1600-6143.2012.04029.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincenti F, Rostaing L, Grinyo J, Rice K, Steinberg S, Gaite L, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med (2016) 374:333–43. 10.1056/NEJMoa1506027 [DOI] [PubMed] [Google Scholar]
- 25.Webster AC, Woodroffe RC, Taylor RS, Chapman JR, Craig JC. Tacrolimus versus ciclosporin as primary immunosuppression for kidney transplant recipients: meta-analysis and meta-regression of randomised trial data. BMJ (2005) 331:810. 10.1136/bmj.38569.471007.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Bruyne R, Bogaert D, De Ruyck N, Lambrecht BN, Van Winckel M, Gevaert P, et al. Calcineurin inhibitors dampen humoral immunity by acting directly on naive B cells. Clin Exp Immunol (2015) 180:542–50. 10.1111/cei.12604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wicker LS, Boltz RC, Jr, Matt V, Nichols EA, Peterson LB, Sigal NH. Suppression of B cell activation by cyclosporin A, FK506 and rapamycin. Eur J Immunol (1990) 20:2277–83. 10.1002/eji.1830201017 [DOI] [PubMed] [Google Scholar]
- 28.Traitanon O, Mathew JM, La Monica G, Xu L, Mas V, Gallon L. Differential effects of tacrolimus versus sirolimus on the proliferation, activation and differentiation of human B cells. PLoS One (2015) 10:e0129658. 10.1371/journal.pone.0129658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger R, Meingassner JG, Knapp W. In vitro effects of cyclosporin A on human B-cell responses. Scand J Immunol (1983) 17:241–9. 10.1111/j.1365-3083.1983.tb00787.x [DOI] [PubMed] [Google Scholar]
- 30.Leibler C, Matignon M, Pilon C, Montespan F, Bigot J, Lang P, et al. Kidney transplant recipients treated with belatacept exhibit increased naive and transitional B cells. Am J Transplant (2014) 14:1173–82. 10.1111/ajt.12721 [DOI] [PubMed] [Google Scholar]
- 31.Shabir S, Girdlestone J, Briggs D, Kaul B, Smith H, Daga S, et al. Transitional B lymphocytes are associated with protection from kidney allograft rejection: a prospective study. Am J Transplant (2015) 15:1384–91. 10.1111/ajt.13122 [DOI] [PubMed] [Google Scholar]
- 32.Svachova V, Sekerkova A, Hruba P, Tycova I, Rodova M, Cecrdlova E, et al. Dynamic changes of B-cell compartments in kidney transplantation: lack of transitional B cells is associated with allograft rejection. Transpl Int (2016) 29:540–8. 10.1111/tri.12751 [DOI] [PubMed] [Google Scholar]
- 33.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, et al. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity (2010) 32:129–40. 10.1016/j.immuni.2009.11.009 [DOI] [PubMed] [Google Scholar]
- 34.Weber M, Stein P, Prufer S, Rudolph B, Kreft A, Schmitt E, et al. Donor and host B cell-derived IL-10 contributes to suppression of graft-versus-host disease. Eur J Immunol (2014) 44:1857–65. 10.1002/eji.201344081 [DOI] [PubMed] [Google Scholar]
- 35.International Summit on Transplant Tourism and Organ Trafficking. The declaration of Istanbul on organ trafficking and transplant tourism. Kidney Int (2008) 74:854–9. 10.1038/ki.2008.388 [DOI] [PubMed] [Google Scholar]
- 36.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant (2010) 10:535–46. 10.1111/j.1600-6143.2009.03005.x [DOI] [PubMed] [Google Scholar]
- 37.Shen J, Townsend R, You X, Shen Y, Zhan P, Zhou Z, et al. Pharmacokinetics, pharmacodynamics, and immunogenicity of belatacept in adult kidney transplant recipients. Clin Drug Investig (2014) 34:117–26. 10.1007/s40261-013-0153-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity (2011) 34:108–21. 10.1016/j.immuni.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He J, Tsai LM, Leong YA, Hu X, Ma CS, Chevalier N, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity (2013) 39:770–81. 10.1016/j.immuni.2013.09.007 [DOI] [PubMed] [Google Scholar]
- 40.Agata Y, Kawasaki A, Nishimura H, Ishida Y, Tsubata T, Yagita H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol (1996) 8:765–72. 10.1093/intimm/8.5.765 [DOI] [PubMed] [Google Scholar]
- 41.Latek R, Fleener C, Lamian V, Kulbokas E, III, Davis PM, Suchard SJ, et al. Assessment of belatacept-mediated costimulation blockade through evaluation of CD80/86-receptor saturation. Transplantation (2009) 87:926–33. 10.1097/TP.0b013e31819b5a58 [DOI] [PubMed] [Google Scholar]
- 42.Di Girolamo N, Visvanathan K, Lloyd A, Wakefield D. Expression of TNF-alpha by human plasma cells in chronic inflammation. J Leukoc Biol (1997) 61:667–78. [DOI] [PubMed] [Google Scholar]
- 43.Denz A, Eibel H, Illges H, Kienzle G, Schlesier M, Peter HH. Impaired up-regulation of CD86 in B cells of “type A” common variable immunodeficiency patients. Eur J Immunol (2000) 30:1069–77. [DOI] [PubMed] [Google Scholar]
- 44.Hoffmann JC, Kruger H, Zielen S, Bayer B, Zeidler H. Human B cell differentiation: dependence on interactions with monocytes and T lymphocytes via CD40, CD80 (B7.1), and the CD2-ligands CD48 and CD58 (LFA-3). Cell Biol Int (1998) 22:21–9. 10.1006/cbir.1997.0208 [DOI] [PubMed] [Google Scholar]
- 45.Wiebe C, Nevins TE, Robiner WN, Thomas W, Matas AJ, Nickerson PW. The synergistic effect of class II HLA epitope-mismatch and nonadherence on acute rejection and graft survival. Am J Transplant (2015) 15:2197–202. 10.1111/ajt.13341 [DOI] [PubMed] [Google Scholar]
- 46.Sellares J, De Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant (2012) 12:388–99. 10.1111/j.1600-6143.2011.03840.x [DOI] [PubMed] [Google Scholar]
- 47.van Gelder T, Silva HT, De Fijter JW, Budde K, Kuypers D, Tyden G, et al. Comparing mycophenolate mofetil regimens for de novo renal transplant recipients: the fixed-dose concentration-controlled trial. Transplantation (2008) 86:1043–51. 10.1097/TP.0b013e318186f98a [DOI] [PubMed] [Google Scholar]
- 48.Grinyo JM, Ekberg H, Mamelok RD, Oppenheimer F, Sanchez-Plumed J, Gentil MA, et al. The pharmacokinetics of mycophenolate mofetil in renal transplant recipients receiving standard-dose or low-dose cyclosporine, low-dose tacrolimus or low-dose sirolimus: the Symphony pharmacokinetic substudy. Nephrol Dial Transplant (2009) 24:2269–76. 10.1093/ndt/gfp162 [DOI] [PubMed] [Google Scholar]
- 49.Kino T, Hatanaka H, Miyata S, Inamura N, Nishiyama M, Yajima T, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot (Tokyo) (1987) 40:1256–65. 10.7164/antibiotics.40.1249 [DOI] [PubMed] [Google Scholar]
- 50.Mayer E, Holzl M, Ahmadi S, Dillinger B, Pilat N, Fuchs D, et al. CTLA4-Ig immunosuppressive activity at the level of dendritic cell/T cell crosstalk. Int Immunopharmacol (2013) 15:638–45. 10.1016/j.intimp.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moret FM, Bijlsma JW, Lafeber FP, Van Roon JA. The efficacy of abatacept in reducing synovial T cell activation by CD1c myeloid dendritic cells is overruled by the stimulatory effects of T cell-activating cytokines. Arthritis Rheumatol (2015) 67:637–44. 10.1002/art.38982 [DOI] [PubMed] [Google Scholar]
- 52.Li J, Lu E, Yi T, Cyster JG. EBI2 augments Tfh cell fate by promoting interaction with IL-2-quenching dendritic cells. Nature (2016) 533:110–4. 10.1038/nature17947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wood KJ, Goto R. Mechanisms of rejection: current perspectives. Transplantation (2012) 93:1–10. 10.1097/TP.0b013e31823cab44 [DOI] [PubMed] [Google Scholar]
- 54.Migita K, Akeda Y, Akazawa M, Tohma S, Hirano F, Ideguchi H, et al. Pneumococcal polysaccharide vaccination in rheumatoid arthritis patients receiving tacrolimus. Arthritis Res Ther (2015) 17:149. 10.1186/s13075-015-0662-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kogure T, Harada N, Tatsumi T, Fujinaga H. Investigation of clinical characteristics as predictive factors for the humoral immune response to the influenza vaccine in patients with rheumatoid arthritis. Clin Rheumatol (2014) 33:323–8. 10.1007/s10067-013-2483-0 [DOI] [PubMed] [Google Scholar]
- 56.Alten R, Bingham CO, III, Cohen SB, Curtis JR, Kelly S, Wong D, et al. Antibody response to pneumococcal and influenza vaccination in patients with rheumatoid arthritis receiving abatacept. BMC Musculoskelet Disord (2016) 17:231. 10.1186/s12891-016-1082-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.