Abstract
To evaluate the effect of recombinant human thrombomodulin (rTM) on sepsis, the levels of nucleosome as well as high-mobility group box 1 (HMGB1) and cytokines in sera and peritoneal fluids were measured in a mouse model of lipopolysaccharide (LPS)-induced sepsis after administration of rTM. C57BL/6 mice were intraperitoneally injected with LPS (15 mg/kg; Escherichia coli O111:B4) with or without the intravenous administration of rTM (3 mg/kg; 30 min prior to or 2 h after LPS injection). The survival rates were evaluated and levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, monocyte chemoattractant protein (MCP)-1, HMGB1 and nucleosome in sera and peritoneal fluids were analyzed by ELISA. Administration of rTM prior to or after LPS improved the survival rate of septic mice. In addition, rTM administered prior to or after LPS suppressed the level of pro-inflammatory cytokine TNF-α in sera at 1–3 h after LPS injection, whereas only the administration of rTM after LPS suppressed the levels of HMGB1 and nucleosome (late-phase mediators of sepsis) (9–12 h) in sera after the LPS injection. Furthermore, administration of rTM prior to or after LPS suppressed the level of TNF-α in the peritoneal fluids at 1–3 h after LPS injection, whereas only the administration of rTM after LPS suppressed the levels of IL-6 and MCP-1 in the peritoneal fluids at 6–9 h after LPS injection. These observations indicated that administration of rTM significantly improves the survival rate and suppresses the increased levels of TNF-α, IL-6, MCP-1, HMGB1 and nucleosome in the LPS-induced septic shock model. Thus, rTM may exert a protective action on sepsis and reduce mortality, possibly by reducing not only the levels of cytokines and chemokine but also the levels of late-phase mediators of sepsis.
Keywords: sepsis, recombinant thrombomodulin, high-mobility group box 1, nucleosome, neutrophil extracellular traps, cytokines, lipopolysaccharide
Introduction
In patients with septic shock, coagulation and fibrinolysis are activated, and excess inflammatory and immune responses eventually lead to disseminated intravascular coagulation (DIC) (1–3).
During septic DIC, damage-associated molecular patterns (DAMPs) such as DNA, high-mobility group box 1 (HMGB1) and histone, as well as pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS), peptidoglycans and lipoteichoic acid are released from the damaged host cells and invading microorganisms, respectively (4). These molecules are recognized by pattern-recognition receptors (PRRs) of immune cells, thereby initiating systemic activation of immune responses and the production of pro-inflammatory cytokines (2,3). As a result, systemic spread of inflammation leads to organ injury and increased mortality (2,3,5,6).
Neutrophils are particularly important in this process, as they work at the frontline of host defense and account for the majority of immune cells (2,5). Neutrophil extracellular traps (NETs) have attracted increased attention over the last decade (6,7). NETs are web-like extracellular structures and are formed with genomic DNA and antimicrobial proteins, including histones, myeloperoxidase and neutrophil elastase, which are expelled from activated neutrophils (6–8). NETs rapidly trap and kill microbial pathogens in the blood and tissues and prevent bacterial dissemination (5–7,9,10). Simultaneously with NET formation, HMGB1 and histone are excessively released from the damaged host cells (7,9,11) and have been shown to be lethal late phase mediators of sepsis (3,12–15).
The efficacy of recombinant human thrombomodulin (rTM) in treating septic DIC has been shown by numerous studies (10,16–18). TM, which is expressed on the endothelial cell surface, is a thrombin-binding anti-coagulant cofactor. The structure of TM comprises five domains, including the three extracellular domains: The N-terminal lectin-like domain TM-D1, followed by epidermal growth factor-like domain TM-D2 and O-glycosylation-rich domain TM-D3. TM binds to thrombin with a high affinity via TM-D2 and inhibits the action of thrombin (19,20). Furthermore, thrombin-TM complexes activate protein C and activated protein C (APC) degrades coagulation factors Va and VIIIa (10,19,21).
In addition to its anti-coagulant activity, TM exhibits an anti-inflammatory action through APC (19,21). APC binds to the endothelial protein C receptor to activate protease-activated receptor 1, thereby eliciting anti-inflammatory and cytoprotective (anti-apoptotic) signaling responses in endothelial cells (21,22). APC downregulates the production of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α) by monocytes via the inhibition of the nuclear translocation of nuclear factor κB (NF-κB) (3,22,23). APC also binds selectins to limit the rolling of neutrophils and monocytes on injured endothelium (18,23,24). Furthermore, HMGB1 is degraded by a thrombin-TM complex, whereas histone is cleaved by APC activated by a thrombin-TM complex (3,19,22). Thus, TM exerts a protective action on sepsis based on its anti-coagulant and anti-inflammatory properties due to inactivation of coagulation factors, suppression of cytokine production and degradation of HMGB1 and histones as DAMPs, ultimately contributing to the improvement of the survival rate (11,17,19).
However, it has remained elusive whether TM modulates other components of DAMPs such as DNA-histone complex (nucleosome) in septic shock. Therefore, the present study evaluated the effect of rTM on the levels of nucleosome as well as HMGB1 and cytokines in sera and peritoneal fluids using in a lipopolysaccharide (LPS)-induced murine model of sepsis.
Materials and methods
Sepsis model
Male C57BL/6 mice (age, 6–8 weeks; weight, 20–25 g; ORIENTAL YEAST Co., Tokyo, Japan) were used. All mice were provided with standard mouse chow and water ad libitum. All of the experimental procedures were performed after obtaining the approval of the Ethical Committee for Animal Experiments of Juntendo University (Tokyo, Japan). Animals were divided into four groups (n=8 each) treated as follows: 15 mg/kg LPS (Escherichia coli 0111:B4; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) diluted with 200 µl sterile physiological saline as a bolus injection into the peritoneal cavity (LPS group); 3 mg/kg rTM (ART-123; Asahi Kasei Pharma Co., Tokyo, Japan) diluted with 200 µl sterile physiological saline was administered as a bolus injection into a caudal vein 30 min before or 2 h after the LPS-injection (rTM pre-administration group or post-administration group, respectively). As a control, an equal volume of physiological saline was administered instead of LPS and rTM (control group). Prior to a bolus injection into a caudal vein, mice were anesthetized using isoflurane (Mylan Seiyaku Co., Osaka, Japan). Survival was monitored for up to 3 days.
Measurement of cytokines, HMGB1 and nucleosome
At the indicated time-points (3, 6, 9 and 12 h after LPS administration in both groups and at 1 h after LPS administration in the rTM pre-administration group), mice were anesthetized using isoflurane and blood and peritoneal fluids were collected: Blood was collected via cardiac puncture and ascites were collected by washing and aspirating the peritoneal cavity with 2 ml cold PBS. Blood and ascites were centrifuged at 1,500 × g for 10 min at 4°C, and stored as serum and peritoneal fluids, respectively, at −30°C until use. For measuring nucleosome, 10 mM EDTA was added to sera and peritoneal fluids at a 1/10 volume ratio to inactivate nuclease, and the mixture was stored at −30°C.
TNF-α in sera was measured using a Mouse TNF Alpha ELISA Ready-Set-Go! (eBioscience, San Diego, CA, USA). HMGB1 in sera and peritoneal fluids was measured using an HMGB1 ELISA kit (Shino-Test Corp., Tokyo, Japan). Nucleosome in sera and peritoneal fluids was measured using a Cell Death Detection ELISA Plus kit (Roche Diagnostics, Indianapolis, IN, USA) and the concentrations were calculated based on the standard curve using a positive control (a DNA-histone complex) contained in the kit. Alternatively, TNF-α, interleukin 6 (IL-6) and monocyte-chemoattractant protein-1 (MCP-1) in peritoneal fluids were measured by cytometric bead array (Mouse Inflammation Kit, BD Biosciences, San Jose, CA, USA).
Statistical analyses
Values are expressed as the means ± standard deviation. All statistical analyses were performed using GraphPad Prism version 5.01 (GraphPad Software Inc., La Jolla, CA, USA). The levels of cytokines, HMGB1 and nucleosome were compared between the control and LSP groups, and between the LPS group and rTM pre-administration group or rTM post-administration group using Student's t-test. A Kaplan-Meier curve was drawn and the survival difference was examined by using the log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
rTM increases the survival of LPS-induced septic model mice
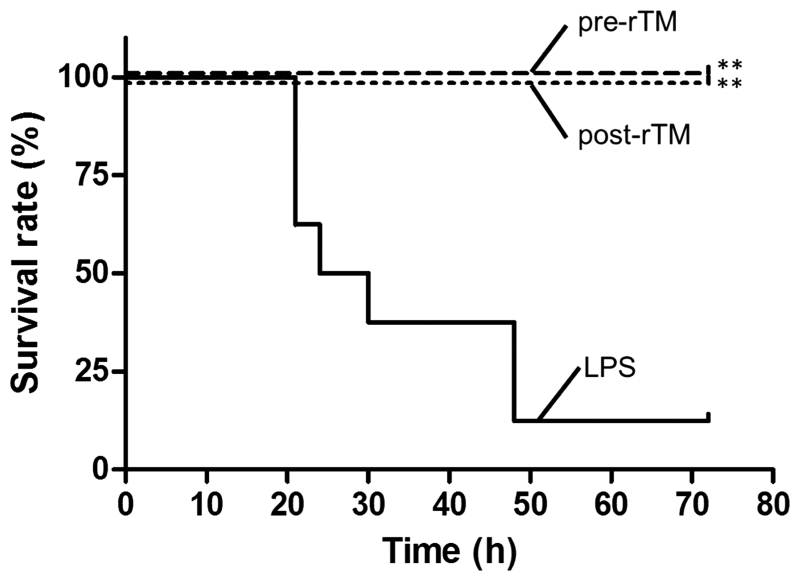
In the LPS-induced septic model, rTM administration significantly improved the survival rate of mice from 12.5 to 100% in the rTM pre-administration and post-administration groups at 72 h after the LPS injection (P<0.01; Fig. 1).
Figure 1.
Protective effect of rTM on survival of LPS-induced septic model mice. LPS (15 mg/kg) was injected into the peritoneal cavity (LPS group); 3 mg/kg of rTM was administered 30 min prior to or 2 h after the LPS-injection (pre-rTM group or post-rTM group, respectively). Survival of LPS, pre-rTM and post-rTM groups (n=8 mice each) was monitored for up to 3 days. Values are compared between LPS group and pre-rTM group or post-rTM group. **P<0.01. LPS, lipopolysaccharide; rTM, recombinant thrombomodulin.
rTM suppresses LPS-induced increases in TNF-α, HMGB1 and nucleosome in sera of septic mice
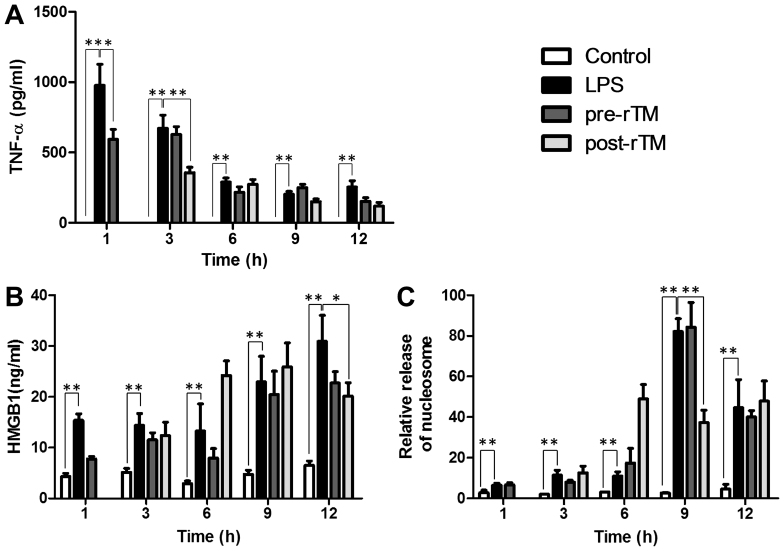
In the control group, TNF-α was not detected in sera. However, in the LPS group, the serum level of TNF-α reached a maximum (977±149 pg/ml) at 1 h after the LPS injection (Fig. 2A). TNF-α levels were significantly suppressed in the rTM pre-administration group at 1 h after the LPS injection (P<0.05) and in the rTM post-administration group at 3 h after the LPS injection (P<0.01).
Figure 2.
Effects of rTM on the levels of TNF-α, HMGB1 and nucleosome in sera. LPS (15 mg/kg) was injected into the peritoneal cavity (LPS group); 3 mg/kg of rTM was administered 30 min before or 2 h after the LPS injection (pre-rTM group or post-rTM group, respectively). As a control, physiological saline was administered instead of LPS and rTM (control group). At the indicated time-points (3, 6, 9 and 12 h after LPS administration in both groups and at 1 h after LPS administration in the rTM pre-administration group), blood was collected and sera were prepared. (A) TNF-α, (B) HMGB1 and (C) nucleosome were measured by ELISA. Values are expressed as the means ± standard deviation, and compared between control group and LSP group, and LPS group and rTM pre-administration group or rTM post-administration group. *P<0.05; **P<0.01. LPS, lipopolysaccharide; rTM, recombinant thrombomodulin; TNF, tumor necrosis factor; HMGB1, high-mobility group box 1.
In the control group, low levels of HMGB1 (~4 ng/ml) and nucleosome (relative concentration, ~3) were detected in sera (Fig. 2B and C). However, in the LPS group, the serum levels of HMGB1 and nucleosome were increased at 1, 3 and 6 h and further enhanced at 9 and 12 h after the LPS injection. Of note, in the rTM post-administration group, the levels of HMGB1 and nucleosome were significantly suppressed at 12 h (P<0.05) and 9 h (P<0.01), respectively, after the LPS injection (Fig. 2B and C).
Effects of rTM on cytokines, HMGB1 and nucleosome in peritoneal fluids of septic mice
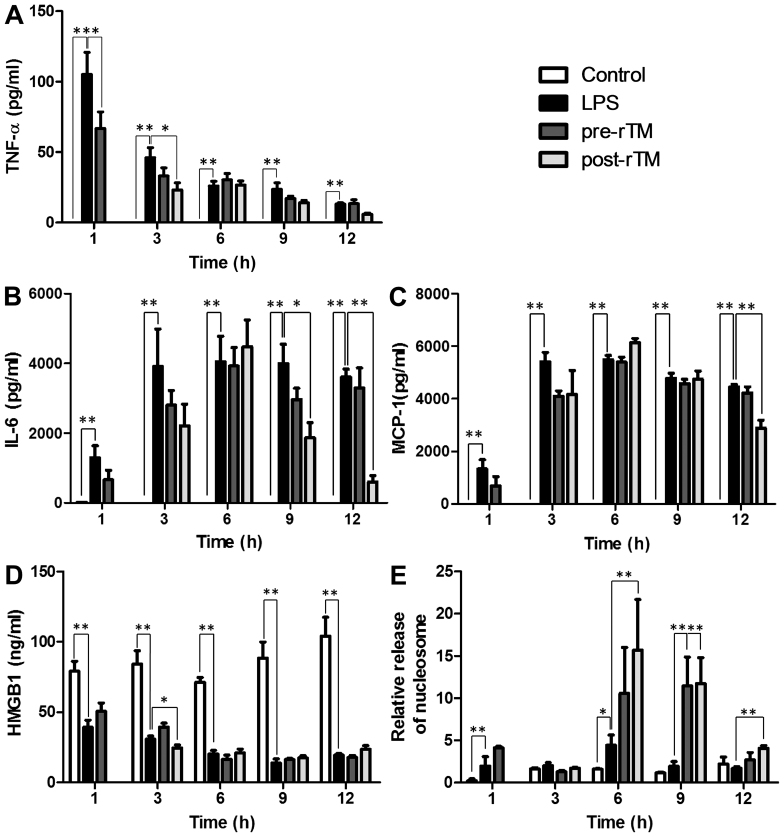
In the control group, TNF-α was not detected in peritoneal fluids. However, in the LPS group, the level of TNF-α reached a maximum level (105±16 pg/ml) at 1 h after LPS injection (Fig. 3A). TNF-α levels were significantly suppressed in the rTM pre-administration group at 1 h after the LPS injection (P<0.05), and in the rTM post-administration group at 3 h after the LPS injection (P<0.05).
Figure 3.
Effects of rTM on the levels of cytokines, HMGB1 and nucleosome in peritoneal fluids. LPS (15 mg/kg) was injected into the peritoneal cavity (LPS group); 3 mg/kg of rTM was administered 30 min before or 2 h after LPS injection (pre-rTM group or post-rTM group, respectively). As a control, physiological saline was administered instead of LPS and rTM (control group). At the indicated time-points (1, 3, 6, 9 and 12 h after LPS administration; except 1 h in the rTM post-administration group), ascites was collected and peritoneal fluids were prepared. (A) TNF-α, (B) IL-6 and (C) MCP-1 were measured by cytometric bead array and (D) HMGB1 and (E) nucleosome were measured by ELISA. Values are expressed as the means ± standard deviation, and compared between control group and LPS group, and LPS group and rTM pre-administration group or rTM post-administration group. *P<0.05 and **P<0.01. LPS, lipopolysaccharide; rTM, recombinant thrombomodulin; TNF, tumor necrosis factor; IL interleukin; MCP, monocyte chemoattractant protein.
In the control group, IL-6 was not essentially detected in peritoneal fluids. However, in the LPS group, the level of IL-6 gradually increased, reached a maximum (3,998±554 pg/ml) at 3 h after the LPS injection and remained almost stable thereafter (Fig. 3B). Of note, the IL-6 level in the rTM post-administration group was significantly suppressed at 9 and 12 h after the LPS injection (P<0.05).
In the control group, MCP-1 was not detected in peritoneal fluids. However, in the LPS group, the level of MCP-1 gradually increased, reached a maximum (5,492±160 pg/ml) at 3 h after the LPS injection and remained almost at the same level thereafter (Fig. 3C). Of note, the MCP-1 level in the rTM post-administration group was significantly suppressed at 12 h after the LPS injection (P<0.05).
At 1, 3, 6, 9 and 12 h after the saline injection, HMGB1 in the peritoneal fluids of control mice was detected to be 80–100 ng/ml. Of note, the HMGB1 level was significantly decreased at 1, 3, 6, 9 and 12 h after the LPS injection regardless of pre-administration and post-administration of rTM (P<0.01) and HMGB1 was significantly decreased in the post-rTM group compared to the LPS group at 3 h after LPS injection (P<0.05; Fig. 3D).
In the control group, low levels of nucleosome (relative concentration, 0.2–2) were detected in the peritoneal fluids of control mice at 1, 3, 6, 9 and 12 h after saline injection (Fig. 3E). Nucleosome levels were slightly increased (relative concentration, 2.5–4) at 1 and 6 h after the LPS injection. Of note, the nucleosome levels were further increased (3- to 5-fold) by pre-administration and post-administration of rTM (P<0.01).
Discussion
In the present study, the effect of rTM on the level of nucleosome as well as HMGB1 and cytokines in sera and peritoneal fluids was evaluated using in a mouse model of LPS-induced sepsis.
Septic shock is the result of an uncontrolled inflammatory response of the host's innate immune system towards invading pathogens (1–3). Macrophages/monocytes recognize DAMPs and PAMPs via PRRs and produce pro-inflammatory cytokines (such as TNF-α and IL-6), chemokines and nitric oxide, which initiate an inflammatory response (3), which directly and indirectly causes widespread tissue injury and organ dysfunction (5,6).
Analysis of pro-inflammatory cytokine production in sepsis has revealed that serum levels of TNF-α peak at the early phase and decrease to undetectable levels in the late phase (3,11,13,14). The release of HMGB1 as a delayed mediator of inflammation occurs considerably later than the secretion of classical early pro-inflammatory mediator such as TNF-α (3,13). In an LPS-induced murine sepsis model, serum HMGB1 levels begin to increase 12–18 h after peaking of TNF-α levels, which occurs at 2 h (3,13,14). In murine models of sepsis induced by LPS or caecal ligation and puncture, inhibition of HMGB1 by specific antibodies protected mice from mortality (3,25), even when the antibody was administered after TNF-α had peaked (3). Furthermore, recombinant HMGB1 protein administration induced lethal organ dysfunction and recapitulated severe sepsis (3).
Originally identified as a chromatin-binding protein associated with chromosomal DNA in the nucleus, HMGB1 is ubiquitous in the nuclei of all eukaryotic cells, where it has a critical role in stabilizing nucleosome formation and regulating transcription by binding to DNA and several transcription factors (3,14,26). Macrophages, monocytes and neutrophils are regarded as main sources of HMGB1 in inflammation (2). When cells undergo necrotic cell death, HMGB1 is released from the nuclei into the extracellular space (11,13). This leads to passive leaking of HMGB1 into the extracellular environment, as the membrane integrity is disrupted during necrosis (3,13,14). Once released into the extracellular environment, HMGB1 acts as a DAMP and promotes inflammation, immune responses and tissue damage (2).
Nucleosomes, which are complexes formed by DNA and histone, are also excessively released from the damaged host cells that have died from apoptosis or necrosis (15,27); nucleosome levels are therefore correlated with the inflammatory response and organ dysfunction in sepsis (11,15). Circulating histones have a highly damaging effect on endothelium and tissues (10).
Recent studies have suggested that rTM directly or indirectly decreases the serum levels of HMGB1, nucleosome and TNF-α (3,19,22,23). HMGB1 binds to the D1 domain of TM in the thrombin-TM complexes and is degraded by thrombin (19). In addition, histones in the form of nucleosome are degraded by APC, which is generated by activation of protein C by thrombin-TM complexes (19,22). Through proteolytic cleavage of extracellular histones, APC exerts cytoprotective effects (19,22,28). In addition, APC downregulates the production of TNF-α by macrophages/monocytes via the inhibition of the nuclear translocation of NF-κB (22,23).
In the present study, rTM administration improved the survival rate of mice in the rTM pre-administration and post-administration groups. rTM also suppressed the circulating levels of pro-inflammatory cytokines such as TNF-α at the early phase after the LPS-injection. In addition, rTM suppressed the levels of HMGB1 and nucleosome in the rTM post-administration group at the late phase after LPS-injection. Significant elevations of HMGB1 and nucleosome in the systemic circulation occur considerably later (9–12 h) than those of early pro-inflammatory cytokines such as TNF-α (1–3 h) after the LPS-injection. The present study revealed that rTM suppressed the serum levels of TNF-α as well as HMGB1 and nucleosome, and eventually improved the survival rate. Based on these findings, it is speculated that rTM has the potential to suppress the levels of TNF-α as an early pro-inflammatory mediator as well as HMGB1 and nucleosome as late-phase mediators, thereby reducing the mortality of LPS-induced septic mice.
The activation of neutrophils induces NETosis, a neutrophil cell death distinct from apoptosis and necrosis (9,11), which is associated with the extracellular release of NETs together with de-condensed chromatin and granular contents (7,9). NETosis reflects the increased levels of circulating nucleosomes (11,15). It has been reported that NETosis is induced at the early phase (180 min) after the LPS stimulation (9,29). In the present study, rTM suppressed serum nucleosome levels in the late phase but not in the early phase. Thus, rTM is unlikely to suppress NETosis, which may possibly have been accountable for increases in the nucleosome level in the early phase after the LPS injection, in the model used.
Furthermore, the effect of rTM on the local inflammatory response was evaluated by measuring the levels of the pro-inflammatory cytokines TNF-α and IL-6, the chemokine MCP-1 as well as HMGB1 and nucleosome in the peritoneal fluids. rTM suppressed the levels of not only pro-inflammatory cytokines but also chemokine in the peritoneal fluids. By contrast, HMGB1 was constitutively detected in the peritoneal fluids of control mice. Of note, HMGB1 levels were significantly decreased by LPS, and the levels were not affected by rTM except at 3 h in the rTM post-administration group. Moreover, the nucleosome level was slightly increased after the LPS-injection, and unexpectedly the level was further increased by rTM. These observations suggested that the rTM-mediated suppression of pro-inflammatory cytokine and chemokine in the local milieu (peritoneal cavity) may contribute to the increased survival rate of LPS-septic mice; however, the effects of rTM on the HMGB1 and nucleosome levels in the peritoneal cavity is unlikely relevant to the survival.
In the present study, administration of rTM prior to or after LPS similarly improved the survival rate of LPS-induced septic mice. However, pre-administration of rTM only suppressed the level of the early pro-inflammatory mediator TNF-α, whereas post-LPS administration of rTM suppressed not only TNF-α but also IL-6 and MCP-1 (mid-phase mediators), as well as HMGB1 and nucleosome (late-phase mediators). Thus, the suppression of early pro-inflammatory mediators by rTM is likely to be more important for improving the survival rate than the suppression of the mid- and late-phase mediators, as the survival in the pre- and post-rTM groups was identical.
In summary, the present study revealed that the administration of rTM significantly improved the survival rate and suppressed the increased levels of TNF-α, HMGB1 and nucleosome in sera, and TNF-α, IL-6 and MCP-1 in peritoneal fluids in the LPS-induced septic shock model. Thus, rTM may exert a protective action on sepsis and reduce the mortality, possibly by reducing not only cytokine and chemokine levels but also the levels of late-phase mediators of sepsis.
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (no. 26460538) and a Grant-in-Aid from the Ministry of Education, Culture, Sport, Science and Technology, Japan for the Foundation of Strategic Research Projects in Private Universities (no. S0991013). The abstract of this work has been registered as a thesis of Dr. Kazuhiro Takehara in Japanese Institutional Repositories Online (http://jairo.nii.ac.jp/0348/00000309/en) in Japanese.
References
- 1.Angus DC, van der Poll T. Severe Sepsis and Septic Shock. N Engl J Med. 2013;369:842–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 2.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shimaoka M, Park EJ. Advances in understanding sepsis. Eur J Anaesthesiol Suppl. 2008;42:146–153. doi: 10.1017/S0265021507003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denk S, Perl M, Huber-Lang M. Damage- and pathogen-associated molecular patterns and alarmins: keys to sepsis? Eur Surg Res. 2012;48:171–179. doi: 10.1159/000338194. [DOI] [PubMed] [Google Scholar]
- 5.Seeley EJ, Matthay MA, Wolters PJ. Inflection points in sepsis biology: From local defense to systemic organ injury. Am J Physiol Lung Cell Mol Physiol. 2012;303:L355–L363. doi: 10.1152/ajplung.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo L, Zhang S, Wang Y, Rahman M, Syk I, Zhang E, Thorlacius H. Proinflammatory role of neutrophil extracellular traps in abdominal sepsis. Am J Physiol Lung Cell Mol physiol. 2014;307:L586–L596. doi: 10.1152/ajplung.00365.2013. [DOI] [PubMed] [Google Scholar]
- 7.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 8.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 9.Kambas K, Mitroulis I, Ritis K. The emerging role of neutrophils in thrombosis-the journey of TF through NETs. Front Immunol. 2012;3:385. doi: 10.3389/fimmu.2012.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iba T, Gando S, Thachil J. Anticoagulant therapy for sepsis-associated disseminated intravascular coagulation: The view from Japan. J Thromb Haemost. 2014;12:1010–1019. doi: 10.1111/jth.12596. [DOI] [PubMed] [Google Scholar]
- 11.Iba T, Miki T, Hashiguchi N, Yamada A, Nagaoka I. Combination of antithrombin and recombinant thrombomodulin attenuates leukocyte-endothelial interaction and suppresses the increase of intrinsic damage-associated molecular patterns in endotoxemic rats. J Surg Res. 2014;187:581–586. doi: 10.1016/j.jss.2013.10.058. [DOI] [PubMed] [Google Scholar]
- 12.Nagato M, Okamoto K, Abe Y, Higure A, Yamaguchi K. Recombinant human soluble thrombomodulin decreases the plasma high-mobility group box-1 protein levels, whereas improving the acute liver injury and survival rates in experimental endotoxemia. Crit Care Med. 2009;37:2181–2186. doi: 10.1097/CCM.0b013e3181a55184. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Yang H, Czura CJ, Sama AE, Tracey KJ. HMGB1 as a late mediator of lethal systemic inflammation. Am J Respir Crit Care Med. 2001;164:1768–1773. doi: 10.1164/ajrccm.164.10.2106117. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 15.Chen Q, Ye L, Jin Y, Zhang N, Lou T, Qiu Z, Jin Y, Cheng B, Fang X. Circulating nucleosomes as a predictor of sepsis and organ dysfunction in critically ill patients. Int J Infect Dis. 2012;16:e558–e564. doi: 10.1016/j.ijid.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Yamakawa K, Ogura H, Fujimi S, Morikawa M, Ogawa Y, Mohri T, Nakamori Y, Inoue Y, Kuwagata Y, Tanaka H, et al. Recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: A multicenter propensity score analysis. Intensive Care Med. 2013;39:644–652. doi: 10.1007/s00134-013-2822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tagami T, Matsui H, Horiguchi H, Fushimi K, Yasunaga H. Recombinant human soluble thrombomodulin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: An observational nationwide study. J Thromb Haemost. 2015;13:31–40. doi: 10.1111/jth.12786. [DOI] [PubMed] [Google Scholar]
- 18.Yamakawa K, Fujimi S, Mohri T, Matsuda H, Nakamori Y, Hirose T, Tasaki O, Ogura H, Kuwagata Y, Hamasaki T, Shimazu T. Treatment effects of recombinant human soluble thrombomodulin in patients with severe sepsis: A historical control study. Crit Care. 2011;15:R123. doi: 10.1186/cc10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito T, Maruyama I. Thrombomodulin: Protectorate God of the vasculature in thrombosis and inflammation. J Thromb Haemost. 2011;9(Suppl 1):S168–S173. doi: 10.1111/j.1538-7836.2011.04319.x. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Kawahara K, Okamoto K, Yamada S, Yasuda M, Imaizumi H, Nawa Y, Meng X, Shrestha B, Hashiguchi T, Maruyama I. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol. 2008;28:1825–1830. doi: 10.1161/ATVBAHA.107.150631. [DOI] [PubMed] [Google Scholar]
- 21.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9:1795–1803. doi: 10.1111/j.1538-7836.2011.04422.x. [DOI] [PubMed] [Google Scholar]
- 22.Rezaie AR. Regulation of the protein C anticoagulant and antiinflammatory pathways. Curr Med Chem. 2010;17:2059–2069. doi: 10.2174/092986710791233706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galligan L, Livingstone W, Volkov Y, Hokamp K, Murphy C, Lawler M, Fukudome K, Smith O. Characterization of protein C receptor expression in monocytes. Br J Haematol. 2001;115:408–414. doi: 10.1046/j.1365-2141.2001.03187.x. [DOI] [PubMed] [Google Scholar]
- 24.Russell JA. Management of sepsis. N Engl J Med. 2006;355:1699–1713. doi: 10.1056/NEJMra043632. [DOI] [PubMed] [Google Scholar]
- 25.Toltl LJ, Beaudin S, Liaw PC, Canadian Critical Care Translational Biology Group Activated protein C up-regulates IL-10 and Inhibits tissue factor in blood monocytes. J Immunol. 2008;181:2165–2173. doi: 10.4049/jimmunol.181.3.2165. [DOI] [PubMed] [Google Scholar]
- 26.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 27.Zeerleder S, Stephan F, Emonts M, de Kleijn ED, Esmon CT, Varadi K, Hack CE, Hazelzet JA. Circulating nucleosomes and severity of illness in children suffering from meningococcal sepsis treated with protein C. Crit Care Med. 2012;40:3224–3229. doi: 10.1097/CCM.0b013e318265695f. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Berghe T Vanden. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]