Abstract
The clinical effects and safety of release and decompression techniques on nerve roots through percutaneous transforaminal endoscopic discectomy (PTED) while treating patients with central lumbar disc herniation (CLDH) were explored. Patient cases of lumbar and leg pain treated in Bethune International Peace Hospital from July 2013 to October 2015 were collected retrospectively. The patients in these cases received imaging examinations such as computed tomography and magnetic resonance imaging. Among these cases, 37 CLDH patients with no other complications were selected for this study. A total of 22 males and 15 females aged 28–54 years, with an average age of 36.8±1.5 years, were the subjects of the study. Their disease course was from 1 month to 3 years, with a median course time of 1.5 years. Visual analogue scale (VAS), Japanese Orthopaedic Association (JOA) scoring and the MacNab method were used to evaluate treatment effects. After permission from patients or their family members, release and decompression techniques of nerve roots were performed through PTED. All patients had successful surgery. Their average surgery time was 41.3 (25.5–57.1) min. A physician followed-up each patient from 0 to 18 months after surgery, with the average follow-up period of 12.1 months. VAS scoring of lower limbs was 7.95±0.82 before surgery and 2.28±0.35, 3 months after surgery. VAS scoring of lower limbs was 2.06±0.58, 1 year after surgery and 2.12±0.23 at the last follow-up appointment. JOA scoring was 12.6±0.72 before surgery and 20.4±1.08, 3 months after surgery. JOA scoring was 21.1±0.82 1 year after surgery and 21.2±0.36 at the last follow-up. Differences are of statistical significance (P<0.05). There were no complications for any of the cases. One patient did not improve after surgery, so a laminectomy and bone grafting internal fixation were performed. Two patients relapsed after surgery and received laminectomy and bone grafting internal fixation. The total percentage of excellent and good rates was 83.5%. In conclusion, release and decompression techniques on nerve roots using PTED while treating CLDH resulted in a safe, effective and less traumatic outcome with fewer complications and quicker pain relief than alternative treatments. Due to the results of this study, the use of these techniques in treating CLDH should be more widely considered.
Keywords: transforaminal endoscopic discectomy, release and decompression techniques of nerve roots, central lumbar disc herniation
Introduction
Clinical symptoms of central lumbar disc herniation (CLDH) are varied (1). Pain symptoms typically occur on one or both sides of lower limbs. Some patients have ‘horse-tail’ nerve symptoms (2). Based on patients' conditions, partial laminectomy on one or both sides (fenestration treatment) is generally used. For patients with spinal canal stenosis, total laminectomy surgery is usually performed (3). However, traditional open surgery results in trauma and substantial bleeding. It also causes damage to normal structures such as muscles, ligaments, fascia and vertebrae (4). In some cases, sequelae is caused, including losing control due to muscle denervation, injured lumbar spinal stability and secondary spinal canal stenosis from spinal canal scarring (5). A series of negative effects are caused to immune systems of important human organs. These effects include increased stress hormones, tissue inflammation, lipolysis and hyperglycemia. Traditional open surgery also requires a long recovery time and risks related to the instability of the lumbar spine (6). Modern medical facilities are advanced and innovative. Many new minimally invasive spine surgery techniques are beginning to be used in treating lumbar disc herniation (7–10). An endoscope is used to help perform release and decompression within the spinal canal through intervertebral foramen, combining traditional posterior-lateral lumbar intervertebral disk treatment techniques and spinal endoscopic techniques. It provides a new solution for treating this type of herniation (8–10). The purpose of this study was to investigate the effects of release and decompression techniques during percutaneous transforaminal endoscopic discectomy (PTED) in treating CLDH.
Materials and methods
Clinical materials
Patients were treated in Bethune International Peace Hospital from July 2013 to October 2015 because of different degrees of lumbar and leg pain. They all received imaging examinations such as computed tomography (CT) and magnetic resonance imaging (MRI). A total of 37 CLDH patients with no other complications were selected, among which there were 22 males and 15 females. Their ages were 28–54 years and their average age was 36.8±1.5 years. Release and decompression techniques through PTED were applied. This study was approved by the Ethics Committee of Bethune International Peace Hospital and the patients signed written informed consents were obtained from all participants before the study.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) Lower limb pain of one or two sides with lumbago or slight lumbago; ii) obvious radicular symptoms - CLDH found under CT and MRI, as well as imaging results consistent with clinical manifestations and able to show the localized responsible intervertebral disk; and iii) no improvement after 6 weeks of conservative treatment. Exclusion criteria were as follows: i) Recurrence after surgery; ii) spinal fracture, malformation, inflammation and tumor; iii) dysfunction of bleeding and blood coagulation mechanisms; and iv) combined with a mental disorder.
Surgical method
During surgery, patients were in the prone position and localized under a C-arm X-ray apparatus (Siemens, Berlin, Germany) (1). Body surface projections in the central line of processus spinosus of vertebra lumbalis and all horizontal lines at the upper edge of the target intervertebral disk were marked. The targeted intervertebral space direction was determined under lateral X-ray fluoroscopy (Siemens). The lines of the trailing edge of the lower vertebra were made along this targeted direction on the body surface. These lines crossed with horizontal lines at the upper edge of the targeted intervertebral disk. The crossing point was the puncture point. Under lateral X-ray fluoroscopy, upper lines of the articular process were marked as safe lines. If a patient had herniation at the L5-S1 segment, then the puncture point was marked as follows: i) Under a normal X-ray perspective, lines of the highest points of the crista iliaca were marked on the body surface, as well as horizontal lines being marked through the lower edges of the L5 lower vertebra; and ii) under lateral X-ray, lateral lines though the S1 superior articular process and the trailing edge of the S1 vertebra that crossed with the lines of the highest crista iliaca points marked served as the puncture point. When L2-3 and L3-4 were herniated, a needle was inserted at a mark 6–10 cm from the siding median line. When L4-5 and L5-S1 were herniated, a needle was inserted at a mark 12–14 cm of the siding median line. After disinfection and draping, the puncture point was anaesthetized partially by 1% lidocaine hydrochloride. A no. 18 puncture needle was inserted under the guidance of the C-arm X-ray apparatus. The needle punctured until the front lower edge of the superior articular process was reached. After removing the needle, a no. 22 puncture needle with front bending was then used to perform intervertebral disk radiography. After removing the puncture needle and putting in a guide wire, the puncture point served as the central point. A 10 mm cut was then made on the skin. A guide rod was injected along with the guide wire to expand the catheter gradually, and then the surgical channel was expanded. After removing the expanded catheter gradually, a zigzag reamer was injected along with the guide rod. Some sclerotins at the outer edges of the superior articular process were cut under fluoroscopy (Siemens). After expanding the intervertebral foramen and removing the trephine, a working cannula was placed along with the guide rod. A transforaminal endoscopic discectomy was performed. After removing the degenerated nucleus pulposus tissues, we found the paper dura cyst was clear. Through further exploration, the nerve root was released until the leg-lifting experiment was negative, which signified complete decompression. Finally, bipolar radiofrequency electrodes were used to perform a shrinking-forming technique on the torn part of the annulus fibrosus. Electric coagulation hemostasis was performed at bleeding points in the surgical field. The surgery was completed by removing the diskoscope, suturing the cutting and then covering the surgical dressing (Figs. 1 and 2).
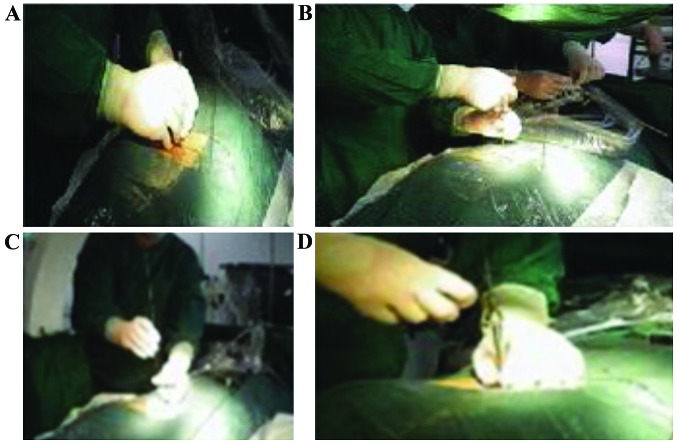
Figure 1.
Basic procedures of release and decompression techniques through PTED. (A) Puncture; (B) puncture through ligamentum flavum and pumpback and air test; (C) planted guide wire; and (D) planted working cannula. PTED, percutaneous transforaminal endoscopic discectomy.
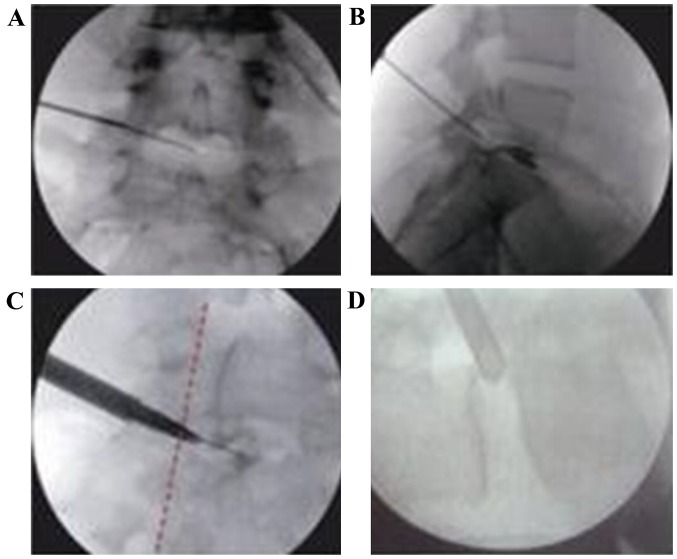
Figure 2.
Basic procedures of release and decompression techniques through PTED under guidance of C-arm. (A) Body surface location; (B) planted guide wire; (C) creating working route; and (D) planted working cannula. PTED, percutaneous transforaminal endoscopic discectomy.
Postoperative management
After surgery, a solution of 7.5 mg imethason and 2 ml lidocaine of volume fraction 1% (both from Yangze Pharm, Taizhou, China) were injected through the working cannula. A working route was removed carefully without damaging surrounding nerves and tissues. Doctors asked patients about their degrees of relief related to lower limb pain after surgery and then asked them to perform leg-lifting experiments to measure improvement. Patients were told to leave their bed after resting for 4 to 6 h. An intravenous injection of wide-spectrum antibiotics was given for 1 day. Instructions were given to avoid heavy manual labor or physical exercise within 3 months after surgery.
Evaluation on effects
The visual analogue scale (VAS), Japanese Orthopaedic Association (JOA) and MacNab scoring methods were used for evaluation. At least 1 year later, a follow-up evaluation was performed for all cases. Telephone and clinical follow-ups were given to record VAS, JOA and MacNab scoring of lumbar and leg pain 3, 6, 12 and 18 months after surgery (2).
Statistical analysis
SPSS 19.0 statistical software (SPSS, Inc., Chicago, IL, USA) was used in data analysis, and t-test was performed for VAS, JOA and MacNab scoring of lumbar and leg pain 3, 6, 12 and 18 months after surgery. P<0.05 was considered to indicate a statistically significant difference.
Results
Comparison of basic patient information
Treatments in all 37 patients were considered successful after follow-up evaluations. Follow-up time was 0–18 months, with an average of 12.1 months. Records were made of imaging data taken before and after surgery, imaging results of transforaminal endoscopic discectomy (Figs. 3–5). In addition, VAS and JOA scoring of lower limbs was recorded before and after surgery (Table I). There were no complications for any of the cases. However, one patient did not improve after surgery, so a laminectomy and bone grafting internal fixation were performed. Also, two patients relapsed after surgery and received a laminectomy and bone grafting internal fixation. A total of 83.5% of the results were in the ‘excellent’ and ‘good’ categories.
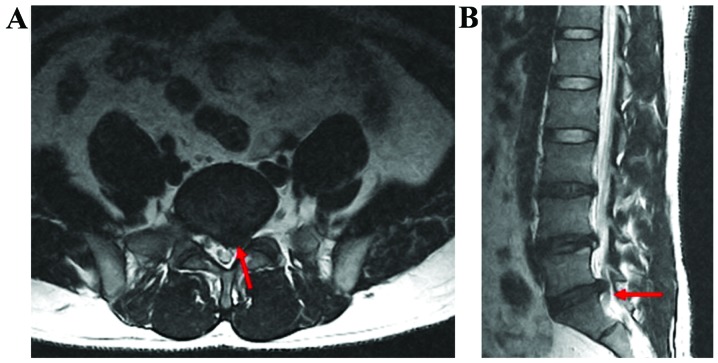
Figure 3.
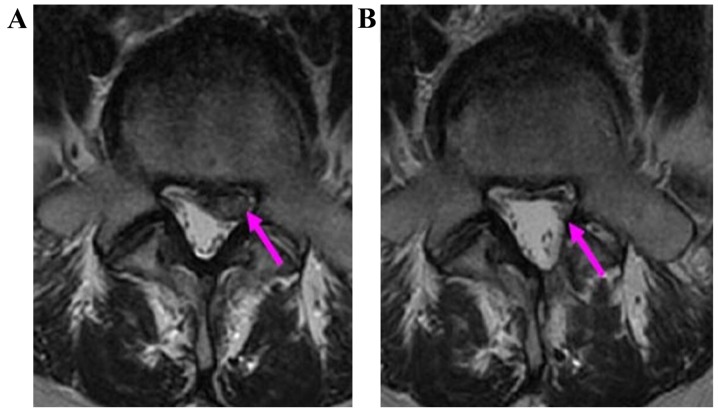
MRI imaging materials for one patient before surgery. (A) Ttransverse section image; (B) saggital section image. Red arrows indicate L5-S1 herniated disk, which is a central type in the front of the spinal canal. MRI, magnetic resonance imaging.
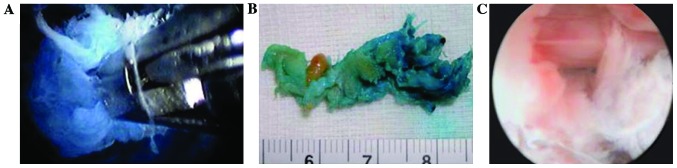
Figure 5.
After release and decompression techniques through PTED, nucleus pulposus pieces are removed and the nerve root is no longer pressured. (A) Removed nucleus pulposus pieces can be seen under transforaminal endoscopic discectomy; (B) removing nucleus pulposus pieces; and (C) nerve root is decompressed after surgery and the structure can be seen clearly under a microscope. PTED, percutaneous transforaminal endoscopic discectomy.
Table I.
Comparison of basic information of 37 LDH patients (n, %).
| Index | n=37 (%) | χ2 value | P-value |
|---|---|---|---|
| Sex | 0.080 | 0.638 | |
| Male | 22 (59) | ||
| Female | 15 (40.1) | ||
| Age (years) | 1.28 | 0.773 | |
| 25 | 10 (27.0) | ||
| 35 | 24 (64.9) | ||
| 45 | 3 (8.1) | ||
| 55 | 0 (0) | ||
| Course of disease (months) | 0.123 | 0.870 | |
| 1 | 5 (13.5) | ||
| 6 | 14 (37.8) | ||
| 12 | 18 (48.6) |
LDH, lumbar disc herniation.
Treatment effects
A total of 37 patients with CLDH who received release and decompression techniques through PTED. VAS and JOA scoring methods were used to evaluate lower limbs (Tables II–IV).
Table II.
VAS and JOA scoring of lower limbs for 37 patients during follow-up periods.
| VAS scoring of lower limbs | Scoring of JOA | ||||||
|---|---|---|---|---|---|---|---|
| Time | n | Mean value | SD | SEM | Mean value | SD | SEM |
| Before surgery | 37 | 7.95 | 0.82 | 0.13 | 12.6 | 0.72 | 0.12 |
| 3 months after surgery | 37 | 2.28 | 0.35 | 0.06 | 20.4 | 1.08 | 0.17 |
| 1 year after surgery | 37 | 2.06 | 0.58 | 0.10 | 21.1 | 0.82 | 0.13 |
| Last follow-up | 37 | 2.12 | 0.23 | 0.38 | 21.2 | 0.36 | 0.10 |
VAS, visual analogue scale; JOA, Japanese Orthopaedic Association; SD, standard deviation; SEM, standard error of the mean.
Table IV.
JOA scoring values of 37 CLDH patients receiving release and decompression techniques through PTED.
| VAS scoring of lower limb | |||||||
|---|---|---|---|---|---|---|---|
| 95% CI | |||||||
| Time | Mean value | SD | SEM | Upper limit | Lower limit | T-value | P-value |
| 3 months after surgery-before surgery | 7.80 | 0.53 | 0.09 | 8.84 | 6.76 | 5.38 | <0.05 |
| 1 year after surgery-before surgery | 8.52 | 0.42 | 0.07 | 9.45 | 7.70 | 6.08 | <0.05 |
| Last follow-up-before surgery | 8.64 | 0.78 | 0.13 | 10.17 | 7.11 | 4.52 | <0.05 |
JOA, Japanese Orthopaedic Association; CLDH, central lumbar disc herniation; PTED, percutaneous transforaminal endoscopic discectomy; CI, confidence interval; SD, standard deviation; SEM, standard error of the mean.
MacNab scoring before and after treatment
Satisfactory MacNab scoring for 37 patients 3 months and 1 year after surgery and at the last follow-up was increased, indicating that treatment effects were of statistical significance (Tables V and VI).
Table V.
MacNab scoring values of 37 CLDH patients receiving release and decompression techniques through PTED.
| Time | Excellent | Good | Fair | Poor | P-value |
|---|---|---|---|---|---|
| Before surgery | 0 | 0 | 2 | 35 | <0.05 |
| 3 months after surgery | 27 | 3 | 7 | 1 | <0.05 |
| 1 year after surgery | 30 | 2 | 5 | 0 | <0.05 |
| Last follow-up | 32 | 1 | 3 | 1 | <0.05 |
CLDH, central lumbar disc herniation; PTED, percutaneous transforaminal endoscopic discectomy.
Table VI.
Effects of release and decompression techniques through PTED on 37 CLDH patients.
| Time | Effectivea | Ineffectiveb | P-value (Fisher) |
|---|---|---|---|
| Before surgery | 0 | 37 | <0.05 |
| 3 months after surgery | 30 | 8 | <0.05 |
| 1 year after surgery | 32 | 5 | <0.05 |
| Last follow-up | 33 | 4 | <0.05 |
Effective, MacNab scores before and after surgery were excellent and good
ineffective, MacNab scores before and after surgery were fair or poor. CLDH, central lumbar disc herniation; PTED, percutaneous transforaminal endoscopic discectomy.
Discussion
Lumbar disc herniation refers to a series of clinical symptoms caused by lumbar intervertebral disk degeneration and the herniation of nucleus pulposus after anular disruption or herniatation until the vertebral plate is negatively affected, causing stimulation or compression of neighboring tissues. Therefore, a series of clinical symptoms are caused such as lumbar pain, numbness and pain of a lower limb on one or both sides occur. Among all cases, the highest incidence is at L4-5 and L5-S1 segments, with a 95% prevalence (11–13). Lumbar disc herniation severely threatens the health of the middle aged and elderly. The main causes are increased pressure, pregnancy, contorted waist position, sudden weight-bearing, catching cold and dampness based on the retrogression of intervertebral disk (12,14). Based on the location of lumbar disc herniation, it can be divided into posterior-lateral herniation, foraminal disc herniation and central herniation (15). CLDH refers to herniated intervertebral disk tissues at the front and central part of the spinal canal. The posterior-lateral intervertebral disk is the weak spot of its anatomical structure, so posterior-lateral herniation is the most common. However, CLDH is also commonly seen clinically, with an incidence rate of 5.4–33.4%. Surgical treatment methods are commonly used for patients after non-effective traditional conservative treatment is attempted (14–16).
With the innovation of spinal endoscopy and the development of surgical instruments, there are over 10 lumbar disc herniation treatment methods. The release and decompression techniques of nerve roots through PTED were developed from the Tessys method of secondary lumbar transforaminal endoscopic discectomy (Thomas Hoogland endoscopic system). In recent years, these techniques have developed from a simple minimally invasive spine surgery technique into broader popularity among physicians in the lumbar transforaminal endoscopic discectomy field. The key to transforaminal endoscopic discectomy is in finding an accurate surgical location and making an accurate puncture. Moreover, clinical symptoms and the location of pain are important references for diagnosis and treatment, along with imaging diagnosis. Based on imaging characteristics and clinical manifestations, an accurate surgical location helps guarantee that working cannula reaches treatment targets to remove the herniated intervertebral disk and nucleus pulposus. Compressed tissues surrounding a nerve root should be cleaned to save space for the nerve root in order to make it sink with dural root sheaths naturally and achieve relaxation and decompression treatment effects (16).
As for surgical instruments, since the appearance of the ‘spiral bone drill’, the safety of the release and decompression techniques in clinical applications has greatly improved (17). Using the spiral bone drill directly over the line of hitch of the entopic vertebral pedicle helps place it into the spinal canal. Its operation is directly within the anterior space of the dural root sheaths, instead of inside the intervertebral disk, resulting in no damage to nerve tissues within the spinal canal. Nerve roots are well exposed in every surgery of this type. Through practice and theoretical innovation, the surgical criteria of proper root release technique can be concluded as follows considering 6 points: i) Space, ensure enough space surrounds the nerve root; ii) collapse, locate the natural depression of the nerve root and dural root sheath; iii) beat, ensure the dural root sheath and nerve root are beating with each heartbeat; iv) blood supply, maintain a rich blood supply on the nerve root; v) slipping, look for slipping of the nerve root, which can be seen during leg-lifting experiments as a positive sign; and vi) symptoms, look for the disappearance of subjective symptoms, which are those only perceptible to the patient (16–18). With the application range of transforaminal endoscopic discectomy growing wider, it likely has broader indications, with no absolute contraindications. It is suitable for further explorative study regarding treatment methods.
Taken together, release and decompression techniques of nerve roots through PTED have wide application, but it requires operators to have certain spine surgery experience and handle indications professionally to reduce complications. It is reported that when patients are suffering from severe lumbar and leg pain, JOA scoring is usually lower than 12 points. Clinical workers should be reminded of extraforaminal, foraminal and dissociative LDH. For narrow nerve root injury, PTED is able to provide a more accurate surgical location, which is good for complete nerve decompression and an improved surgical outcome (19,20).
There were 37 patients with CLDH. After performing release and decompression techniques through PTED, there was only one lower-limb pain case with no obvious improvement. After a two-year follow-up, two cases relapsed. The reasons for these relapses possibly include severe retrogression of the intervertebral disk, less moisture content, incomplete nucleus pulposus removal, poor surgical location and potential undetermined unstable conditions. For the two relapsed cases, although the patients were considered for second release and decompression techniques through PTED, a laminectomy (fenestration) was performed on single or both sides after considering the safety and treatment effects and getting permission from the patients or their family members (11,12,21). For the remaining 34 cases, lower limb pain symptoms improved among them, and their quality of life improved as well. Total laminectomy surgery was performed on patients with spinal canal stenosis (21,22). Good effects were achieved after surgery. In previous studies, removal of herniated target points under transforaminal endoscopic discectomy and the use of ozone injections inside and outside of lumbar disks were demonstrated (23). During minimally invasive surgery of transforaminal endoscopic discectomy, there was improvement of liquid nucleus pulposus retention caused by annulus fibrosus repair. Moreover, stability of the spine was not influenced by removing normal nucleus pulposus, and the prognosis was not influenced (24–26). In addition, although transforaminal endoscopic discectomy has significant advantages, the surgical location is not precise. Plus, extraforaminal and dissociated lumbar disc herniation may occur; therefore, this kind of discectomy should be used cautiously. In conclusion, basic treatment principles should guide all surgeries, including striving for no negative effects, no recurrence and no extra medical costs - all while also improving the patient's quality of life (27–30). Patients receiving release and decompression techniques through PTED were all suffering from CLDH. Their clinical symptoms and imaging diagnoses were accurate. Based on advanced surgical equipment and present medical conditions, this minimally invasive spine surgery technique has become a trend of the future. It is believed that in the near future, this minimally invasive treatment method will become the prevailing way to treat CLDH.
Figure 4.
CT plain scan imaging of patients before and after surgery. (A) Before surgery, the herniated part is at the front of the spinal canal; (B) after surgery, removing the pressured objects, then normal space of the nerve root is recovered and clinical symptoms of the patient are improved. Purple arrows indicate the compression site. CT, computed tomography.
Table III.
VAS scoring values of 37 CLDH patients receiving release and decompression techniques through PTED.
| VAS scoring of lower limb | |||||||
|---|---|---|---|---|---|---|---|
| 95% CI | |||||||
| Time | Mean value | SD | SEM | Upper limit | Lower limit | T-value | P-value |
| Before surgery-3 months after surgery | 5.67 | 0.63 | 0.10 | 6.90 | 4.44 | 3.38 | <0.05 |
| Before surgery-1 year after surgery | 5.89 | 0.52 | 0.09 | 6.91 | 4.87 | 2.31 | <0.05 |
| Before surgery-last follow-up | 5.83 | 0.38 | 0.06 | 6.57 | 5.08 | 4.12 | <0.05 |
VAS, visual analogue scale; CLDH, central lumbar disc herniation; PTED, percutaneous transforaminal endoscopic discectomy; CI, confidence interval; SD, standard deviation; SEM, standard error of the mean.
References
- 1.Bärlocher CB, Krauss JK, Seiler RW. Central lumbar disc herniation. Acta Neurochir (Wien) 2000;142:1369–1374. doi: 10.1007/s007010070007. [DOI] [PubMed] [Google Scholar]
- 2.Topuz K, Eroglu A, Simsek H, Atabey C, Cetinkal A, Colak A. Demographical aspects of central large lumbar disc herniation. Turk Neurosurg. 2016;26:111–118. doi: 10.5137/1019-5149.JTN.7320-12.2. [DOI] [PubMed] [Google Scholar]
- 3.Fager CA. Role of laminectomy in lumbar disk herniation. Mt Sinai J Med. 1991;58:133–138. [PubMed] [Google Scholar]
- 4.Chen HC, Lee CH, Wei L, Lui TN, Lin TJ. Comparison of percutaneous endoscopic lumbar discectomy and open lumbar surgery for adjacent segment degeneration and recurrent disc herniation. Neurol Res Int. 2015;2015:791943. doi: 10.1155/2015/791943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valen B, Rolfsen LC. Complications of lumbar disk herniation surgery. Tidsskr Nor Laegeforen. 2003;123:640–641. (In Norwegian) [PubMed] [Google Scholar]
- 6.Cristante AF, Rocha ID, MartusMarcon R, Filho TE. Randomized clinical trial comparing lumbar percutaneous hydrodiscectomy with lumbar open microdiscectomy for the treatment of lumbar disc protrusions and herniations. Clinics (Sao Paulo) 2016;71:276–280. doi: 10.6061/clinics/2016(05)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payer M. ‘Minimally invasive’ lumbar spine surgery: a critical review. Acta Neurochir (Wien) 2011;153:1455–1459. doi: 10.1007/s00701-011-1023-4. [DOI] [PubMed] [Google Scholar]
- 8.Kim CH, Chung CK, Jahng TA, Yang HJ, Son YJ. Surgical outcome of percutaneous endoscopic interlaminar lumbar diskectomy for recurrent disk herniation after open diskectomy. J Spinal Disord Tech. 2012;25:E125–E133. doi: 10.1097/BSD.0b013e31825bd111. [DOI] [PubMed] [Google Scholar]
- 9.Cong L, Zhu Y, Tu G. A meta-analysis of endoscopic discectomy versus open discectomy for symptomatic lumbar disk herniation. Eur Spine J. 2016;25:134–143. doi: 10.1007/s00586-015-3776-6. [DOI] [PubMed] [Google Scholar]
- 10.Kim CH, Chung CK, Sohn S, Lee S, Park SB. The surgical outcome and the surgical strategy of percutaneous endoscopic discectomy for recurrent disk herniation. J Spinal Disord Tech. 2014;27:415–422. doi: 10.1097/BSD.0b013e3182a180fc. [DOI] [PubMed] [Google Scholar]
- 11.Kitagawa Y, Sairyo K, Shibuya I, Kitahama Y, Kanamori Y, Koga S, Matsumoto H, Sumita T, Yamada A, Dezawa A. Minimally invasive and simultaneous removal of herniated intracanal and extracanal lumbar nucleus pulposus with a percutaneous spinal endoscope. Asian J Endosc Surg. 2012;5:183–186. doi: 10.1111/j.1758-5910.2012.00144.x. [DOI] [PubMed] [Google Scholar]
- 12.Wood KB, Devine J, Fischer D, Dettori JR, Janssen M. Vascular injury in elective anterior lumbosacral surgery. Spine. 2010;35(Suppl 9):S66–S75. doi: 10.1097/BRS.0b013e3181d83411. [DOI] [PubMed] [Google Scholar]
- 13.Dragojlovic N, Stampas A, Kitagawa RS, Schmitt KM, Donovan W. Communicating hydrocephalus due to traumatic lumbar spine injury: case report and literature review. Am J Phys Med Rehabil. 2016 doi: 10.1097/PHM.0000000000000540. Jun 17. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.Nellensteijn J, Ostelo R, Bartels R, Peul W, van Royen B, van Tulder M. Transforaminal endoscopic surgery for symptomatic lumbar disc herniations: a systematic review of the literature. Eur Spine J. 2010;19:181–204. doi: 10.1007/s00586-009-1272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yunhong H, Yan L, Chengen Z, Honghai Z. Progress on location study of Hu Yougu zone of lumbar disc herniation. Chinese Journal of Traditional Medical Traumatology and Orthopedics. 2013;8:68–70. (In Chinese) [Google Scholar]
- 16.Sencer A, Yorukoglu AG, Akcakaya MO, Aras Y, Aydoseli A, Boyali O, Sencan F, Sabanci PA, Gomleksiz C, Imer M, et al. Fully endoscopic interlaminar and transforaminal lumbar discectomy: short-term clinical results of 163 surgically treated patients. World Neurosurg. 2014;82:884–890. doi: 10.1016/j.wneu.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Nunley P, Sandhu F, Frank K, Stone M. Neurological complications after lateral transpsoas approach to anterior interbody fusion with a novel flat-blade spine-fixed retractor. BioMed Res Int. 2016;2016:8450712. doi: 10.1155/2016/8450712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith N, Masters J, Jensen C, Khan A, Sprowson A. Systematic review of microendoscopic discectomy for lumbar disc herniation. Eur Spine J. 2013;22:2458–2465. doi: 10.1007/s00586-013-2848-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattei TA, Rehman AA, Teles AR, Aldag JC, Dinh DH, McCall TD. The ‘Lumbar Fusion Outcome Score’ (LUFOS): a new practical and surgically oriented grading system for preoperative prediction of surgical outcomes after lumbar spinal fusion in patients with degenerative disc disease and refractory chronic axial low back pain. Neurosurg Rev. 2016;1:1–15. doi: 10.1007/s10143-016-0751-6. [DOI] [PubMed] [Google Scholar]
- 20.Yongjun Z, Le Y, Xiangrui W. Short-term outcomes of percutaneous transforaminal endoscopic discectomy for the treatment of lumbar disc herniation in 30 patients. Shanghai Med J. 2012;6:473–475. (In Chinese) [Google Scholar]
- 21.Hikata T, Kamata M, Furukawa M. Risk factors for adjacent segment disease after posterior lumbar interbody fusion and efficacy of simultaneous decompression surgery for symptomatic adjacent segment disease. J Spinal Disord Tech. 2014;27:70–75. doi: 10.1097/BSD.0b013e31824e5292. [DOI] [PubMed] [Google Scholar]
- 22.Birkenmaier C, Komp M, Leu HF, Wegener B, Ruetten S. The current state of endoscopic disc surgery: review of controlled studies comparing full-endoscopic procedures for disc herniations to standard procedures. Pain Physician. 2013;16:335–344. [PubMed] [Google Scholar]
- 23.Pan Z, Ha Y, Yi S, Cao K. Efficacy of transforaminal endoscopic spine system (TESSYS) technique in treating lumbar disc herniation. Med Sci Monit. 2016;22:530–539. doi: 10.12659/MSM.894870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei J, Li W, Pei Y, Shen Y, Li J. Clinical analysis of preoperative risk factors for the incidence of deep venous thromboembolism in patients undergoing posterior lumbar interbody fusion. J Orthop Surg. 2016;11:68. doi: 10.1186/s13018-016-0403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korse NS, Jacobs WC, Elzevier HW, Vleggeert-Lankamp CL. Complaints of micturition, defecation and sexual function in cauda equina syndrome due to lumbar disk herniation: a systematic review. Eur Spine J. 2013;22:1019–1029. doi: 10.1007/s00586-012-2601-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn Y. Transforaminal percutaneous endoscopic lumbar discectomy: technical tips to prevent complications. Expert Rev Med Devices. 2012;9:361–366. doi: 10.1586/erd.12.23. [DOI] [PubMed] [Google Scholar]
- 27.Cho JY, Lee SH, Lee HY. Prevention of development of postoperative dysesthesia in transforaminal percutaneous endoscopic lumbar discectomy for intracanalicular lumbar disc herniation: floating retraction technique. Minim Invasive Neurosurg. 2011;54:214–218. doi: 10.1055/s-0031-1287774. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Yang L, Chen J, Xie H, Tian W, Cao X. Magnetic resonance imaging-based anatomical study of the multifidus-longissimus cleavage planes in the lumbar spine. Am J Transl Res. 2016;8:109–116. [PMC free article] [PubMed] [Google Scholar]
- 29.Gibson JN, Cowie JG, Iprenburg M. Transforaminal endoscopic spinal surgery: the future ‘gold standard’ for discectomy? - A review. Surgeon. 2012;10:290–296. doi: 10.1016/j.surge.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Schleich C, Müller-Lutz A, Eichner M, Schmitt B, Matuschke F, Bittersohl B, Zilkens C, Wittsack HJ, Antoch G, Miese F. Glycosaminoglycan chemical exchange saturation transfer of lumbar intervertebral discs in healthy volunteers. Spine. 2016;41:146–152. doi: 10.1097/BRS.0000000000001144. [DOI] [PubMed] [Google Scholar]