Abstract
Purpose
Controversies remain surrounding the choice of hyaluronic acid products and patient selection. A study was conducted to report the long-term survivorship of intra-articular injection effect of high molecular weight hyaluronic preparation hylan GF-20 (Synvisc-One) for patients with symptomatic knee osteoarthritis.
Materials and Methods
A retrospective observational analysis of a single therapeutic series was carried out. The analysis was conducted to determine therapeutic effect survivorship taking arthroplasty and any other surgical interventions as endpoint results.
Results
Seventy-seven consecutive patients (82 knees) were followed up for five years. At one-year follow-up, 71 knees (87%) responded to treatment and only 8 knees (10%) were offered arthroplasty due to persistence of symptoms. At five-year follow-up, 41 (50%) were still considered responders. During the study period, repeat injection was given in 9 knees (11%). Arthroplasty (either total or unicompartmental) was required in 26 (31%). Kaplan-Meier survivorship analysis of therapeutic effect demonstrated 67% survival at 5 years with arthroplasty as endpoint and 58% survival at 5 years with all secondary interventions as endpoint.
Conclusions
This study demonstrates a significantly longer duration of clinical benefit of hylan GF-20 injection. Present results may suggest a notion of an ideal delay therapeutic strategy for patients not ready to receive an arthroplasty. Further studies will be required to help characterise these subsets of patients.
Keywords: Knee, Osteoarthritis, Intra-articular injections, Viscosupplementation, Hyaluronic acid, Treatment outcome, Survival
Introduction
Osteoarthritis (OA) represents one of the most important causes of musculoskeletal disability and is a major burden to the health-care system. In the United Kingdom (UK), 8.5 million people have pain reportedly attributed to arthritis1). It results in 36 million working days lost annually in the UK and an estimated 115,000 hospital admissions1). Knee OA is additionally the most common form of OA causing disability in the UK. The symptomatic treatment of OA focuses mainly on physical therapy, analgesics, non-steroidal anti-inflammatory drugs (NSAIDs) and intra-articular injections of corticosteroids2). However, the side-effects of NSAIDs and intra-articular steroid injections have directed the interest towards alternative forms of treatment, such as viscosupplementation with hyaluronic acid (HA)3,4).
HA has been shown to increase elasticity and reduce viscosity in high shear, as well as reduce elasticity and increase viscosity in low shear stress5–7). In addition to mechanical properties, experimental studies have shown a chondro-protective effect attributed to antiprotease and antiapoptotic actions as well as an upregulation of endogenous HA8,9). Kim et al.10) demonstrated improved osteochondral defect healing in animal models treated with intraarticular HA. Both native and cross-linked hyaluronan are being used for the treatment of human OA. Lower molecular weight preparations (e.g. Artzal, Fass-verksamheten, Stockholm, Sweden) generally range in molecular weight from 0.5 to 1 million dalton, while the molecular weight of cross-linked preparations (e.g. Synvisc-One, Sanofi-Aventis, Ridgefield, NJ, USA) is considerably higher at 6,000 kilodaltons (kDa)11,12). New evidence has recently also demonstrated the relative importance of molecular weight and the concentration of hyaluronan for its efficacy13). Even though previous trials have reported that intra-articular hyaluronan is a safe and well-tolerated treatment, controversies remain surrounding choice of products and patient selection14–17). In addition, there has been paucity of studies demonstrating longer duration of clinical benefit following viscosupplementation in the treatment of knee OA18). Consequently, conditional recommendations have been put forward in relation to its utilisation according to a variety of specialist societies such as Osteoarthritis Research Society International, the European League Against Rheumatism and the American College of Rheumatology, and the American Academy of Orthopedic Surgeons19). Reviewed recommendations also suggest attention to patient selection due to different phenotypes of OA19).
A retrospective observational analysis of a single centre single therapeutic clinical series was conducted in order to evaluate long-term results following intra-articular injections of high molecular weight hyaluronic preparation hylan GF-20 (Synvisc-One) in selected patients with symptomatic knee OA. The objective of the study was to report the five-year therapeutic effect survivorship taking knee arthroplasty and any other surgical intervention as endpoints.
Materials and Methods
From 2010 to 2011 a total of 77 consecutive patients received Synvisc-One knee intra-articular injection carried out in a specialist orthopaedic knee outpatient clinic. Selection criteria were derived from a multidisciplinary management algorithm. All patients were treated with initial medical management of OA, which includes weight loss, analgesia and activity modification advice. Inclusion criteria consisted of symptomatic knee OA and radiologically confirmed disease on standard weight bearing knee radiographic views. All patients included had confirmed arthritic symptoms following clinical evaluation in the knee clinic. In addition to those having radiographs confirming tibiofemoral compartment location of disease, patients not medically fit for surgery, considered too young for arthroplasty, or patients whose occupation would have precluded them from having an arthroplasty were included in the intervention protocol. Using data from our National Joint Replacement Registry (NJR), the categories too-young and not-fit for surgery were respectively benchmarked at <40 years and American Society of Anaesthesiologists grade 4 (severe systemic disease that is a constant threat to life). The latter categories both represented an outlying cohort of <1% on the NJR database20).
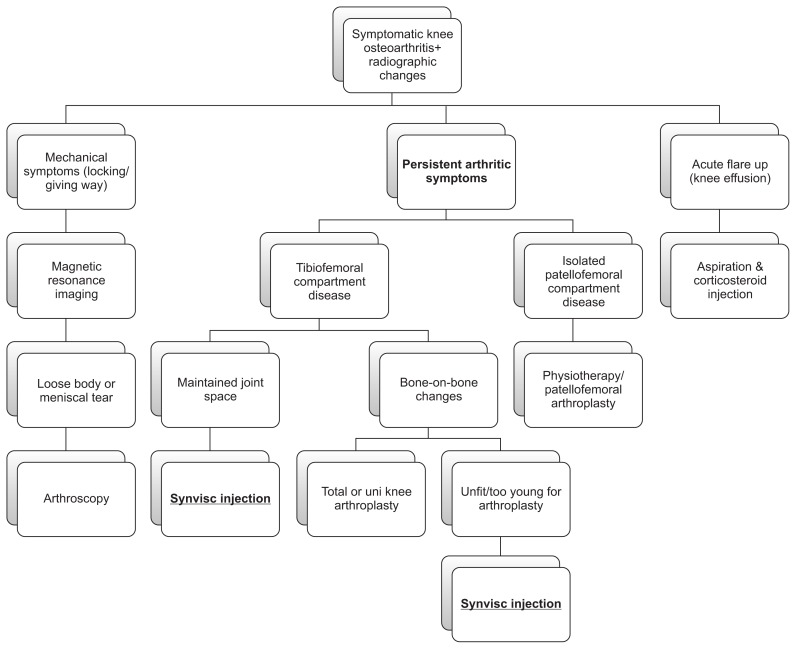
Exclusion criteria consisted of patients with predominantly mechanical symptoms and well preserved joint who required further imaging in the form of magnetic resonance imaging and progression to arthroscopic treatment where indicated. Acute cases of flare-up were treated with aspiration and injection of intra-articular corticosteroid and subsequently managed according to the management algorithm outlined in Fig. 1. In addition, patients with a predominantly patellofemoral location of disease were considered for physiotherapy or patellofemoral arthroplasty depending on the extent of functional loss and severity of degenerative changes. Rheumatoid arthritis or other inflammatory joint diseases were excluded. Furthermore, patients were not included if they had a known allergy to any substance related to the injection. The study analysis included only patients who were treated with Synvisc-One from the outset.
Fig. 1.
Symptomatic knee osteoarthritis management algorithm.
The HA injection used was hylan GF-20 with a molecular weight of 6,000 kDa (Sanofi-Aventis). The technique for injection followed a standardised method of aseptic no touch technique. The skin is prepared with alcoholic chlorhexidine and allowed to air dry. The knee joint is positioned in extension and the injection portal follows the patellofemoral compartment to allow easy access to the anterior portion of the joint space. The injection is carried out using a prefilled sterile packed syringe and ‘white’ hypodermic needle. The injection site is covered with a simple dry dressing and the patient is allowed to mobilise fully and discharged back to primary care. Repeat of injection was not routinely carried out unless patients experienced symptomatic benefit for a minimum period of nine months following the initial injection. As per our local protocol, if there was no response or limited response, patients needed to be referred back to secondary care to consider other surgical interventions.
Baseline characteristics and diagnostic data were recorded at the initial visit and patients were entered into a prospectively collected database for evaluation of clinical outcomes at one year and at five years after the initial injection. The primary efficacy parameter at one year and during the total five-year duration of the study was defined as absence of subsequent referral to secondary care other than for repeat Synvisc-One injection. These patients were defined as responders to the injection therapeutic effect if they had not returned for a secondary intervention excluding repeat Synvisc-One injection. The parameters were recorded from the hospital electronic medical record. If no hospital records were identified, clinical data was checked with the primary care general practice in case the patients had re-presented with knee symptoms and/or referred to another centre. The secondary efficacy parameter consisted of time to clinical failure as defined by the need for secondary treatment for the study knee excluding repeat Synvisc-One injection. Secondary interventions included arthroscopic procedures or arthroplasty during the study period as well as patients awaiting arthroplasty surgery at the end of the five-year study period. Similarly, these parameters were recorded from the trust electronic medical record and checked with the primary care practice in case patients were referred for treatment in another centre in order to reduce errors and loss of cases. All patients who received the initial intervention were accounted for at five years of follow-up. Finally, duration of clinical benefit was measured using survival analysis taking arthroplasty and all secondary interventions as endpoint results. Safety of injections was evaluated by the presence of any adverse reaction immediately after the treatment and the presence of any subsequent complications related to the injection.
In terms of ethical provisions, the study was conducted in accordance with the principles of good clinical practice and in accordance with the declaration of Helsinki, and was approved by the trust audit department (ID/647). Approval for product utilisation had been obtained from the regional clinical commissioning group and regional prescribing committee. Results were initially collated online and tabulated using Excel 2010 (Microsoft, Redmond, WA, USA). Multiple regression analysis was used to assess correlation between baseline characteristics and clinical outcomes. Kaplan-Meier survivorship analysis was performed to evaluate duration of clinical benefit.
Results
A total of 77 consecutive patients (82 knees) were eligible for the intervention and had clinical outcome measures recorded and follow-up of outcomes at one year and five years following initial injection of Synvisc-One. The cohort comprised 36 male (47%) and 41 female (53%) patients. The mean age at the time of index intervention was 58 years (standard deviation [SD], 11.9; range, 32 to 88 years) (95% confidence interval, 51.5–73.2). Multivariate regression analyses of the baseline characteristics and potential confounding factors were carried out. There was no significant relationship between gender and clinical outcomes in the form of secondary interventions (p=0.41). Mean ages per intervention group were: 58.8 years (SD, 13) in the arthroplasty group; 57.5 years (SD, 14.9) among patients who had no further interventions; and 57.9 years (SD, 9.4) among patients who had a repeat injection. There was no significant difference in mean age in the different clinical outcome groups at five-year follow-up for any subsequent intervention (p=0.72) and for all interventions grouped together (p=0.64).
At one year, 71 (87%) of knees, which received the intervention, responded to treatment and only 8 (10%) were listed for arthroplasty due to persistence of symptoms. Within the responders, a total of 53 (65%) were reviewed and 18 (22%) were discharged. According to our protocol, these cases were considered responders in terms of primary therapeutic efficacy. At five-year follow-up, 41 (50%) of knees, which received the initial injection, were classed as responders. These included 32 (39%) asymptomatic and 9 (11%) who required a repeat of one injection only as per protocol. All cases which received a repeat injection did so within the time frame of six to nine months postulated in the original treatment plan. During the five-year period, 26 (31%) required arthroplasty, either total or unicompartmental. Clinical outcomes are outlined in Table 1. Within secondary surgical procedures carried out during the study period, there was one arthroscopic microfracture at one year and two arthroscopic debridements of meniscal tears at five years due to development of mechanical symptoms. There were no records of adverse reactions to the injections reported during the study period. One patient was not available for follow-up at one year but was later identified and reviewed at five years. This particular case did not require further intervention and remained asymptomatic at five years.
Table 1.
Outcomes at 1 Year and 5 Years of Follow-up
| Variable | No. (%) |
|---|---|
| Outcomes at 1 year | |
| Responders | 71 (87) |
| Waiting list arthroplasty | 8 (10) |
| Arthroscopic procedure | 1 (1) |
| Reviewed+symptomatic | 1 (1) |
| No follow-up records | 1 (1) |
| Outcomes at 5 years | |
| Responders | 41 (50) |
| Total knee arthroplasty | 19 (23) |
| Unicompartmental knee arthroplasty | 6 (7) |
| Patellofemoral joint arthroplasty | 1 (1) |
| Waiting list arthroplasty | 5 (6) |
| Symptomatic | 3 (4) |
| Arthroscopic procedure | 2 (3) |
| Patient deceased | 5 (6) |
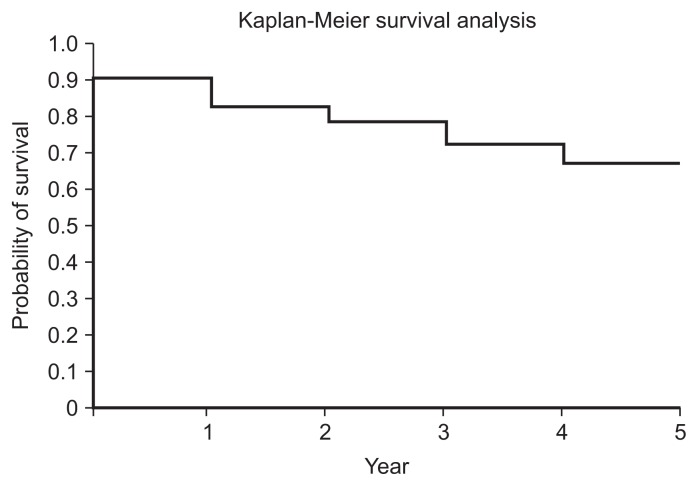
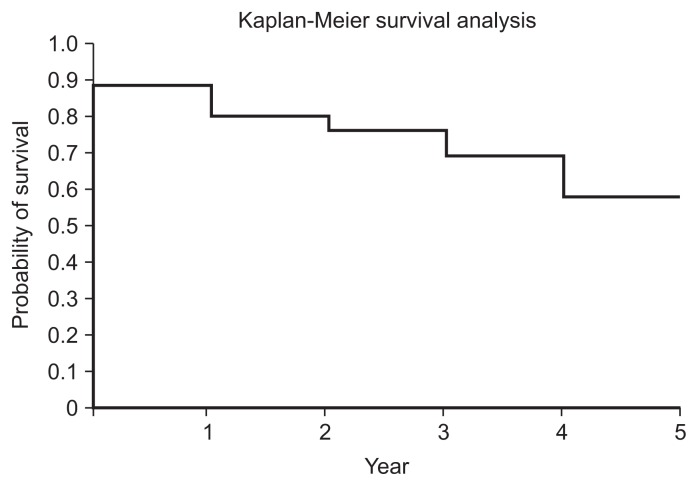
Kaplan-Meier failure-time curves were used to analyse the cumulative probability of patients not requiring additional treatment for their study knee during the follow-up period. The analysis demonstrated 67% survival at 5 years with arthroplasty as the endpoint and 58% survival at 5 years with all secondary interventions, excluding repeat Synvisc-One injection, as the endpoint (Tables 2 and 3, Figs. 2 and 3). Throughout the five-year study period, there were no adverse reaction or long-term complications related to the injection of HA.
Table 2.
Cumulative Probability of Patients Not Requiring Arthroplasty
| Period (yr) | At risk | Censored | Arthroplasty | Survived | Kaplan-Meier (%) |
|---|---|---|---|---|---|
| 1 | 80 | 2 | 8 | 72 | 0.90 |
| 2 | 71 | 1 | 6 | 65 | 0.82 |
| 3 | 63 | 2 | 3 | 60 | 0.79 |
| 4 | 60 | 0 | 5 | 55 | 0.72 |
| 5 | 55 | 0 | 4 | 51 | 0.67 |
Values are presented as number.
Table 3.
Cumulative Probability of Patients Not Requiring Any Secondary Interventiona)
| Period (yr) | At risk | Censored | Secondary intervention | Survived | Kaplan-Meier (%) |
|---|---|---|---|---|---|
| 1 | 80 | 2 | 10 | 70 | 0.88 |
| 2 | 69 | 1 | 6 | 63 | 0.80 |
| 3 | 61 | 2 | 3 | 58 | 0.76 |
| 4 | 58 | 0 | 5 | 53 | 0.69 |
| 5 | 53 | 0 | 9 | 44 | 0.58 |
Values are presented as number.
Excluding repeat Synvisc-One injections.
Fig. 2.
Therapeutic effect survivorship analysis with arthroplasty as the endpoint.
Fig. 3.
Therapeutic effect survivorship analysis with any secondary intervention as the endpoint.
Discussion
The pain-relieving mechanism of intra-articular injections of HA is yet to be fully elucidated. It has been suggested that the injections may stimulate the synthesis of endogenous HA and act as a scavenger, reducing the amount of inflammatory degradation products in the joint. Furthermore, the viscoelastic and anti-inflammatory functions of the synovial fluid may be improved by the treatment. The utilisation of this treatment has been closely monitored within our unit. The present study constitutes a detailed long-term analysis of consecutive patients treated by a multidisciplinary team and followed up for five years. The principal findings were the long-term efficacy and safety of intra-articular injections of hyaluronic preparation (Synvisc-One/hylan GF-020) for patients with symptomatic knee OA. The present study demonstrates a significantly longer duration of clinical benefit. One third of patients required arthroplasty surgery and over half of the patients did not require any further secondary intervention at five years of follow-up. In a pragmatic setting, our primary and secondary efficacy parameters support the use of this intervention in adequately selected patients with purely arthritic symptoms, who are either ‘not ready’ or ‘not willing’ to undergo joint replacement surgery.
The subject of HA injection in the treatment of OA has been vastly studied. Within the literature a large number of clinical trials, systematic reviews and meta-analyses had sought to answer the question of HA injection efficacy and how it compares to other treatment modalities. Wang et al.15) conducted a meta-analysis, which confirmed the therapeutic efficacy and safety of intraarticular injection of HA for the treatment of OA of the knee. Bellamy et al.16) demonstrated a therapeutic effect superior to placebo but not significantly different to NSAIDs or intra-articular corticosteroid injections. This meta-analysis, which included 76 trials looking at 20 different HA products, echoes our results of sustained efficacy over a long period of time in adequately selected patients. Lo et al.17) demonstrated a moderate therapeutic effect when compared to placebo in a meta-analysis of 22 selected trials. Results were more significant with high molecular weight HA injections17). Nevertheless, publication bias and heterogeneity of the studies included mitigated these results. Similar results were widely reported in the international literature, with added evidence of favourable cost-effectiveness in the utilisation of HA injection for symptomatic arthritic “dry” knees21,22). A more recent meta-analysis reported similar methodological limitations. The authors suggested a significant association between cross-linked hyaluronan and long-term results, yet not beyond the two-year mark18). A recent randomised controlled trial compared hylan GF-20 single shot injection with corticosteroid injection. The authors reported that both groups had similar improvement in pain, knee function, and range of motion during the 6-month follow-up (p<0.001)23,24). In contrast, an earlier study by Anand et al.25) showed that multiple-dose injections of HA when given in advanced arthritis lasted for up to three years and delayed the need for knee arthroplasty in 58% of the cases.
The scope of our clinical series was not to replicate the results previously published in the literature. Our present study adds valuable observations, which can easily be applied in other institutions. In adequately selected patients with knee OA who are not medically fit or ready for arthroplasty according to the criteria defined above and reports from the NJR database20), Synvisc-One injection can constitute a satisfactory treatment modality even in the long-term. Patient selection remains a difficult challenge. We demonstrated results following the implementation of a rigorous management algorithm. We believe that a more liberal approach could have attenuated the overall therapeutic effect within this patient population. Factors predicting long-term efficacy of injection have been previously reported. Conrozier et al.26) reported results at 14 months with a 78% global therapeutic effect lasting up to that point. Factors significantly associated with a favourable response in their series were: moderate effusion, injection lateral to the patella, joint space loss in a single compartment, and radiological meniscal calcinosis26).
All studies investigating the efficacy of HA injections appear to share numerous and recurring limitations such as: sample size, clinical environment, outcome measures, disease duration, radiological grade, and rescue medications. Even in the most controlled environment, such factors will inadvertently mitigate the interpretation of evidence reported. The present study followed a pragmatic approach, reflecting a normal clinical environment to which patients are exposed. The principal limitation of the present study was therefore the lack of filter for all possible confounding factors, which could influence the results of treatment. Nevertheless, it is the authors’ viewpoint that this contributes to evidence for the efficacy of HA injection within this heterogeneous patient population. It is often difficult to translate evidence extracted from the very tightly regulated environment of randomised controlled studies and apply to the realism of everyday practice. It can be attributed to the very fact that outside the controlled environment represented in such studies, patients are exposed to numerous confounding factors. This is especially true for knee OA, a condition with a complex natural history. Consequently, our results characterise a pragmatic approach to the question. The efficacy parameters reported above may have been heavily influenced by external factors. However, when patients are adequately selected, such approach adds external validity to the results reported in our study. Instead of becoming a single therapeutic answer, the long-term results presently reported can become an addition in the armamentarium of the orthopaedic surgeon when discussing treatment options with their patients.
Conclusions
The majority of patients with symptomatic knee OA who were treated with hylan GF-20 injection using our protocol showed clinical improvement. Our treatment algorithm incorporating a single product Synvisc-One injection demonstrated long-term effect when arthroplasty and any other surgical intervention were measured as endpoints. This therapeutic series demonstrates a significantly longer duration of clinical benefit for Synvisc-One injection compared to previous studies. Only a third of patients required arthroplasty surgery and over half of the patients did not require any further surgical intervention at five-year follow-up. Results are echoed in the literature, most likely attributed to molecular weight of preparation and improved patient selection. These results can suggest a notion of an ideal delay therapeutic strategy for patients not ready to receive an arthroplasty. The present study should also pave the way for further research with attention to product and patient selection, in order to determine whether such a pragmatic approach can be widely implemented.
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.National Institute for Health and Clinical Excellence. Osteoarthritis: care and management [Internet] London: National Institute for Health and Clinical Excellence; 2014. [cited 2016 Aug 26]. Available from: https://www.nice.org.uk/Guidance/cg177. [Google Scholar]
- 2.Garcia Rodríguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994;343:769–72. doi: 10.1016/S0140-6736(94)91843-0. [DOI] [PubMed] [Google Scholar]
- 3.Rashad S, Revell P, Hemingway A, Low F, Rainsford K, Walker F. Effect of non-steroidal anti-inflammatory drugs on the course of osteoarthritis. Lancet. 1989;2:519–22. doi: 10.1016/S0140-6736(89)90651-X. [DOI] [PubMed] [Google Scholar]
- 4.Bradley JD, Brandt KD, Katz BP, Kalasinski LA, Ryan SI. Treatment of knee osteoarthritis: relationship of clinical features of joint inflammation to the response to a nonsteroidal antiinflammatory drug or pure analgesic. J Rheumatol. 1992;19:1950–4. [PubMed] [Google Scholar]
- 5.Ghosh P, Read R, Armstrong S, Wilson D, Marshall R, McNair P. The effects of intraarticular administration of hyaluronan in a model of early osteoarthritis in sheep. I. Gait analysis and radiological and morphological studies. Semin Arthritis Rheum. 1993;22(6 Suppl 1):18–30. doi: 10.1016/S0049-0172(10)80016-2. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig TE, McAllister JR, Lun V, Wiley JP, Schmidt TA. Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: restoration through proteoglycan 4 supplementation. Arthritis Rheum. 2012;64:3963–71. doi: 10.1002/art.34674. [DOI] [PubMed] [Google Scholar]
- 7.Ludwig TE, Hunter MM, Schmidt TA. Cartilage boundary lubrication synergism is mediated by hyaluronan concentration and PRG4 concentration and structure. BMC Musculoskelet Disord. 2015;16:386. doi: 10.1186/s12891-015-0842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh P, Read R, Numata Y, Smith S, Armstrong S, Wilson D. The effects of intraarticular administration of hyaluronan in a model of early osteoarthritis in sheep. II: cartilage composition and proteoglycan metabolism. Semin Arthritis Rheum. 1993;22(6 Suppl 1):31–42. doi: 10.1016/S0049-0172(10)80017-4. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira MZ, Albano MB, Namba MM, da Cunha LA, de Lima Gonçalves RR, Trindade ES, Andrade LF, Vidigal L. Effect of hyaluronic acids as chondroprotective in experimental model of osteoarthrosis. Rev Bras Ortop. 2014;49:62–8. doi: 10.1016/j.rbo.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SS, Kang MS, Lee KY, Lee MJ, Wang L, Kim HJ. Therapeutic effects of mesenchymal stem cells and hyaluronic Acid injection on osteochondral defects in rabbits’ knees. Knee Surg Relat Res. 2012;24:164–72. doi: 10.5792/ksrr.2012.24.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall KW. The current status of hylan therapy for the treatment of osteoarthritis. Todays Ther Trends. 1997;15:99–108. [Google Scholar]
- 12.Aviad AD, Houpt JB. The molecular weight of therapeutic hyaluronan (sodium hyaluronate): how significant is it? J Rheumatol. 1994;21:297–301. [PubMed] [Google Scholar]
- 13.Puttick MP, Wade JP, Chalmers A, Connell DG, Rangno KK. Acute local reactions after intraarticular hylan for osteoarthritis of the knee. J Rheumatol. 1995;22:1311–4. [PubMed] [Google Scholar]
- 14.Karlsson J, Sjogren LS, Lohmander LS. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis: a controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology (Oxford) 2002;41:1240–8. doi: 10.1093/rheumatology/41.11.1240. [DOI] [PubMed] [Google Scholar]
- 15.Wang CT, Lin J, Chang CJ, Lin YT, Hou SM. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee: a meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2004;86:538–45. doi: 10.2106/00004623-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;(2):CD005321. doi: 10.1002/14651858.CD005321.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA. 2003;290:3115–21. doi: 10.1001/jama.290.23.3115. [DOI] [PubMed] [Google Scholar]
- 18.Jevsevar D, Donnelly P, Brown GA, Cummins DS. Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. J Bone Joint Surg Am. 2015;97:2047–60. doi: 10.2106/JBJS.N.00743. [DOI] [PubMed] [Google Scholar]
- 19.Migliore A, Bizzi E, Herrero-Beaumont J, Petrella RJ, Raman R, Chevalier X. The discrepancy between recommendations and clinical practice for viscosupplementation in osteoarthritis: mind the gap! Eur Rev Med Pharmacol Sci. 2015;19:1124–9. [PubMed] [Google Scholar]
- 20.National Joint Registry. National Joint Registry for England and Wales: 13th annual report [Internet] Hemel Hempstead: National Joint Registry; 2016. [cited 2016 Aug 26]. Available from: http://www.njrcentre.org.uk/njrcentre/Reports,PublicationsandMinutes/Annualreports/tabid/86/Default.aspx. [Google Scholar]
- 21.Uthman I, Raynauld JP, Haraoui B. Intra-articular therapy in osteoarthritis. Postgrad Med J. 2003;79:449–53. doi: 10.1136/pmj.79.934.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahan A, Lleu PL, Salin L. Prospective randomized study comparing the medicoeconomic benefits of hylan GF-20 vs. conventional treatment in knee osteoarthritis. Joint Bone Spine. 2003;70:276–81. doi: 10.1016/S1297-319X(03)00043-5. [DOI] [PubMed] [Google Scholar]
- 23.Tammachote N, Kanitnate S, Yakumpor T, Panichkul P. Intra-articular, single-shot hylan GF-20 hyaluronic acid injection compared with corticosteroid in knee osteoarthritis: a double-blind, randomized controlled trial. J Bone Joint Surg Am. 2016;98:885–92. doi: 10.2106/JBJS.15.00544. [DOI] [PubMed] [Google Scholar]
- 24.Khanasuk Y, Dechmaneenin T, Tanavalee A. Prospective randomized trial comparing the efficacy of single 6-ml injection of hylan GF-20 and hyaluronic acid for primary knee arthritis: a preliminary study. J Med Assoc Thai. 2012;95(Suppl 10):S92–7. [PubMed] [Google Scholar]
- 25.Anand A, Balduini F, Rogers K. Hyaluronic acid in management of advanced osteoarthritis of the knee: retrospective analysis. Euro J Orthop Surg Traumatol. 2010;20:645–9. doi: 10.1007/s00590-010-0635-3. [DOI] [Google Scholar]
- 26.Conrozier T, Mathieu P, Schott AM, Laurent I, Hajri T, Crozes P, Grand P, Laurent H, Marchand F, Meignan F, Noel E, Rozand Y, Savoye JF, Vignon E. Factors predicting long-term efficacy of hylan GF-20 viscosupplementation in knee osteoarthritis. Joint Bone Spine. 2003;70:128–33. doi: 10.1016/S1297-319X(03)00005-8. [DOI] [PubMed] [Google Scholar]