Abstract
Total knee arthroplasty (TKA) has been much improved recently and it is regarded as one of the most common and successful surgical procedures that provides pain relief and improves function in patients with severe knee arthritis. However, recent studies have reported that 15%–20% of patients are not satisfied after TKA without evident clinical or radiological findings and the most common causes of patient dissatisfaction include residual pain and limited function. The evaluation and treatment of painful TKA relies on a thorough understanding of the origin by careful evaluation, and a systematic approach is essential to efficiently and effectively resolve the pain. Periarticular injections (PAIs) and nerve blocks are gaining popularity because they are associated with less side effects than systemic regimens. The analgesic efficacy and safety of PAI compared with nerve blocks for postoperative pain management still remain controversial. Therefore, more study is needed to determine if any changes in the regimen of the injection or technique could provide added benefit to long-term functional improvement beyond the perioperative period.
Keywords: Knee, Arthroplasty, Pain, Management
Introduction
Total knee arthroplasty (TKA) has been much improved recently and it is regarded as one of the most common and successful surgical procedures that provides pain relief and improves function in patients with severe knee arthritis1–4). However, recent studies have reported that 15%–20% of patients are not satisfied after TKA in spite of no evident clinical or radiological findings, and the most common causes of patient dissatisfaction include residual pain and limited function5–7). Therefore, appropriate pain control after TKA can be expected to enhance early functional recovery and improve patient satisfaction8).
Nowadays, several risk factors and mechanisms of persistent pain after TKA are being proposed and targeting of the patients at risk might help to manage these patients. Significant progress has also been made in improving analgesia and pain control, but various protocols are still not well understood9,10). In this review article, risk factors and mechanisms of painful TKA will be assessed and pain management protocols would be summarized.
Definition and Mechanism of Painful TKA
Pain could be defined as an “unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage”11). It could imply that pain is a complex and multifactorial experience that involves multiple organ systems. An increased amplification of pain is related to tissue injury, blood pressure, impaired pain regulatory systems and proinflammatory states. All chronic pain was once acute, but not all acute pain becomes chronic. The transition is complex and involves pre-, intra- and postoperative, psychosocial, socioenvironmental and patient-related genetic factors6).
Five predictors contribute to chronic postsurgical pain (CPSP): preoperative pain at the operated area, preoperative pain elsewhere in the body (such as spine or hip), acute postoperative pain, capacity overload, and comorbid stress symptoms such as anxiety, rumination, magnification, and helplessness12). Nociceptive and neuropathic pain would be closely related to this process. Nociceptive pain is caused by the ongoing activation of sensory neurons in response to a noxious stimulus such as injury, disease, or inflammation. On the contrary, neuropathic pain is caused by aberrant signal processing in the peripheral or central nervous system and is broadly categorized as peripheral or central in origin11).
Patients with TKA frequently show pain elsewhere in the body13). Chang et al.14) reported that lumbar radiating pain during an activity may be a source of poor outcome after TKA. The risk factors for the development of CPSP are controversial, but more intense postoperative pain is seen in association with central sensitization, younger age, obesity, and female gender15). Sensitization is a non-adaptive processing of nociceptive inputs and has a vital role in the etiology of neuropathic pain6,11). This contributes to pain in patients with osteoarthritis of the knee; this explains the discordance between the intensity of pain and the severity of the osteoarthritis on radiographs as well as the discrepancy between a satisfactory postoperative radiographic appearance and unexplained persistent pain after TKA16–18). Chronic use of the opioid also causes a state of nociceptive sensitization called ‘opioid-induced hyperalgesia’19). Those who have the possibility of developing neuropathic pain might benefit from specific management with antihyperalgesic drugs such as ketamine or gabapentinoids.
A recent study showed that chronic non-orthopedic conditions such as fibromyalgia, migraine, irritable bowel syndrome, chronic low back pain, a head injury or stroke are more commonly found in patients with poor outcome after TKA. Those conditions are all causes of stress, leading to a combination of psychological distress and amplification of pain. These mechanisms are poorly understood, but are characterized by pain associated with abnormalities of motor function, autonomic balance, neuro-endocrine function and sleep6,20).
Evaluation of Painful TKA
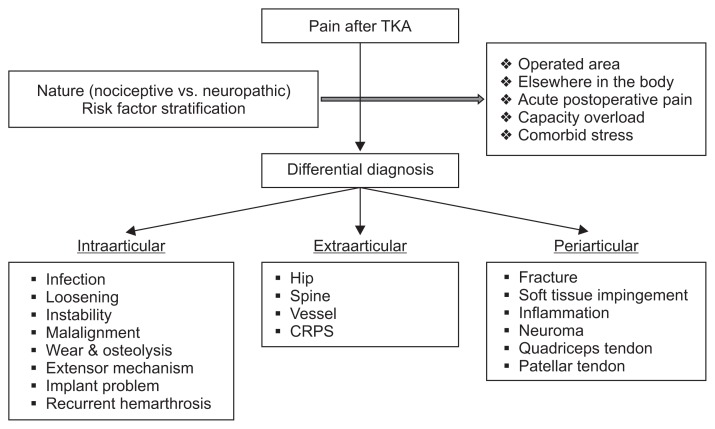
Full assessment of both the surgical and non-surgical factors that can cause pain after TKA is essential in addition to multidisciplinary team approaches that involve orthopedic surgeons, physical therapists, pain management physicians, and primary medical doctors21). Laskin22) categorized etiologies of pain after TKA based on their temporal associations as follows: start-up pain, pain on weight bearing, early postoperative pain, pain associated with full flexion, pain with stair climbing or descent, rest pain, and continuous postoperative pain. Start-up pain may be related with loosening of components. Pain that is activity related may indicate a mechanical etiology and suggest instability if it is associated with recurrent swelling. In contrast, pain that is constant and not alleviated with rest and activity modification should raise suspicion of underlying periprosthetic joint infection. Pain with stair climbing can be caused by extensor mechanism pathology. Pain that begins within the first year after TKA suggests infection, instability, malpositioned implants, or soft tissue impingement. In contrast, pain that begins more than 1 year postoperatively suggests wear, osteolysis, aseptic loosening, or infection. Extraarticular sources of knee pain include the spine, hip, vessels, and complex regional pain syndrome. If pain was radiated to the thigh or down to the foot, it may have been referred from the lumbar spine or hip. Pain described as burning, tingling, prickling, shooting, electric shock-like, squeezing, spasm, or cold may indicate a neuropathic origin11) (Fig. 1).
Fig. 1.
Flow chart of pain evaluation. TKA: total knee arthroplasty, CRPS: chronic postsurgical pain.
Regimens for Pain Control after TKA
Pain control regimens after TKA include oral or parenteral anesthesia, intravenous patient-controlled analgesia (PCA), nerve blocks, periarticular injections (PAIs), and continuous epidural or intraarticular analgesia23–26). Among them, nerve blocks and PAIs are gaining popularity because they are associated with little side effects than systemic regimens and this review article will focus on these regimens. PCA is widely used with morphine, but its drawbacks include somnolence, nausea, vomiting, ileus, constipation, pruritis, urinary retention, hypotension, and respiratory depression which can also affect the patient’s ability to effectively participate in physical therapy27,28). Epidural analgesia may produce spinal headache, neurogenic bladder, hypotension, and contralateral leg numbness27,29).
1. Periarticular Injection
PAI has been reported to be effective in controlling immediate postoperative pain without the systemic side effects associated with systemic opioids24,30–32). The goal of PAI is to decrease pain at the central and peripheral levels while minimizing side effects, facilitating patient participation in postoperative rehabilitation, allowing earlier discharge, and improving overall function outcome27,33). The agents used in PAIs typically include local anesthetics, opioids, non-steroidal anti-inflammatory drugs, and corticosteroids optionally34–36). However, the advantages of each medication and the additive or synergistic effects of each medication are still not well known.
Kelly et al.27) performed a randomized controlled trial (RCT) to compare the clinical efficacy of multimodal approaches with ropivacaine, epinephrine, clonidine, and ketorolac. They concluded that the multimodal pain control protocol involving an intraoperative PAI containing ropivacaine, epinephrine, clonidine, and ketorolac showed better early postoperative pain control than the protocol used in a control group (ropivacaine and epinephrine). However, the difference between group A (ropivacaine, epinephrine, ketorolac, and clonidine) and group B (ropivacaine, epinephrine, and ketorolac) was questionable. In a RCT of Kim et al.37), the gold standard for drug combination was evaluated. Among 6 tested protocols, a combination of ropivacaine, morphine, and ketorolac in a PAI showed a significantly stronger and sufficiently synergistic analgesic effect without adding methylprednisolone in TKA.
Another clinical trial examined the effect of liposomal bupivacaine in the use of PAI38). Liposomal bupivacaine is a novel composition in which the drug is dissolved into liposomes that release the medication in a controlled fashion slowly over a period of 96 hours. It was shown to reduce opioid use after the surgery when it was used with femoral nerve block (FNB) in TKA38–40). Therefore, it was assumed that it would improve the effect of PAI. However, it was not found to be superior to standard PAI in opioid-dependent patients undergoing TKA38).
Addition of corticosteroids in PAI is another controversial issue. Conceptually, a corticosteroid is believed to be a key component because of its local anti-inflammatory effects and ability to reduce the local stress responses to surgery34,36). However, its efficacy and association with risk of infection remain points of controversy: some studies41,42) do not support the efficacy of corticosteroids in PAI while other studies43–46) do. Recent analyses have shown that intraarticular steroid injections provide short-term advantages in pain relief and antiemetic effects without increasing the infection risk. However, the optimal dose and long-term effects of steroid injection need to be confirmed by numerous studies47).
2. Nerve Blocks (Regional Anesthesia)
Regional anesthesia techniques seem to be an ideal method to deliver intraoperative analgesia and to minimize postoperative pain. However, it has been questioned by some authors because of side effects, such as impairment of motor function and risk of falls, risk of infection, and neurological complications48). With the ongoing evolution of regional anesthesia and the development of ultrasound guidance, success rates have increased, complications have been minimized and various techniques have become more popular48). However, the optimal nerve block, combination with other blocks or other modalities, and single- or multiple-shot techniques are still being debated.
FNB is frequently used to control postoperative pain after TKA. It showed superior outcomes to opioid-based analgesia. Compared with epidural analgesia, it showed similar analgesia but caused less nausea and vomiting and better patient satisfaction49). However, continuous blockade of the femoral nerve induces weakness of the quadriceps that raises the risk for falls and increases the risk of infection8). However, a recent cohort study indicated that peripheral nerve blocks do not necessarily increase the likelihood for falls50). The superiority of single shot vs. continuous FNB is questionable, but continuous blockade could induce weakness of the quadriceps more easily. Therefore, adjustment of continuous infusion with a rather low-dose regimen may effectively minimize quadriceps weakness51). In case of insufficient pain control despite FNB, sciatic nerve block appeals to be a suitable option to optimize pain management48). However, the use of an additional sciatic nerve block is controversial and it should be determined by individuals based on the consideration of advantages (improved analgesia and reduction of opioids) and disadvantages (risk of nerve injury and motor weakness)48,52–54).
Adductor canal block (ACB) was devised with the idea of performing a conduction block on the sensory branches of the femoral nerve and avoiding the disadvantage of motor impairment48). However, clinical studies are only recently available and still sparse. A recent meta-analysis reported that ACB remains an attractive alternative to FNB for pain control and motor strength preservation after TKA, but the anatomical location of the adductor canal needs to be better defined to ensure consistency in the type of block performed. It was concluded that they cannot safely suggest that an ACB provides optimal outcomes in comparison to FNB until aforementioned factor is completely understood55). Therefore, it is too early to recommend ACB for pain management after TKA even though it has the potential to replace FNB as a gold standard of pain management after TKA56).
The analgesic efficacy and safety of PAI compared with FNB for postoperative pain management in TKA still remains controversial. Recent two meta-analyses also reported a little different conclusions. In a meta-analysis performed by Wang et al.57), single shot FNB showed better pain relief in the early postoperative period compared with single shot PAI and continuous PAI provided postoperative analgesia comparable to that of continuous FNB. No significant difference was seen in regard to the complications between the two methods. However, in the meta-analysis of the Albrecht et al.58), there were no clinical differences in functional outcome or rates of complications. PAI provided similar postoperative analgesia after TKA to FNB; however, they pointed out the low number of trials that sought complications. One interesting article was published by Youm et al.8). They reported that PAI was more effective than FNB during the early (0–8 hours) postoperative period after TKA. However, patients treated with PAI experienced rebound pain at 24 hours. Therefore, they concluded that the combination of PAI and FNB may provide greater postoperative pain management than either alone for the first 24 hours after TKA.
Conclusions
The evaluation and treatment of painful TKA relies on a thorough understanding of the origin by careful evaluation, and a systematic approach is essential to efficiently and effectively resolve the pain. The preoperative assessment of risk factors might lead to individualization of perioperative management. PAIs and nerve blocks are gaining popularity because they are associated with little side effects than systemic regimens. The superiority blocks with regard to analgesic efficacy and safety for postoperative pain management in TKA still remains controversial. Therefore, more research is needed to determine if an addition or a change in the regimen of the injection or technique could provide added benefit to long-term functional improvement beyond the perioperative period.
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Sakellariou VI, Poultsides LA, Ma Y, Bae J, Liu S, Sculco TP. Risk assessment for chronic pain and patient satisfaction after total knee arthroplasty. Orthopedics. 2016;39:55–62. doi: 10.3928/01477447-20151228-06. [DOI] [PubMed] [Google Scholar]
- 2.Bugala-Szpak J, Kusz D, Dyner-Jama I. Early evaluation of quality of life and clinical parameters after total knee arthroplasty. Ortop Traumatol Rehabil. 2010;12:41–9. [PubMed] [Google Scholar]
- 3.Gonzalez Sáenz de Tejada M, Escobar A, Herrera C, Garcia L, Aizpuru F, Sarasqueta C. Patient expectations and health-related quality of life outcomes following total joint replacement. Value Health. 2010;13:447–54. doi: 10.1111/j.1524-4733.2009.00685.x. [DOI] [PubMed] [Google Scholar]
- 4.Quintana JM, Escobar A, Arostegui I, Bilbao A, Azkarate J, Goenaga JI, Arenaza JC. Health-related quality of life and appropriateness of knee or hip joint replacement. Arch Intern Med. 2006;166:220–6. doi: 10.1001/archinte.166.2.220. [DOI] [PubMed] [Google Scholar]
- 5.Wylde V, Bruce J, Beswick A, Elvers K, Gooberman-Hill R. Assessment of chronic postsurgical pain after knee replacement: a systematic review. Arthritis Care Res (Hoboken) 2013;65:1795–803. doi: 10.1002/acr.22050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavand’homme P, Thienpont E. Pain after total knee arthroplasty: a narrative review focusing on the stratification of patients at risk for persistent pain. Bone Joint J. 2015;97(10 Suppl A):45–8. doi: 10.1302/0301-620X.97B10.36524. [DOI] [PubMed] [Google Scholar]
- 7.Choi YJ, Ra HJ. Patient Satisfaction after Total Knee Arthroplasty. Knee Surg Relat Res. 2016;28:1–15. doi: 10.5792/ksrr.2016.28.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youm YS, Cho SD, Cho HY, Hwang CH, Jung SH, Kim KH. Preemptive femoral nerve block could reduce the rebound pain after periarticular injection in total knee arthroplasty. J Arthroplasty. 2016;31:1722–6. doi: 10.1016/j.arth.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Liu SS, Buvanendran A, Rathmell JP, Sawhney M, Bae JJ, Moric M, Perros S, Pope AJ, Poultsides L, Della Valle CJ, Shin NS, McCartney CJ, Ma Y, Shah M, Wood MJ, Manion SC, Sculco TP. Predictors for moderate to severe acute postoperative pain after total hip and knee replacement. Int Orthop. 2012;36:2261–7. doi: 10.1007/s00264-012-1623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu SS, Buvanendran A, Rathmell JP, Sawhney M, Bae JJ, Moric M, Perros S, Pope AJ, Poultsides L, Della Valle CJ, Shin NS, McCartney CJ, Ma Y, Shah M, Wood MJ, Manion SC, Sculco TP. A cross-sectional survey on prevalence and risk factors for persistent postsurgical pain 1 year after total hip and knee replacement. Reg Anesth Pain Med. 2012;37:415–22. doi: 10.1097/AAP.0b013e318251b688. [DOI] [PubMed] [Google Scholar]
- 11.McDowell M, Park A, Gerlinger TL. The painful total knee arthroplasty. Orthop Clin North Am. 2016;47:317–26. doi: 10.1016/j.ocl.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Althaus A, Hinrichs-Rocker A, Chapman R, Arranz Becker O, Lefering R, Simanski C, Weber F, Moser KH, Joppich R, Trojan S, Gutzeit N, Neugebauer E. Development of a risk index for the prediction of chronic post-surgical pain. Eur J Pain. 2012;16:901–10. doi: 10.1002/j.1532-2149.2011.00090.x. [DOI] [PubMed] [Google Scholar]
- 13.Ayers DC, Li W, Oatis C, Rosal MC, Franklin PD. Patient-reported outcomes after total knee replacement vary on the basis of preoperative coexisting disease in the lumbar spine and other nonoperatively treated joints: the need for a musculoskeletal comorbidity index. J Bone Joint Surg Am. 2013;95:1833–7. doi: 10.2106/JBJS.L.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CB, Park KW, Kang YG, Kim TK. Coexisting lumbar spondylosis in patients undergoing TKA: how common and how serious? Clin Orthop Relat Res. 2014;472:710–7. doi: 10.1007/s11999-013-3298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puolakka PA, Rorarius MG, Roviola M, Puolakka TJ, Nordhausen K, Lindgren L. Persistent pain following knee arthroplasty. Eur J Anaesthesiol. 2010;27:455–60. doi: 10.1097/EJA.0b013e328335b31c. [DOI] [PubMed] [Google Scholar]
- 16.Petersen KK, Arendt-Nielsen L, Simonsen O, Wilder-Smith O, Laursen MB. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain. 2015;156:55–61. doi: 10.1016/j.pain.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 17.Petersen KK, Simonsen O, Laursen MB, Nielsen TA, Rasmussen S, Arendt-Nielsen L. Chronic postoperative pain after primary and revision total knee arthroplasty. Clin J Pain. 2015;31:1–6. doi: 10.1097/AJP.0000000000000146. [DOI] [PubMed] [Google Scholar]
- 18.Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, Campbell CM, Haythornthwaite JA, Edwards RR, Smith MT. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013;65:363–72. doi: 10.1002/art.34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zywiel MG, Stroh DA, Lee SY, Bonutti PM, Mont MA. Chronic opioid use prior to total knee arthroplasty. J Bone Joint Surg Am. 2011;93:1988–93. doi: 10.2106/JBJS.J.01473. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs CA, Christensen CP, Karthikeyan T. Chronic non-orthopedic conditions more common in patients with less severe degenerative changes that have elected to undergo total knee arthroplasty. J Arthroplasty. 2015;30:1146–9. doi: 10.1016/j.arth.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 21.Toms AD, Mandalia V, Haigh R, Hopwood B. The management of patients with painful total knee replacement. J Bone Joint Surg Br. 2009;91:143–50. doi: 10.1302/0301-620X.91B2.20995. [DOI] [PubMed] [Google Scholar]
- 22.Laskin RS. The patient with a painful total knee replacement. In: Lotke PA, Garino JP, editors. Revision total knee arthroplasty. Philadelphia, PA: Lippincott-Raven; 1999. pp. 91–107. [Google Scholar]
- 23.Andersen LO, Husted H, Otte KS, Kristensen BB, Kehlet H. High-volume infiltration analgesia in total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. Acta Anaesthesiol Scand. 2008;52:1331–5. doi: 10.1111/j.1399-6576.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 24.Busch CA, Shore BJ, Bhandari R, Ganapathy S, MacDonald SJ, Bourne RB, Rorabeck CH, McCalden RW. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006;88:959–63. doi: 10.2106/JBJS.E.00344. [DOI] [PubMed] [Google Scholar]
- 25.Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin MC, Varin F. A multimodal analgesia protocol for total knee arthroplasty: a randomized, controlled study. J Bone Joint Surg Am. 2006;88:282–9. doi: 10.2106/00004623-200602000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Goyal N, McKenzie J, Sharkey PF, Parvizi J, Hozack WJ, Austin MS. The 2012 Chitranjan Ranawat award: intraarticular analgesia after TKA reduces pain: a randomized, double-blinded, placebo-controlled, prospective study. Clin Orthop Relat Res. 2013;471:64–75. doi: 10.1007/s11999-012-2596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28:1274–7. doi: 10.1016/j.arth.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998;87:88–92. doi: 10.1097/00000539-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Mahoney OM, Noble PC, Davidson J, Tullos HS. The effect of continuous epidural analgesia on postoperative pain, rehabilitation, and duration of hospitalization in total knee arthroplasty. Clin Orthop Relat Res. 1990;(260):30–7. [PubMed] [Google Scholar]
- 30.Affas F, Nygards EB, Stiller CO, Wretenberg P, Olofsson C. Pain control after total knee arthroplasty: a randomized trial comparing local infiltration anesthesia and continuous femoral block. Acta Orthop. 2011;82:441–7. doi: 10.3109/17453674.2011.581264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lombardi AV, Jr, Berend KR, Mallory TH, Dodds KL, Adams JB. Soft tissue and intra-articular injection of bupivacaine, epinephrine, and morphine has a beneficial effect after total knee arthroplasty. Clin Orthop Relat Res. 2004;(428):125–30. doi: 10.1097/01.blo.0000147701.24029.cc. [DOI] [PubMed] [Google Scholar]
- 32.Mauerhan DR, Campbell M, Miller JS, Mokris JG, Gregory A, Kiebzak GM. Intra-articular morphine and/or bupivacaine in the management of pain after total knee arthroplasty. J Arthroplasty. 1997;12:546–52. doi: 10.1016/S0883-5403(97)90178-9. [DOI] [PubMed] [Google Scholar]
- 33.Maheshwari AV, Blum YC, Shekhar L, Ranawat AS, Ranawat CS. Multimodal pain management after total hip and knee arthroplasty at the Ranawat Orthopaedic Center. Clin Orthop Relat Res. 2009;467:1418–23. doi: 10.1007/s11999-009-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6 Suppl 2):33–8. doi: 10.1016/j.arth.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 35.Tsukada S, Wakui M, Hoshino A. Pain control after simultaneous bilateral total knee arthroplasty: a randomized controlled trial comparing periarticular injection and epidural analgesia. J Bone Joint Surg Am. 2015;97:367–73. doi: 10.2106/JBJS.N.00373. [DOI] [PubMed] [Google Scholar]
- 36.Tsukada S, Wakui M, Hoshino A. The impact of including corticosteroid in a periarticular injection for pain control after total knee arthroplasty: a double-blind randomised controlled trial. Bone Joint J. 2016;98:194–200. doi: 10.1302/0301-620X.98B2.36596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim TW, Park SJ, Lim SH, Seong SC, Lee S, Lee MC. Which analgesic mixture is appropriate for periarticular injection after total knee arthroplasty? Prospective, randomized, double-blind study. Knee Surg Sports Traumatol Arthrosc. 2015;23:838–45. doi: 10.1007/s00167-014-3366-x. [DOI] [PubMed] [Google Scholar]
- 38.Schwarzkopf R, Drexler M, Ma MW, Schultz VM, Le KT, Rutenberg TF, Rinehart JB. Is there a benefit for liposomal bupivacaine compared to a traditional periarticular injection in total knee arthroplasty patients with a history of chronic opioid use? J Arthroplasty. 2016;31:1702–5. doi: 10.1016/j.arth.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 39.Baxter R, Bramlett K, Onel E, Daniels S. Impact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from ten prospective, controlled clinical studies. Clin Ther. 2013;35:312–20. doi: 10.1016/j.clinthera.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Surdam JW, Licini DJ, Baynes NT, Arce BR. The use of exparel (liposomal bupivacaine) to manage postoperative pain in unilateral total knee arthroplasty patients. J Arthroplasty. 2015;30:325–9. doi: 10.1016/j.arth.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Chia SK, Wernecke GC, Harris IA, Bohm MT, Chen DB, Macdessi SJ. Peri-articular steroid injection in total knee arthroplasty: a prospective, double blinded, randomized controlled trial. J Arthroplasty. 2013;28:620–3. doi: 10.1016/j.arth.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 42.Christensen CP, Jacobs CA, Jennings HR. Effect of periarticular corticosteroid injections during total knee arthroplasty: a double-blind randomized trial. J Bone Joint Surg Am. 2009;91:2550–5. doi: 10.2106/JBJS.H.01501. [DOI] [PubMed] [Google Scholar]
- 43.Ikeuchi M, Kamimoto Y, Izumi M, Fukunaga K, Aso K, Sugimura N, Yokoyama M, Tani T. Effects of dexamethasone on local infiltration analgesia in total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22:1638–43. doi: 10.1007/s00167-013-2367-5. [DOI] [PubMed] [Google Scholar]
- 44.Kwon SK, Yang IH, Bai SJ, Han CD. Periarticular injection with corticosteroid has an additional pain management effect in total knee arthroplasty. Yonsei Med J. 2014;55:493–8. doi: 10.3349/ymj.2014.55.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sean VW, Chin PL, Chia SL, Yang KY, Lo NN, Yeo SJ. Single-dose periarticular steroid infiltration for pain management in total knee arthroplasty: a prospective, double-blind, randomised controlled trial. Singapore Med J. 2011;52:19–23. [PubMed] [Google Scholar]
- 46.Yue DB, Wang BL, Liu KP, Guo WS. Efficacy of multimodal cocktail periarticular injection with or without steroid in total knee arthroplasty. Chin Med J (Engl) 2013;126:3851–5. [PubMed] [Google Scholar]
- 47.Cui Z, Liu X, Teng Y, Jiang J, Wang J, Xia Y. The efficacy of steroid injection in total knee or hip arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2015;23:2306–14. doi: 10.1007/s00167-014-3049-7. [DOI] [PubMed] [Google Scholar]
- 48.Bauer MC, Pogatzki-Zahn EM, Zahn PK. Regional analgesia techniques for total knee replacement. Curr Opin Anaesthesiol. 2014;27:501–6. doi: 10.1097/ACO.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 49.Chan EY, Fransen M, Parker DA, Assam PN, Chua N. Femoral nerve blocks for acute postoperative pain after knee replacement surgery. Cochrane Database Syst Rev. 2014;(5):CD009941. doi: 10.1002/14651858.CD009941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Memtsoudis SG, Danninger T, Rasul R, Poeran J, Gerner P, Stundner O, Mariano ER, Mazumdar M. Inpatient falls after total knee arthroplasty: the role of anesthesia type and peripheral nerve blocks. Anesthesiology. 2014;120:551–63. doi: 10.1097/ALN.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 51.Ilfeld BM, Turan A, Ball ST. Not all “continuous femoral nerve blocks” are equivalent. J Arthroplasty. 2015;30:896–7. doi: 10.1016/j.arth.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 52.Abdallah FW, Brull R. Is sciatic nerve block advantageous when combined with femoral nerve block for postoperative analgesia following total knee arthroplasty? A systematic review. Reg Anesth Pain Med. 2011;36:493–8. doi: 10.1097/AAP.0b013e318228d5d4. [DOI] [PubMed] [Google Scholar]
- 53.Ilfeld BM, Madison SJ. The sciatic nerve and knee arthroplasty: to block, or not to block: that is the question. Reg Anesth Pain Med. 2011;36:421–3. doi: 10.1097/AAP.0b013e31822940d2. [DOI] [PubMed] [Google Scholar]
- 54.Wegener JT, van Ooij B, van Dijk CN, Hollmann MW, Preckel B, Stevens MF. Value of single-injection or continuous sciatic nerve block in addition to a continuous femoral nerve block in patients undergoing total knee arthroplasty: a prospective, randomized, controlled trial. Reg Anesth Pain Med. 2011;36:481–8. doi: 10.1097/AAP.0b013e318228c33a. [DOI] [PubMed] [Google Scholar]
- 55.Hussain N, Ferreri TG, Prusick PJ, Banfield L, Long B, Prusick VR, Bhandari M. Adductor canal block versus femoral canal block for total knee arthroplasty: a meta-analysis: what does the evidence suggest? Reg Anesth Pain Med. 2016;41:314–20. doi: 10.1097/AAP.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 56.Mariano ER, Perlas A. Adductor canal block for total knee arthroplasty: the perfect recipe or just one ingredient? Anesthesiology. 2014;120:530–2. doi: 10.1097/ALN.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 57.Wang C, Cai XZ, Yan SG. Comparison of periarticular multimodal drug injection and femoral nerve block for postoperative pain management in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2015;30:1281–6. doi: 10.1016/j.arth.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Albrecht E, Guyen O, Jacot-Guillarmod A, Kirkham KR. The analgesic efficacy of local infiltration analgesia vs femoral nerve block after total knee arthroplasty: a systematic review and meta-analysis. Br J Anaesth. 2016;116:597–609. doi: 10.1093/bja/aew099. [DOI] [PubMed] [Google Scholar]