Abstract
Drug tolerance, lacking liver regenerative activity and inconclusive inhibition of steatosis and cirrhosis by silymarin treatment during chronic liver injury have increased the demand for novel alternative or synergistic treatments for liver damage. Litchi fruit is abundant in polyphenolic compounds and is used in traditional Chinese medicine for treatments that include the strengthening of hepatic and pancreatic functions. Unique polyphenolic compounds obtained from litchi pericarp extract (LPE) were studied in vitro and in vivo for hepatoprotection. Epicatechin (EC) and procyanidin A2 (PA2) of LPE were obtained by fractionated-extraction from pulverized litchi pericarps. All fractions, including LPE, were screened against silymarin in carbon tetrachloride (CCl4)-treated murine embryonic liver cell line (BNL). The effects of daily gavage-feeding of LPE, silymarin (200 mg/kg body weight) or H2O in CCl4-intoxicated male ICR mice were evaluated by studying serum chemicals, liver pathology and glutathione antioxidative enzymes. The effects of EC and PA2 on liver cell regenerative activity were investigated using a scratch wound healing assay and flow cytometric cell cycle analysis; the results of which demonstrated that LPE protected BNL from CCl4-intoxication. Gavage-feeding of LPE decreased serum glutamic oxaloacetate transaminase and glutamic pyruvic transaminase levels, and exhibited superior retention of the hexagonal structure of hepatocytes and reduced necrotic cells following liver histopathological examinations in CCl4-intoxicated ICR mice. Glutathione peroxidise and glutathione reductase activities were preserved as the normal control level in LPE groups. EC and PA2 were principle components of LPE. PA2 demonstrated liver cell regenerative activity in scratch wound healing assays and alcohol-induced liver cell injury in vitro. The present findings suggest that litchi pericarp polyphenolic extracts, including EC and PA2, may be a synergistic alternative to silymarin in hepatoprotection and liver cell regeneration.
Keywords: carbon tetrachloride, litchi, lychee, liver injury, procyanidin, epicatechin, hepatoprotection
Introduction
Oxidative stress is a primary cause of toxic hepatitis and may result in chronic liver disease. Carbon tetrachloride (CCl4)-induced hepatotoxicity is widely used, experimentally, as a chemical toxicant for rapid induction of liver injury and the creation of liver fibrosis models of chronic liver damage. CCl4 induces liver injury through the destruction of the microsomal triglyceride transfer protein and through the metabolism of hepatic microsomal liver cytochrome P450, which produces free radicals and, ultimately, results in liver cell injury and inflammation (1).
Milk thistle, which is an antioxidative plant, produces silymarin containing four flavonolignan isomers (silybin, isosilybin, silydianin and silychristin). Silymarin is currently the first-line prescribed drug in clinics for the treatment of various symptoms of hepatitis. Silymarin has been reported to exhibit hepatoprotection due to its antioxidant and anti-inflammatory properties (2) and through the stimulation of protein synthesis, resulting in decreased serum glutamic oxaloacetate transaminase (GOT) and glutamic pyruvic transaminase (GPT) levels. However, drug tolerance (3) and inconsistent outcomes in the inhibition of liver fibrosis and steatosis (4) have hindered silymarin efficacy. Therefore, there is an increasing demand for novel alternative treatments to cure liver diseases or to use as synergistic health supplements for liver protection. Recently, B type procyanidin extract, primarily from grape seeds (5), and anthocyanidins (6) have exhibited effective inhibition of liver fibrosis and steatosis.
Litchi fruit, a member of the Sapindaceae family, is abundant in polyphenolic compounds and is commonly used as a Chinese traditional herbal ingredient for liver tonics to strengthen hepatic and pancreatic functions (7). Fruit pericarp and seeds have been reported to possess anti-inflammatory and anti-carcinogenic properties (8,9). Polyphenolic compounds, such as anthocyanins, tannins and many other ortho-diphenolic compounds, have been identified recently (10,11). Further investigation in controlled studies is required for the evaluation of the unique polyphenolic contents of litchi for medicinal purposes and efficacy in hepatoprotection. Therefore, the present study investigated the hepatoprotective properties of litchi pericarp extract in vitro and in vivo liver injury models.
Materials and methods
Ethics statement
Laboratory animal protocols performed in the present study were in compliance with the regulations of the Institutional Animal Care and Use Committee (IACUC), National Chiayi University (Chiayi, Taiwan). All animal experimentations and protocols were approved by the IACUC.
Materials, isolation, purification and identification of bioactive compounds in litchi pericarp
Pulverized pericarps of Litchi chinensis Sonn. fruit were extracted with methanol (4l; ×2), and the solution was filtered. The filtrate was concentrated by evaporation and, subsequently, freeze-dried to yield the litchi pericarp methanol extract (280 g). A 100-g portion of the methanol extract was dissolved in water and subjected to chromatography over a Diaion HP-20 column (9.5×82 cm; Mitsubishi Chemical Corporation, Tokyo, Japan) with proportion of water to methanol (0, 20, 40 and 60% methanol) and final elute with 70% acetone to yield five fractions. From the five fractions, 40% methanol elute (LPE) was screened for bioactivity and subjected to further chromatography using Waters high-performance liquid chromatography equipment (Waters Corp., Milford, MA, USA) in settings and conditions described in the study by Chang (12). LPE was subjected to chromatography using a LiChroprep RP-18 column (2.5 cm i.d.x51 cm; Merck Millipore, Darmstadt, Germany) from which five sub-fractions, of 10 g each, were derived. Sub-fractions one and two underwent additional LiChroprep RP-18 column chromatography to yield compound A, epicatechin, [EC; 71.2 mg; C15H14O6; molecular weight (MW), 290.27] and compound B, procyanidin A2 (PA2; 168.5 mg; C30H24O12; MW, 576.50), respectively. The chemical structures of the two compounds were originally identified by nuclear magnetic resonance (NMR; Bruker Avance DRX 500 instrument), conducted according to the study by Chang (12).
In vitro assessments
Cell culture
The murine embryonic liver BNL CL2 cell line (BNL; #60180) and the monocyte/macrophage RAW264.7 cell line (RAW; #60001) were obtained from the Bioresource Collection and Research Center (Hsinchu, Taiwan). All cell lines were maintained in RPMI-1,640 supplemented with 10% fetal bovine serum, 100 mg/l streptomycin, and 100 U/ml penicillin (Sigma-Aldrich; Merck Millipore) at 37°C and 5% CO2 in a humidified incubator. Cell culture plastics were purchased from Corning Inc., (Corning, NY, USA). Chemicals were purchased from Sigma-Aldrich (Merck Millipore).
Rescue cell viability of CCl4-injured BNL CL2 cells
Assessment of the cytotoxicity of LPE on BNL and RAW264.7 monocytic cell lines was conducted with both 3-(4-, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and lactate dehydrogenase (LDH; both Sigma-Aldrich; Merck Millipore) release assays, as described previously (13). LPE did not exhibit any cytotoxicity at the maximum concentration of 200 µg/ml (cytotoxicity data not shown). Rescued cell viability was performed by LDH release assay. Briefly, 1×106 cells/ml (200 µl) were seeded onto 96-well culture plates overnight at 37°C and in a humidified incubator containing 5% CO2. LPE was subsequently added at concentrations of 6.25, 12.5, 50, and 100 µg/ml for a further 24 h incubation at 37°C, in a humidified incubator containing 5% CO2. Subsequently, 20 µl MTT reagent or 50 µl LDH reagents, including 0.2 M Tris/HCl buffer containing 0.0054 M L-(+)-lactic acid, 1.3 mM β-nicotinamide adenine dinucleotide, 0.66 mM 2-p-iodophenyl-3-p-nitrophenyl-5-phenyl tetrazolium chloride and 0.28 mM N-methylphenazonium methyl sulfate (all Sigma-Aldrich; Merck Millipore) were added to each well for 30 min incubation at room temperature in the dark. Stop solution (1 M acetic acid) was then added and briefly mixed to allow for colorimetric detection. The specific absorbance at 570 nm for MTT assay and 490 nm for LDH assay were detected using a 96-well plate ELISA reader (BioTek Instruments, Inc., Winooski, UT, USA). To determine the liver cell protecting effect of LPE on CCl4-induced cell injury in vitro, BNL cells were treated with 10 nM CCl4 (in dimethyl sulfoxide) for 1 h prior to treatment with LPE at concentrations of 6.25, 12.5, 50, or 100 µg/ml or medium only. Silymarin at 50 µg/ml was used for positive control.
Scratch wound healing assay
The methodology was modified from the study performed by Liang et al (14). BNL cells were seeded in a 24-well plate and cultured in the same conditions described previously. Subsequently, a fixed tip width disposable plastic cell scraper was scratched across the cell monolayer, creating a gap in each well. Following this, EC, PA2 or silymarin were randomly assigned to the 24 wells for an additional 48-h culture. Images were captured, using a CCD-equipped inverted microscope, of the ability of the BNL cells to fill the gap. Images were analyzed for the cell-free area (mm2) by Image Pro-Plus 5.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Flow cytometry analysis of cell integrity and cell cycle of alcohol-injured BNL cells
Confluence BNL cells were pre-treated in medium containing 10% alcohol for 1 h at 37°C. Subsequently, the cell culture was washed twice and placed in fresh medium. Following a 37°C overnight culture in normal media containing no treatment, or media containing 1, 5, 10, 25 or 50 µg/ml PA2, the cells were harvested and fixed with 70% ethanol and stored overnight at 4°C for subsequent flow cytometry. Prior to acquisition, cells were centrifuged and stained by adding propidium iodide solution. Following staining for 30 min, the samples were vortexed and ready for cell acquisition of at least 10,000 cells. Data were analyzed using WinMDI free software.
CCl4-intoxicated mouse liver-injury model
Animals
A total of 48 8-week old male ICR mice were weighed (initial body weight, 28±0.54 g) and randomly assigned to the normal control (house-keeping control) and CCl4-intoxicated treatment groups. Mice were housed in a temperature controlled environment at 23±1°C with a 12-h light/dark cycle. The basal diet was using commercial rodent diet (Labdiet, St. Louis, MO, USA). Water and feed were provided ad libitum throughout the experiment. The treatment groups included: Distilled water (H2O) vehicle control; silymarin [200 mg/kg body weight (BW)/day]; and three levels of LPE (20, 100, and 200 mg/kg BW/day). With the exception of the normal control group, all treatments were orally administered every morning. For CCl4 intoxication, mice were injected with 10 µl CCl4 (80% v/v in olive oil) intraperitoneally during the afternoon on Monday and Thursday, twice a week for an entire 6-week period.
Serum biochemical analyses
Peripheral blood samples were collected in heparinized haematocrit tubes. Serum was obtained by brief spin-down of blood samples. Biochemical analysis was conducted on serum samples at 1, 3 and 6 weeks. Serum aspartate aminotransferase (GOT, also known as aspartate amino transferase), alanine aminotransferase (GPT, also known as alanine amino tranferase), total cholesterol (T-Chol), total protein (T-p), albumin, globulin, total bilirubin (T-bili), and triglycerides (TG) were analyzed using kits employing photometrical methods with a blood autoanalyser (Roche Cobas Mira Plus; Roche Diagnostics, Basel, Switzerland). Quantitative determinations were obtained within established limits. The referenced ranges of all analyzed parameters were taken from the study by Quimby and Luong (15). Serum analysis was also conducted on normal control group mice that were not treated with CCl4. Statistical comparisons were performed with data collected from the animals with CCl4-induced liver injury.
Gross liver examinations and histopathology assessments
Following animal experimentation, animals were euthanized via CO2 overdose, which first induced unconsciousness prior to sacrifice. Entire liver from the animals in each treatment group were harvested, weighed and immediately immersed in 37% formaldehyde (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Forlmaldehyde was replaced with fresh formaldehyde at 48 h. Liver tissues were sampled by taking vertical cross section of the largest liver lobe (lobus sinister lateralis heptis). Samples were subsequently embedded in paraffin. Tissue blocks were sliced into 5-µm sections, layered onto glass slides and subsequently stained with haematoxylin and eosin for microscopic assessment. Tissue sectioning and staining were performed by the Animal Technology Laboratories at the Agricultural Technology Research Institute (Miaoli, Taiwan).
Liver antioxidative enzyme activity
An aliquot of fresh liver sample (~0.2 g) was collected from each animal and stored at −86°C until analysis. Samples were weighed and homogenized in a cold-water bath by adding 1 M PBS (containing 1% anhydrous ethanol and 1% Triton X-100). Protein concentration was quantified via the Lowry assay using a Protein Quantification kit (#PE0021; Gene Research Lab Co., Ltd., Taipei, Taiwan) following centrifugation at 1,000 × g for 10 min at 4°C. Subsequently, samples were justified for equal protein concentration. The glutathione peroxidase (GSHpx) and reductase (GSHred) activities of the tissue samples were determined using a Glutathione Peroxidase Assay kit (#703102) and a Glutathione Reductase Assay kit (#703202; Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturer's instructions. The rate of change of absorbance (optical density, 340 nm) per minute was expressed as nmol/min/ml.
Statistical analysis
All data were expressed as the mean ± standard deviation and analyzed using one-way analysis of variance (SAS Institute Inc., Cary, NC, USA). Treatment comparisons were performed using Duncan's multiple tests. P<0.05 was considered to indicate a statistically significant difference.
Results and Discussion
LPE increases cell viability of CCl4-injured BNL CL2 liver cells in vitro
The D40M fraction (LPE) was fractionated from litchi pericarps using a LiChroprep RP-18 column and was subjected to chromatography as shown in Fig. 1A. The LPE was enriched with the primary polyphenolic compounds EC and PA2, identified by NMR (Fig. 1B). The rich polyphenolic content of litchi pericarps was first reported in a study by Sarni-Manchado et al (10) and the antioxidant properties of polymeric proanthocyanidins extracted from litchi pericarp have been addressed (16). Flavonoids are able to regulate cellular DNA, RNA and protein synthesis (17).
Figure 1.
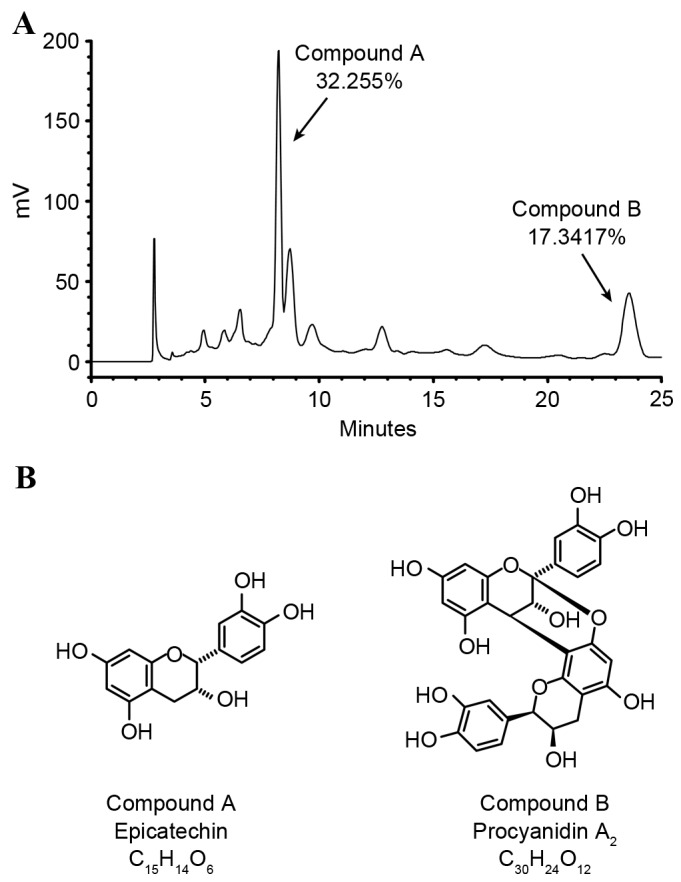
Sample chromatogram of the D40M fraction of LPE detected by high-performance liquid chromatography. (A) Primary compounds in LPE identified by nuclear magnetic resonance. (B) Compound A is epicatechin C15H14O6 (MW, 290.27) and compound B is procyanidin A2 C30H24O12 (MW, 576.50). LPE, litchi pericarp extract; MW, molecular weight.
In the present study, the efficacy of LPE in comparison with silymarin in the recovery of cell viability of CCl4 (10 nM) injured BNL cells in vitro was investigated. As demonstrated in Fig. 2, treatment with CCl4 reduced cell viability to an average of 60% of that of the untreated (medium only) cell population. Following LPE administration to the CCl4-injured cells for an additional 6-h co-incubation, a dose-dependent trend in the rejuvenation of CCl4-injured BNL cells was observed. Multiple hepatoprotective mechanisms of silymarin have been demonstrated (18–20), supporting its use as a first-line drug for various symptoms associated with chronic liver injury. Silymarin's primary mechanism of protection is attributed to free radical scavenging. It is understood that silymarin scavenges the CCl3˙ radical derived from cytochrome P450-metabolised CCl4 (1); however, it has been demonstrated that poor uptake and absorption of silymarin results in only 16% protection against CCl4-induced liver necrosis (2). LPE containing EC and PA2 exert potent free radical scavenging activities with IC50 <5 µg/ml (21). The present study has demonstrated that the viability of CCl4-injured BNL cells is improved more effectively by LPE treatments compared with treatments of silymarin at all levels.
Figure 2.
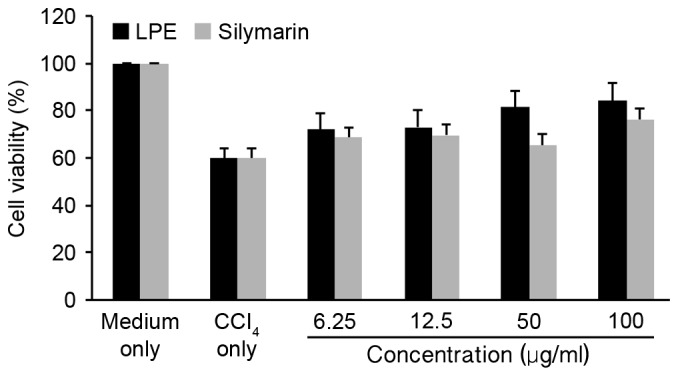
Effect of LPE on recovery of cell viability in low-level CCl4 (10 nM) injured BNL CL2 liver cells in vitro. Data are expressed as the mean ± standard deviation of at least three independent experiments. LPE dose-dependently rescued cell viability more effectively than silymarin, at all levels. LPE, litchi pericarp extract; CCl4, carbon tetrachloride.
LPE reduces liver inflammation and normalizes serum biomarkers of CCl4-induced liver injury in mice
It has been reported that procyanidin dimers A1, A2 and B2 are readily absorbed through the small intestine of rats, whereas A-type trimers are not absorbed (22). In addition, absorbed PA2 remains in its native form and is not conjugated or methylated (23). In the present study, in vivo evaluation of the hepatoprotective effect of LPE rich in EC and PA2 was investigated in comparison with silymarin in a CCl4-induced liver injury mouse model for a duration of 6 weeks. Injection of the chemical toxicant CCl4-induced liver injury, as observed by clinical signs, including the presence of serum biochemical markers of acute hepatitis, early liver tissue necrosis, metabolic dysfunction, chronic signs of hepatic fibrosis and the formation of hepatocellular carcinoma (24). At 6-weeks, CCl4-treated animals gavage-fed with a high dose of LPE (200 mg/kg BW/day) exhibited the lowest relative liver weights, whereas animals treated with silymarin and the animals from the H2O vehicle-control group demonstrated an increased mean liver weight (Table I), which implied the presence of liver inflammation.
Table I.
Serum biochemical analyses performed at 1, 3 and 6-week sampling periods in CCl4-intoxicated mice.
| Serum component | Referenced range | House keeping control | Sampling week | Vehicle control | Silymarin | Low LPE | Middle LPE | High LPE |
|---|---|---|---|---|---|---|---|---|
| GOT (IU/l) | 69–191 | 141.23±20.5 | 1 | 69.83±8.35a,b | 80.62±7.29a,b | 48.36±5.63b | 77.45±14.5a,b | 87.46±16.73a |
| 3 | 4298.09±546.27a | 3982.85±504.79a | 1620.58±578.42b | 962.56±512.04b | 1879.40±585.41b | |||
| 6 | 2099.56±361.43a | 2169.08±358.68a | 821.94±297.18b | 402.82±56.31b | 1168.6±389.25b | |||
| GPT (IU/l) | 26–120 | 28.09±4.19 | 1 | 43.83±9.67b | 39.85±2.88b | 33.09±4.79b | 46.09±3.96b | 86.92±19.22a |
| 3 | 1455.51±402.45a | 1247.49±383.78a,b | 586.89±336.78a,b | 312.25±291.82b | 303.90±185.50b | |||
| 6 | 2569.26±492.33a,b | 3214.47±527.85a | 1514.42±484.47b,c | 928.39±232.72c | 1620.00±433.67b,c | |||
| T-chol (mg/dl) | 63–174 | 55.86±4.72 | 1 | 124.58±11.05 | 114.62±5.40 | 102.55±9.77 | 101.27±9.86 | 106.38±8.39 |
| 3 | 78.09±5.78a,b | 83.19±9.81b | 88.55±9.69a | 71.42±6.73a,b | 93.77±11.25a,b | |||
| 6 | 112.28±9.11 | 102.14±8.02 | 116.67±9.44 | 111.42±8.26 | 92.93±8.88 | |||
| TG (mg/dl) | 26–145 | 23.86±4.82 | 1 | 26.33±2.91c | 28.62±2.68b,c | 32.27±2.57b,c | 54.36±5.66a | 38.85±4.23b |
| 3 | 17.16±1.91a,b | 19.20±2.33b | 32.27±3.63a | 22.33±1.88a | 21.15±3.94a | |||
| 6 | 32.26±7.09 | 27.64±4.00 | 45.61±11.50 | 37.85±4.32 | 27.33±6.99 | |||
| T-p (g/ml) | 4.3–6.4 | 4.07±0.42 | 1 | 4.93±0.31b | 5.88±0.26a | 4.31±0.26b | 4.83±0.22b | 4.90±0.27b |
| 3 | 3.83±0.29c | 3.97±0.37b,c | 5.19±0.4a | 5.02±0.45a,b | 5.90±0.45a | |||
| 6 | 5.56±0.42a,b | 5.32±0.28a,b | 5.51±0.30a,b | 6.20±0.32a | 4.64±0.22b | |||
| Albumin (g/dl) | 2.5–4.8 | 1.95±0.19 | 1 | 2.73±0.16a,b | 2.99±0.11a | 2.45±0.15b | 2.73±0.17a,b | 2.75±0.18a,b |
| 3 | 2.04±0.18b | 2.14±0.19b | 2.84±0.24a | 2.69±0.26a,b | 3.01±0.26a | |||
| 6 | 2.83±0.20b,c | 2.68±0.15b,c | 3.07±0.16a,b | 3.34±0.18a | 2.52±0.10c | |||
| Globulin (g/dl) | 0.6 | 2.12±0.24 | 1 | 2.23±0.18b | 2.89±0.19a | 1.85±0.15b | 2.17±0.17b | 2.15±0.17b |
| 3 | 1.79±0.12b | 1.82±0.19b | 2.36±0.18a,b | 2.49±0.26a | 2.89±0.26a | |||
| 6 | 2.73±0.23a | 2.64±0.16a,b | 2.44±0.16a,b | 2.86±0.23a | 2.12±0.13b | |||
| T-bili (mg/dl) | 0.3–0.9 | 0.29±0.02 | 1 | 0.77±0.04 | 0.81±0.04 | 0.70±0.04 | 0.77±0.05 | 0.80±0.04 |
| 3 | 0.79±0.09 | 0.66±0.06 | 0.94±0.09 | 0.80±0.08 | 0.76±0.08 | |||
| 6 | 0.30±0.02b | 0.32±0.02a,b | 0.29±0.01b | 0.36±0.02a | 0.29±0.01b | |||
| Mean body weight (g) | – | 37.57±1.02 | 40.34±0.98 | 37.16±1.70 | 36.83±0.97 | 37.83±1.03 | 40.28±0.68 | |
| Liver weight (g) | – | 2.11±0.07 | 2.42±0.11 | 2.23±0.12 | 2.19±0.11 | 2.23±0.08 | 2.29±0.11 | |
| Relative liver weight | – | 5.62±0.08 | 5.99±0.27 | 6.01±0.35 | 5.97±0.24 | 5.92±0.18 | 5.67±0.31 |
Data are presented as mean ± standard deviation (n=8).
Represent comparisons within the same row, where different superscripts indicate significant differences at P<0.05. Referenced ranges of all serum biochemical components listed refer to Clinical chemistry of the laboratory mouse in The Mouse in Biomedical Research. The treatment groups were mice injected with CCl4 twice weekly and subjected to daily gavage-feeding of H2O (vehicle control), silymarin (200 mg/kg BW), a low (20 mg/kg BW), middle (100 mg/kg BW) or high dose (200 mg/kg BW) of LPE. The normal control group mice were not injected with CCl4. Serum samples were collected and analyzed at 1, 3 and 6 weeks. Body and liver weight were measured on the date of euthanasia and relative liver weight was calculated as the proportion of liver weight to body weight. LPE, litchi pericarp extract; CCl4, carbon tetrachloride; BW, body weight; GOT, glutamic oxaloacetate transaminase; GPT, glutamic pyruvic transaminase; TG, triglycerides; T-chol, total cholesterol; T-p, total protein; T-bili, total bilirubin.
Twice-weekly injections of CCl4-induced sub-acute hepatotoxicity in mice. As demonstrated in Table I, serum GOT levels markedly increased, with levels reaching their peak at 3 weeks. GPT levels increased steadily throughout the 6 weeks in all groups. Silymarin (200 mg/kg BW) administration exhibited a moderate reduction in serum GOT and GPT levels at weeks 1 and 3; however, no long-term effects were observed. Mice gavage-fed daily with LPE with doses between 20 and 200 mg/kg BW exhibited significantly (P<0.05) decreased serum GOT and GPT levels compared with those of the silymarin group, and the middle level LPE group, which was fed 100 mg/kg BW and LPE, exhibited the most effective protective outcomes. Moreover, the TG level was significantly reduced (P<0.05) in the H2O vehicle-control groups compared with the TG level of the middle and high level LPE groups. By week 3, the TG level of the silymarin group did not significantly differ from that of the H2O vehicle-control group; however, the TG level of the silymarin group was significantly lower (P<0.05) compared with that of all three LPE groups. Pan et al (1) reported that CCl4-induced steatosis resulted from proteosomal degradation of microsomal TG transfer protein, resulting in dysfunctional TG synthesis. Despite the results of the present study indicating that LPE groups had higher serum TG level, all measured values were within the normal range. Liver dysfunction in fatty acid metabolism as the sign of hepatocyte steatosis was only observed in the liver histology. CCl4 may induce liver cell protein synthesis dysfunction, resulting in reduced serum T-p levels. Although the silymarin group exhibited a significantly higher serum T-P level (P<0.05) than all other groups in the first week, the LPE-treated animals demonstrated normalized serum T-P levels by week 3. Furthermore, calculated results demonstrated that the albumin/globulin (A/G) ratio gradually decreased over the sampling period time in the H2O vehicle-control group, indicating a gradual increase in the severity of CCl4-induced hepatotoxicity (data not shown). In general, low-level LPE (20 mg/kg BW) treatment demonstrated promising results in retaining a relatively unchanged A/G ratio compared with the other LPE groups. Obstructed bile formation is frequently observed in chronic liver diseases along with increasing serum bilirubin level. By week 6, an overall reduction of T-bili was observed in all groups, which may be a result of physiological compensation. Further investigations are required to confirm this.
Liver gross anatomy and histological assessments affected by LPE
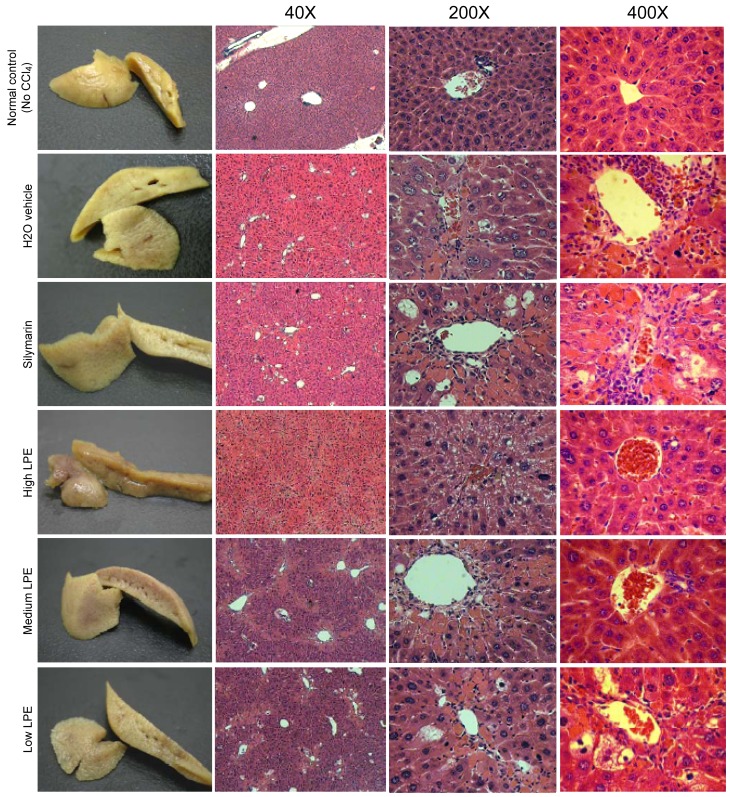
Macroscopic examination of the largest transverse plane section of the primary liver lobule (lobus sinister lateralis hepatis), allowing observation of the central portal veins and bile ducts, was performed. The outer appearance of the entire caudal lobe was examined after formaldehyde fixation, as exhibited in the left column of Fig. 3. Under CCl4 intoxication, high-level LPE treatment preserved the smooth outer capsule, similar to that observed in the liver from normal control mice. The cross section of the primary liver lobes demonstrated no sign of focal hepatic lesion in any group. Evidence of a swollen liver in the sectional views of the silymarin, H2O vehicle-control and low-LPE groups was observed. In addition, the rough segmental outer appearance and fibrotic septa were less apparent in the high-level LPE and normal control groups compared with all other groups. Furthermore, the central venous architectures in the middle of the cross section of the primary liver lobe were readily observed in the H2O vehicle-control and the low-level LPE groups; however, they were not clearly observed in the silymarin, middle or high-level LPE groups. The results of the present study demonstrated that LPE containing rich procyanidins reduced hepatic fibrosis, similar to the effects of procyanidins found in grape seed (25).
Figure 3.
The gross appearance and microscopic histopathology (haematoxylin and eosin staining; magnification, ×40, ×200, and ×400) of liver tissue from the normal animal group (control) and treatment groups of silymarin, H2O, high-dose LPE (200 mg/kg BW/day), middle-dose LPE (100 mg/kg BW/day), and low-dose LPE (20 mg/kg BW/day) in mice with CCl4-induced liver injury. Based on the gross appearance of the liver, the normal and high LPE groups had similar smooth outer capsules compared with those of other groups. Histopathological analysis demonstrated the hepatocyte edges formed pentagonal lobule architecture were retained in all treatments; however, steatosis can be seen in the silymarin, H2O, medium-LPE and low-LPE groups (magnification, ×40). CCl4-intoxication resulted in swollen hepatocytes and impaired lipid synthesis, resulting in the formation of a fatty vacuole (macrosteatosis) in the low-level LPE, silymarin and H2O vehicle-control groups (magnification, ×200). Furthermore, eosinophil infiltration (eosinophilia) along the central portal veins extending to the lobular venous tract and dark-purple/blue stained Kupffer cells infiltration along the central portal vein were evident in the in the low-LPE, silymarin and H2O groups. However, fewer focal observations were noted in the middle and high-LPE samples (magnification, ×400). LPE, litchi pericarp extract; BW, body weight; CCl4, carbon tetracholoride.
Histopathological assessments were performed, as exhibited in microscopic sections in Fig. 3. When examined at lower magnification (x40), fewer lipid droplet holes or spaces were observed in the high-level LPE group compared with all other groups. The portal area of hexagonal architecture, formed by aligning hepatocyte edges, was retained in all treatment groups. White fibrotic connective tissues surrounding the portal vein areas extending along the sinusoid was clearly observed in the silymarin and H2O vehicle-control groups, indicating early liver cirrhosis. Eosinophilia (red colour stain) was observed in all tissue sections of CCl4-induced hepatotoxicity (magnification, ×200). The high-level LPE group exhibited less eosinophilic infiltration than the other groups. Eosinophilic infiltration is an early sign of chronic inflammation and results in chemotaxis of interleukin 5 secreted from Kupffer cells (26). Further investigation is required to determine whether LPE may exhibit immunomodulatory potential.
Additionally, differing severities of steatosis were observed in all groups of CCl4-intoxication, as indicated by the white arrowheads in Fig. 3. Macrosteatosis was clearly observed in the silymarin, low-level LPE and H2O vehicle-control groups (magnification, ×200), whereas steatosis was less evident along the portal vein area of the middle and high-level LPE groups. Furthermore, infiltrated darker, dense nuclei-stained Kupffer cells were clearly observed in the sinusoids of the H2O, silymarin and low-LPE groups. It is understood that Kupffer cells are responsible for multiple mediators in the activation of stellate cells, which induce liver fibrosis (27). The in situ results of the present study have posed additional questions on modulatory properties of LPE in the healing process of Kupffer cells and eosinophils.
LPE upregulates antioxidative enzyme activity in the liver
The antioxidative activity of liver tissues is facilitated by multiple enzymatic systems. The glutathione system governs lipid integrity by scavenging radicals for transfer to nicotinamide adenine dinucleotide phosphate (28). Studies have demonstrated that phenolic compounds are able bind to antioxidant response elements and increase the gene transcription of antioxidant enzymes in the liver (29,30). The present study exhibited that both GSHpx and GSHred activity were rescued by silymarin or LPE administration; however, the vehicle group treated with H2O only demonstrated diminished GSHpx and GSHred activity as a result of CCl4-intoxication (Table II). The high-level LPE group exhibited a similar GSHred activity to that of normal control mice, which was significantly higher (P<0.05) than the silymarin group. Potent free radical scavenging activity of litchi pericarp polyphenols, EC and procyanidins, in the 2, 2-diphenyl-1-picrylhydrazyl assay has been reported (31). In the present study, ex vivo analysis of liver antioxidative enzyme systems demonstrated that LPE dramatically preserved hepatic GSHred activity, supporting other studies on hepatoprotective antioxidants (32,33).
Table II.
LPE rescued CCL4-induced liver injury by preserving the glutathione antioxidative system.
| Groups | GSH peroxidase (nmol/min/ml) | GSH reductase (nmol/min/ml) |
|---|---|---|
| Normal control | 70.19±3.37c | 233.51±25.18a,b |
| H2O vehicle | 61.42±2.98d | 174.04±27.24d |
| Silymarin | 75.95±5.06a,b | 218.86±20.69b,c |
| Low LPE (20 mg/kg BW) | 82.21±1.32a | 180.20±38.41c,d |
| Middle LPE (100 mg/kg BW) | 68.93±3.02c | 193.03±39.18c |
| High LPE (200 mg/kg BW) | 72.73±7.39b,c | 265.24±48.78a |
Data are presented as mean ± standard deviation (n=8).
represent the comparisons among treatments in the same column, with different superscripts indicating significant differences at P<0.05. Mice were injected with CCl4 twice weekly, apart from mice in the normal control group, and subjected to daily gavage-feeding of the same volume of either H2O or silymarin (200 mg/kg BW), or a low level (20 mg/kg BW), middle level (100 mg/kg BW) or high level dose (200 mg/kg BW) of LPE. LPE, litchi pericarp extract; GSH, glutathione; CCl4, carbon tetrachloride; BW, body weight; NC, normal house-keeping control group.
PA2 isolated from LPE promotes scratch wound healing and rescues alcohol-injured BNL CL2 liver cells
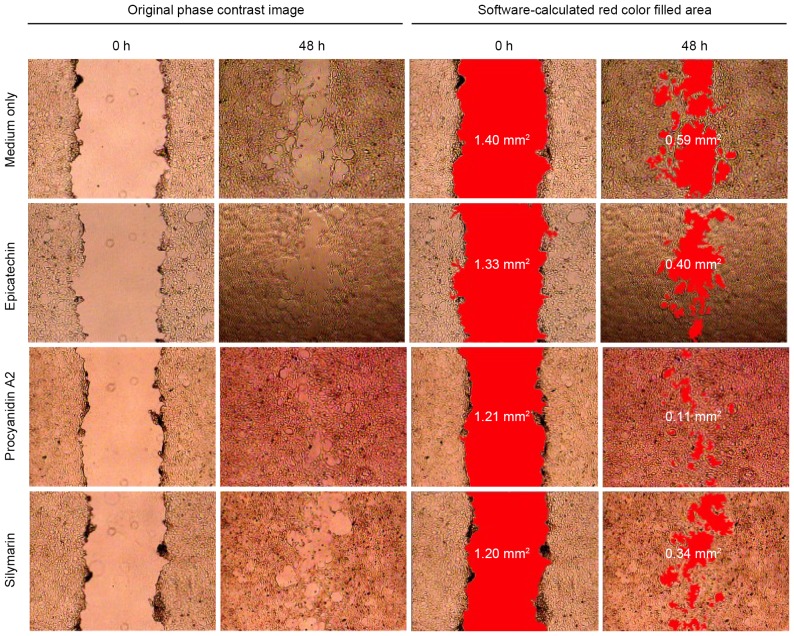
To further elucidate the functional mechanisms of LPE, and its containing EC and PA2, in association to injured-liver recovery, the scratch wound healing assay was utilized to assess their effects on cell migration, growth and proliferation. By quantitative analysis of the recovery of the scratch wound gap, it has been reported that the silibinin in milk thistle fractions has protective and curative effects on liver damage (34). In the present study, the BNL cells in culture medium with EC, PA2 or silymarin exhibited smaller cell-free areas than that of the control group BNL cells in culture medium only (Fig. 4). PA2 treatment resulted in the fastest scratch wound healing.
Figure 4.
BNL CL2 murine embryonic liver cell recovery with the scratch wound healing assay. The scratch area and the gap recovery were quantitatively analyzed (red colour filled in the cell-free gap). The results demonstrate that cells treated with procyanidin A2 had averagely improved wound recovery than those treated with either epicatechin or silymarin. The data represent one of five independent trials.
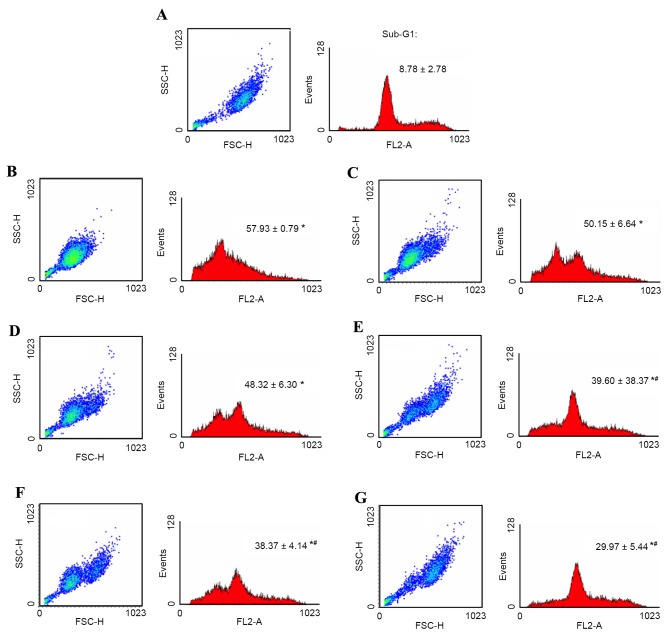
Furthermore, in order to determine whether accelerated wound healing activity by PA2 could result from an increase in cell proliferation, and decreased cell apoptosis, the present study analyzed the cell cycle of PA2-treated alcohol-injured BNL cells in vitro. Alcohol exposure may result in increased mitochondrial production of reactive oxygen species in liver cells (35). In the present study, BNL cells treated with ethanol exhibited free radical damage, resulting in cell oxidative stress. Under these circumstances, cells underwent apoptosis and the cell cycle was halted. BNL cells retracted for detoxification and DNA repairing, as exhibited in cell scatter (SSC vs. FSC) dot plots in a condensed population (Fig. 5). Furthermore the histogram of cell cycle analysis demonstrated a significant dose-dependent reduction of cells in sub-G1 phase with increasing PA2 levels (P<0.05). Additionally, high-level PA2 (50 µg/ml) treatment demonstrated accelerated self-repairing of injured BNL cells by retaining the G1 and cell mitosis phases of the cell cycle.
Figure 5.
Cell integrity, cell cycle and quantified sub-G1 phase of alcohol-injured BNL murine embryonic liver cells treated with PA2. Cells were protected dose-dependently by PA2. All samples were pre-treated with 10% alcohol for 1 h, except for the (A) negative control sample. The alcohol-containing medium was then replaced with fresh medium with either (B) 0, (C) 1, (D) 5, (E) 10, (F) 25 or (G) 50 µg/ml PA2 for an additional 24-h culture. Cell integrity (dot plot; x-axis, cell-size; y-axis, granularity) and accumulated DNA content (histogram plot) of cell cycle were obtained by flow cytometry. PA2 dose-dependently reduced the proportion of sub-G1 phase cells (quantitative data value) and normalized cell-integrity. The sub-G1 values are presented as mean ± standard deviation, n=3. *P<0.05 vs. negative control group; #P<0.05 vs. alcohol-containing medium group; PA2, procyanidin A2.
In conclusion, chemically identified LPE rich in EC and PA2 are understood to be potent antioxidants. LPE gavage-fed to CCl4-intoxicated mice resulted in the recovery of normalized serum biochemical and GSHred levels in the liver. Liver histopathological assessments demonstrated that LPE was more effective than silymarin at reducing liver steatosis and fibrosis. Both EC and PA2 of LPE were able to accelerate cell recovery in injured liver cells. PA2 exhibited superior liver protection compared to EC and silymarin. The findings of the present study support the traditional Chinese herbal medical practice of prescribing litchi as a tonic ingredient for the treatment of liver damage.
Acknowledgements
The present study was supported by internal funding from the National Chiayi University and partial financial support was provided by the Council of Agriculture, Taiwan (grant no. 992101010503-030103Z1).
Glossary
Abbreviations
- BNL
BNL CL2 murine embryonic liver cell line
- LPE
litchi pericarp extract derived from D40M fraction
- A/G
albumin/globulin
- CCl4
carbon tetrachloride
- GOT
glutamic oxaloacetate transaminase
- GPT
glutamic pyruvic transaminase
- T-Chol
total cholesterol
- T-p
total protein
- T-bili
total bilirubin
- TG
triglyceride
- GSHpx
glutathione peroxidise
- GSHred
glutathione reductase
- EC
epicatechin
- PA2
procyanidin A2
References
- 1.Pan X, Hussain FN, Lqbal J, Feuerman MH, Hussain MM. Inhibiting proteasomal degradation of microsomal triglyceride transfer protein prevents CCl4-induced steatosis. J Biol Chem. 2007;282:17078–17089. doi: 10.1074/jbc.M701742200. [DOI] [PubMed] [Google Scholar]
- 2.Pradhan SC, Girish C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J Med Res. 2006;124:491–504. [PubMed] [Google Scholar]
- 3.Yang Z, Zhuang L, Lu Y, Xu Q, Chen X. Effects and tolerance of silymarin (Milk thistle) in chronic hepatitis C virus infection patients: A meta-analysis of randomized controlled trials. Biomed Res Int. 2014;2014:941085. doi: 10.1155/2014/941085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joseph A, Knight A. Criss call for new preventive medicine: Emerging effects of lifestyle on morbidity and mortality. Published by World Scientific Publishing Co. Pte. Ltd.; 2004. pp. 632–634. [Google Scholar]
- 5.Yousef MI, Saad AA, El-Shennawy LK. Protective effect of grape seed procyanidin extract against oxidative stress induced by cisplatin in rats. Food Chem Toxicol. 2009;47:1176–1183. doi: 10.1016/j.fct.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Domitrović R, Jakovac H. Antifibrotic activity of anthocyanidin delphinidin in carbon tetrachloride-induced hepatotoxicity in mice. Toxicology. 2010;272:1–10. doi: 10.1016/j.tox.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Chiu NY, Chang KH. The illustrated medicinal plants of Taiwan. SMC Publishing, Inc. Taipei; Taiwan ROC: 1986. p. 129. [Google Scholar]
- 8.Besra SE, Sharma RM, Gomes A. Antiinflammatory effect of petroleum ether extract of leaves of litchi chinensis Gaertn. (Sapindaceae) J Ethnopharmacol. 1996;54:1–6. doi: 10.1016/0378-8741(96)01440-7. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Yuan S, Wang J, Lin P, Liu G, Lu Y, Zhang J, Wang W, Wei Y. Anticancer activity of litchi fruit pericarp extract against human breast cancer in vitro and in vivo. Toxicol Appl Pharmacol. 2006;215:168–178. doi: 10.1016/j.taap.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Sarni-Manchado P, Le Roux E, Le Guernevé C, Lozano Y, Cheynier V. Phenolic composition of litchi fruit pericarp. J Agric Food Chem. 2000;48:5995–6002. doi: 10.1021/jf000815r. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Cao S, Xu Y, Zhang M, Xiao G, Deng Q, Xie B. Oxidation of (−)-epicatechin is a precursor of litchi pericarp enzymatic browning. Food Chem. 2010;118:508–511. doi: 10.1016/j.foodchem.2009.05.019. [DOI] [Google Scholar]
- 12.Chang CW. Identification and investigation on the hepatoprotective properties of the pericarps extract of Litchi (Chinensis Sonn) Taiwan, ROC: 2009. [Google Scholar]
- 13.Weng BB, Lin WS, Chang JC, Chiou RY. The phytoestrogenic stilbenes, arachidin-1 and resveratrol, modulate regulatory T cell functions responsible for successful aging in aged ICR mice. Int J Mol Med. 2016;38:1895–1904. doi: 10.3892/ijmm.2016.2792. [DOI] [PubMed] [Google Scholar]
- 14.Liang CC, Park AY, Guan JL. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 15.Quimby FW, Luong RH. Clinical chemistry of the laboratory mouse. In: Fox JG, Davisson M, Qunimby F, Barthold S, Newcomer C, Smith A, editors. The Mouse in Biomedical Research. 2nd. Elsevier; 2007. pp. 117–216. [Google Scholar]
- 16.Zhou HC, Lin YM, Li YY, Li M, Wei SD, Chai WM, Tam NF. Antioxidant properties of polymeric proanthocyanidins from fruit stones and pericarps of litchi chinensis Sonn. Food Res Int. 2011;44:613–620. doi: 10.1016/j.foodres.2010.12.016. [DOI] [Google Scholar]
- 17.Hirano T, Gotoh M, Oka K. Natural flavonoids and lignans are potent cytostatic agents against human leukaemic HL-60 cells. Life Sci. 1994;55:1061–1069. doi: 10.1016/0024-3205(94)00641-5. [DOI] [PubMed] [Google Scholar]
- 18.Dixit N, Baboota S, Kohli K, Ahmad S, Ali J. Silymarin: A review of pharmacological aspects and bioavailability enhancement approaches. Indian J Pharmacol. 2007;39:172–179. doi: 10.4103/0253-7613.36534. [DOI] [Google Scholar]
- 19.Kim M, Yang SG, Kim JM, Lee JW, Kim YS, Lee JI. Silymarin suppresses hepatic stellate cell activation in a dietary rat model of non-alcoholic steatohepatitis: Analysis of isolated hepatic stellate cells. Int J Mol Med. 2012;30:473–479. doi: 10.3892/ijmm.2012.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surai PF. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants (Basel) 2015;4:204–247. doi: 10.3390/antiox4010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu L, Xie B, Cao S, Yang E, Xu X, Guo S. A-type procyanidins from litchi chinensis pericarps with antioxidant activity. Food Chem. 2007;105:1446–1451. doi: 10.1016/j.foodchem.2007.05.022. [DOI] [Google Scholar]
- 22.Appeldoorn MM, Vincken JP, Gruppen H, Hollman PC. Procyanidin dimers A1, A2, and B2 are absorbed without conjugation or methylation from the small intestine of rats. J Nutr. 2009;139:1469–1473. doi: 10.3945/jn.109.106765. [DOI] [PubMed] [Google Scholar]
- 23.Ma Q, Xie H, Li S, Zhang R, Zhang M, Wei X. Flavonoids from the pericarps of litchi chinensis. J Agric Food Chem. 2014;62:1073–1078. doi: 10.1021/jf405750p. [DOI] [PubMed] [Google Scholar]
- 24.Plaa GL. Chlorinated methanes and liver injury: Highlights of the past 50 years. Annu Rev Pharmacol Toxicol. 2000;40:42–65. doi: 10.1146/annurev.pharmtox.40.1.43. [DOI] [PubMed] [Google Scholar]
- 25.Dulundu E, Ozel Y, Topaloglu U, Toklu H, Ercan F, Gedik N, Sener G. Grape seed extract reduces oxidative stress and fibrosis in experimental biliary obstruction. J Gastroenterol Hepatol. 2007;22:885–892. doi: 10.1111/j.1440-1746.2007.04875.x. [DOI] [PubMed] [Google Scholar]
- 26.Tsuda K, Maeda T, Tominaga A, Watanabe Y, Miyazaki E, Enzan H, Akisawa N, Iwasaki S, Saibara T, Onishi S. Eosinophil-induced liver injury: An experimental model using IL-5 transgenic mice. J Hepatol. 2001;34:270–277. doi: 10.1016/S0168-8278(00)00027-1. [DOI] [PubMed] [Google Scholar]
- 27.Roberts RA, Ganey PE, Ju C, Kamendulis LM, Rusyn I, Klaunig JE. Role of the kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol Sci. 2007;96:2–15. doi: 10.1093/toxsci/kfl173. [DOI] [PubMed] [Google Scholar]
- 28.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Han X, Shen T, Lou H. Dietary polyphenols and their biological significance. Int J Mol Sci. 2007;8:950–988. doi: 10.3390/i8090950. [DOI] [Google Scholar]
- 30.Martin MA, Serrano AB Grando, Ramos S, Pulido MI, Bravo L, Goya L. Cocoa flavonoids up-regulated antioxidant enzyme activity via the ERK1/2 pathway to protect against oxidative stress-induced apoptosis in HepG2 cells. J Nutri Biochem. 2010;21:196–205. doi: 10.1016/j.jnutbio.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Castellain RC, Gesser M, Tonini F, Schulte RV, Demessiano KZ, Wolff FR, Delle-Monache F, Netz DJ, Cechinel-Filho V, de Freitas RA, et al. Chemical composition, antioxidant and antinociceptive properties of litchi chinensis. J Pharm Pharmacol. 2014;66:1796–1807. doi: 10.1111/jphp.12309. [DOI] [PubMed] [Google Scholar]
- 32.Dai N, Zou Y, Zhu L, Wang HF, Dai MG. Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (CCl4)-induced steatosis and liver injury in rats via CYP2E1 regulation. J Med Food. 2014;17:663–669. doi: 10.1089/jmf.2013.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang BY, Zhang XY, Guan SW, Hua ZC. Protective effect of procyanidin B2 against CCl4-induced acute liver injury in mice. Molecules. 2015;20:12250–12265. doi: 10.3390/molecules200712250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yarrow JC, Perlman ZE, Westwood NJ, Mitchison TJ. A high throughput cell migration assay using scratch wound healing, a comparison of image-based readout method. BMC Biotechnol. 2004;4:21. doi: 10.1186/1472-6750-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey SM, Cunningham CC. Acute and chronic ethanol increase reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology. 1998;28:1318–1326. doi: 10.1002/hep.510280521. [DOI] [PubMed] [Google Scholar]