Abstract
The neuroprotective mechanisms of miR-124 activating phosphoinositide 3-kinase (PI3K)/Akt signaling pathway in ischemic stroke were investigated. The oxygen-glucose deprivation model of nerve cells induced by PC12 cells was established in vitro, then miR-124 mimics or inhibitor was transfected and synthesized by liposome. Cells were divided into the blank control, model, mimics and inhibitor groups, and the apoptotic rate was determined using flow cytometry. Additionally, the expression levels of PI3K, Akt, Bax, Bcl-2, caspase-3 mRNA and protein were tested by quantitative PCR and western blot analysis at 0, 3, 6, 12 and 24 h, respectively. The apoptotic rate at each time-point in the blank control group was not significantly different. The apoptotic rate of the model and inhibitor groups increased over time, whereas the mimics group decreased (P<0.05). The apoptotic rate at each time-point in the mimics group was significantly lower than that of the model and inhibitor groups, and the rate of the inhibitor group was higher than that of the model group (P<0.05). PI3K, Akt and Bcl-2 mRNA and protein expression levels at the different time-points in the mimics group were significantly higher than those of the remaining groups (P<0.05). The expression levels of Bax and caspase-3 mRNA and protein in the inhibitor group were the highest, followed by the model and mimics groups, while that of the blank control group was the lowest (P<0.05). The results suggest that miR-124 participates in the neural protection of ischemic stroke by activating the PI3K/Akt signaling pathway.
Keywords: miR-124, phosphoinositide 3-kinase/Akt signaling pathway, ischemic stroke, oxygen-glucose deprivation model, apoptosis rate, Bax, Bcl-2, caspase-3
Introduction
Apoptosis of ischemic stroke is a principal mechanism that participates in nerve injury and recovery (1). As a cascade reaction, apoptosis is regulated by intracellular genes and extracellular factors (2). miR-124 is abundantly expressed in neuronal cells (3). As the richest miRNAs, miR-124 accounts for 25–48% of total miRNAs in the brain (3). Additionally, it participates in a series of significant physiological activities including neuronal cell cycle regulation, cell differentiation, spinal cord development and the regulation of adult neurogenesis (4).
Previous findings showed that the anoxic environment of cerebral stroke can stimulate the stable upregulation of miR-124 expression, which is reported to be closely related to the phosphoinositide 3-kinase (PI3K)/Akt pathway (5). The PI3K/Akt signaling pathway is an important surviving and anti-apoptotic signal transduction pathway, which plays a significant biological function in cell apoptosis, survival, proliferation, cytoskeleton change and other activities (6). Ischemic brain injury can activate the neural stem cells (NSCs) that gather within the brain when in a resting state, and induce proliferation and differentiate to neurons, which plays a role in repairing the nerve function (7).
The aim of the present study was to examine the neuroprotective mechanisms of miR-124 activating the PI3K/Akt signaling pathway in ischemic stroke. The results showed that miR-124 activation of the PI3K/Akt signaling pathway was involved in the induction and differentiation of NSCs and serves as a potentially significant target for the treatment of ischemic stroke.
Materials and methods
Establishment of oxygen-glucose deprivation (OGD) model of nerve cells
The neuron-like PC12 cell (conserved by Cell Laboratory of The Affiliated Hospital of Zunyi Medical College) with the appropriate growth condition (cell volume 85–90%) was selected and washed 3 times using Dulbecco's modified Eagle's medium (DMEM) without glucose containing 10% fetal bovine serum and 1.0 mmol/l sodium hydrosulfide for culture. The cell culture flask was placed in the anoxic tank and incubated at 37°C for 0, 3, 6, 12 and 24 h.
miR-124 mimics or inhibitor was transfected and synthesized by liposome
The miR-124 expression vector was constructed by BLOCK-iT™ Pol II miR-RNAi expression vector kit with EmGFP, and then the miR-124 expression vector or negative control plasmid was transfected using Lipofectamine 2000 reagent (both from Invitrogen Life Technologies, Carlsbad, CA, USA) into the PC12 cell. The negative control plasmid contained a sequence that can form a similar precursor miRNA. Twenty-four hours after transfection, 3 nnsf blasticidin (Sigma-Aldrich, St. Louis, MO, USA) was added to the culture media for continuous screening for 10 days. The resistant clones were selected using a fluorescence microscope and thereafter, the clones were expanded and re-cultured.
Experimental grouping
Cells were divided into four groups: Blank control group (PC12 cell), model group (PC12 cell OGD model), mimics group and inhibitor group, and the apoptotic rate was determined using flow cytometry (FCM). The expression levels of p-PI3K, p-Akt, Bax, Bcl-2, caspase-3 mRNA and protein were examined using RT-PCR and western blotting, at 0, 3, 6, 12 and 24 h.
FCM technique
The cells were suspended in PBS and centrifuged at 850 × g for 5 min. Annexin V-FITC combination liquid (500 µl) was added into each tube to resuspend the cells, followed by 5 µl Annexin V-FITC and 10 µl PI dye liquor (all from Beijing Zhongshan Biological Technology Co., Ltd., Beijing, China). After gentle mixing, the cells were incubated for 10 min at room temperature in the dark, and the apoptotic rate was determined by FCM (Beckman Coulter, Inc., Brea, CA, USA).
qPCR test
Total RNA was extracted by miRNA isolation kit, and the RNA was removed by DNase I (both from Ambion, Austin, TX, USA) to test concentration, purity and integrity. RNA was reverse transcribed to cDNA by Reverse Transcription kit (Qiagen GmbH, Hilden, Germany). SYBR-Green Fluorescent dye (Sigma-Aldrich) was used for qPCR, performed on LightCycler (Roche Diagnostics GmbH, Mannheim, Germany) apparatus. Primers were designed according to the GenBank website, with internal reference for company, and the RNA was removed using DNase I (Ambion) Technology Co., Ltd. PCR reaction conditions were: Pre-denaturation at 94°C for 5 min, 94°C for 30 sec, 63°C for 30 sec, 72°C for 30 sec, a total of 35 cycles, with overall elongation for 7 min at 72°C. The results were expressed by 2−ΔΔCq method.
Western blotting
Total protein was extracted by RIPA lysate (Biyuntian Biotechnology Institute, Beijing, China), and the purity was determined by BCA reagent (Wuhan Boshide Biological Engineering Co., Ltd., Wuhan, China). Protein (10 µg) was loaded, separated by 12% SDS-PAGE and transferred onto NC membranes (both from R&D Systems, Inc., Minneapolis, MN, USA), with blocking at room temperature for 1 h. The membranes were incubated with rabbit polyclonal PI3K antibody (dilution, 1:500; cat. no. ab182651), rabbit polyclonal Akt antibody (dilution, 1:500; cat. no. ab38449), rabbit monoclonal Bax antibody (dilution, 1:500; cat. no. ab32503), rabbit monoclonal Bcl-2 antibody (dilution, 1:500; cat. no. ab32124), rabbit polyclonal caspase-3 antibody (dilution, 1:500; cat. no. ab13847) and rabbit polyclonal β-actin antibody (dilution, 1:500; cat. no. ab8227) (all purchased from Abcam, Cambridge, MA, USA) overnight at 4°C. The membrane was washed three times using TBST, and secondary goat anti-rabbit (HRP) IgG antibody (dilution: 1/2000; Abcam, Cambridge, MA, USA; Catlog#: ab6721) was added. The membrane was then incubated at room temperature for 1 h, washed with TBST three times, and chemiluminescence (ECL; Biyuntian Biotechnology Institute) was performed. Grayscale bands were observed using an optical microscope (Olympus, Tokyo, Japan).
Statistical analysis
Using SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA) for data analysis, measurement data were expressed as mean ± standard deviation. Single factor analysis of variance (ANOVA) was used to compare indexes among all groups, and repeated measurements ANOVA was applied to compare data in each group. P<0.05 was considered to indicate a statistically significant difference.
Results
Comparison of apoptotic rate
The apoptotic rate at each time-point in the blank control group was not significantly different. The apoptotic rate of the model and inhibitor groups increased over time, whereas that of the mimics group decreased at each time-point, and the difference in each group was statistically significant (P<0.05). The apoptotic rate at each time-point in the mimics group was significantly lower than that of the model and inhibitor groups, while the inhibitor group was higher than the model group, and the difference was statistically significant (P<0.05) (Table I).
Table I.
Comparison of apoptotic rate (%).
| Groups | 0 | 3 h | 6 h | 12 h | 24 h | F-value | P-value |
|---|---|---|---|---|---|---|---|
| Blank | 0.06±0.01 | 0.08±0.01 | 0.09±0.02 | 0.10±0.02 | 0.08±0.02 | 0.635 | 0.427 |
| Model | 32.6±10.3 | 43.6±15.2a | 49.7±18.4a | 56.3±19.3a | 64.8±20.3a | 9.468 | <0.001 |
| Mimics | 15.3±5.2 | 12.6±5.5b | 10.5±5.6b | 9.4±4.3b | 8.6±4.0b | 8.352 | <0.001 |
| Inhibitor | 40.5±12.6 | 45.9±16.2a | 54.2±15.9a | 59.7±21.2a | 66.5±24.6a | 9.123 | <0.001 |
| F-value | 12.306 | 14.524 | 16.529 | 20.321 | 25.426 | ||
| P-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Indicates increase with time in comparison to 0 h
indicates decrease with time in comparison to 0 h.
Comparison of expression levels of mRNA in PI3K/Akt signaling pathway
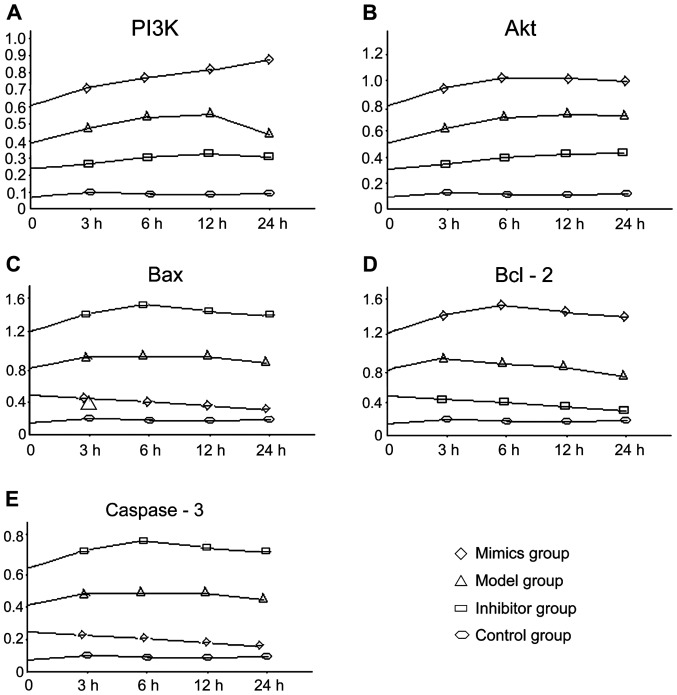
The expression levels of PI3K, Akt and Bcl-2 mRNA at each time-point in the mimics group were significantly higher than those of the other groups, followed by the model group and inhibitor group, and the blank control group, which had the lowest expression levels. Differences among the groups were statistically significant (P<0.05). The expression levels of Bax and caspase-3 mRNA in the inhibitor group were the highest overall, followed by the model and the mimics groups, with the blank control group having the lowest expression levels. Differences among the groups were statistically significant (P<0.05). The changing trend at different time-points in each group is shown in Fig. 1.
Figure 1.
Comparison of mRNA expression levels using RT-PCR in PI3K/Akt signaling pathway. PI3K, phosphoinositide 3-kinase.
Comparison of the expression level of proteins in the PI3K/Akt signaling pathway
At 12 h, a comparison of expression level of protein in each group and among all groups coincided with the changing trend of mRNA (Fig. 2).
Figure 2.

Comparison of protein expression levels using western blotting in PI3K/Akt signaling pathway. PI3K, phosphoinositide 3-kinase.
Discussion
The mutual intervention and regulation at different levels have led to identification of numerous transduction and regulation pathways of apoptosis. PI3K is a lipid kinase and PI3K family is related to cell proliferation, anti-apoptosis, cell migration, membrane vesicle transport, cell carcinoma transformation and other processes (8). Protein kinase B (PKB), also known as Akt, is a serine/threonine kinase. Activated Akt can regulate cell function by phosphorylating various enzymes, kinases and transcription factors. Activation of Akt phosphorylation mainly depends on the PI3K activation, through the phosphorylation and inactivation of its downstream substrate. Activated Akt can promote cell survival via various avenues. After brain injury, multiple neurotrophic factors play a protective effect on the brain by activating the PI3K/Akt signaling pathway (9). Another study indicated that the Akt expression in the brain tissue of the adult rabbit was a trace, while the expression of p-Akt increased 1 h after traumatic brain injury, and the immune response was enhanced at the edge of the lesion (10). The difference of expression of p-Akt area in the whole brain may lead to a different extent of cell injury, suggesting that the PI3K/Akt signaling pathway participated in the regulation of the pathological process of traumatic brain injury, while the expression amount of p-Akt was related to the extent of brain injury (11). The study found that ischemic brain tissue can express erythropoietin and plays a protective role in the brain by activating ERK1/2 and Akt signaling pathways (12).
There are various expression types in the nervous system, the specific miRNAs in brain tissue contains miR-9, miR-124a, miR-124b, miR-135, miR-153, miR-183, miR-219 (13). In their study, Lagos-Quintana et al (14) found a specific expression of miR-101, miR-124, miR-127, miR-128, miR-131 and miR-132 in mouse brain tissue, with miR-124 expression being the most obvious and most highly expressed in the cerebral cortex and cerebellum (13). These miRNAs may be closely related to the differentiation of developmental neuron of brain tissue and the performance of advanced neural function (15), such as miR-124 overexpression of P19 cells of mouse which in a state of differentiation can promote axonal growth; blocking miR-124 expression could decrease the expression levels of acetylated-tubulin and affect axon growth. The expression profile of miRNAs in the tissues and plasma of patients with ischemic stroke was significant. Dharap et al (16) tested the expression of 238 miRNAs in the brain tissues of rats for 3 h to 3 days after the model of focal cerebral ischemia reperfusion injury was established. They found that the expression amount of 8 miRNAs increased significantly and the expression of 12 miRNAs evidently decreased. The change of miRNA expression profile after cerebral ischemia was specific, and the change of miRNAs in plasma was consistent with that in tissue (17), which indicated that miRNAs are potential markers for diagnosis of ischemic stroke.
Based on the results of the present study, we can conclude that the apoptotic rate in the model and inhibitor groups increased over time, although the mimics group decreased over time, and the difference in each group was statistically significant. The apoptotic rate at each time-point in the mimics group was significantly lower than that of the model and inhibitor groups, while the inhibitor group was higher than the model group, and the difference was statistically significant. The expression levels of PI3K, Akt and Bcl-2 mRNA and protein at each different time-point in the mimics group were significantly higher than those of other groups, followed by the model and inhibitor groups, with the blank control group was the lowest, and the difference among the groups was statistically significant. The expression levels of Bax and caspase-3 mRNA and protein in the inhibitor group were the highest versus the model and mimics groups, while the blank control group was the lowest, and the difference among the groups was statistically significant. The results of the present study revealed that miR-124 participated in the neural protection of ischemic stroke by activating the PI3K/Akt signaling pathway, while the intervention of miR-124 or PI3K/Akt signaling pathway is a significant target for the treatment of ischemic stroke and repair of neural function.
Acknowledgements
The present study was supported by the Zunyi Science and Technology Plan, Compliance with Zunyi City Branch (2014) 78. We thank all partners and staff who helped us in the process of this study.
References
- 1.Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 2.Bergmann A. Autophagy and cell death: no longer at odds. Cell. 2007;131:1032–1034. doi: 10.1016/j.cell.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149:515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao FB. Posttranscriptional control of neuronal development by microRNA networks. Trends Neurosci. 2008;31:20–26. doi: 10.1016/j.tins.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu XS, Chopp M, Zhang RL, Tao T, Wang XL, Kassis H, Hozeska-Solgot A, Zhang L, Chen C, Zhang ZG. MicroRNA profiling in subventricular zone after stroke: miR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS One. 2011;6:e23461. doi: 10.1371/journal.pone.0023461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao S, Fu J, Liu X, Wang T, Zhang J, Zhao Y. Activation of Akt/GSK-3beta/beta-catenin signaling pathway is involved in survival of neurons after traumatic brain injury in rats. Neurol Res. 2012;34:400–407. doi: 10.1179/1743132812Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 7.Richardson RM, Singh A, Sun D, Fillmore HL, Dietrich DW, III, Bullock MR. Stem cell biology in traumatic brain injury: effects of injury and strategies for repair. J Neurosurg. 2010;112:1125–1138. doi: 10.3171/2009.4.JNS081087. [DOI] [PubMed] [Google Scholar]
- 8.Gu X, Meng S, Liu S, Jia C, Fang Y, Li S, Fu C, Song Q, Lin L, Wang X. miR-124 represses ROCK1 expression to promote neurite elongation through activation of the PI3K/Akt signal pathway. J Mol Neurosci. 2014;52:156–165. doi: 10.1007/s12031-013-0190-6. [DOI] [PubMed] [Google Scholar]
- 9.Lang Q, Ling C. miR-124 suppresses cell proliferation in hepatocellular carcinoma by targeting PIK3CA. Biochem Biophys Res Commun. 2012;426:247–252. doi: 10.1016/j.bbrc.2012.08.075. [DOI] [PubMed] [Google Scholar]
- 10.Wang ZG, Cheng Y, Yu XC, Ye LB, Xia QH, Johnson NR, Wei X, Chen DQ, Cao G, Fu XB, et al. bFGF protects against blood-brain barrier damage through junction protein regulation via PI3K-Akt-Rac1 pathway following traumatic brain injury. Mol Neurobiol. 2015 Dec 21; doi: 10.1007/s12035-015-9583-6. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang L, Ding K, Wang H, Wu Y, Xu J. Traumatic brain injury-induced neuronal apoptosis is reduced through modulation of PI3K and autophagy pathways in mouse by FTY720. Cell Mol Neurobiol. 2016;36:131–142. doi: 10.1007/s10571-015-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilic E, Kilic U, Soliz J, Bassetti CL, Gassmann M, Hermann DM. Brain-derived erythropoietin protects from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt pathways. FASEB J. 2005;19:2026–2028. doi: 10.1096/fj.05-3941fje. [DOI] [PubMed] [Google Scholar]
- 13.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci. 2009;12:399–408. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/S0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 15.Åkerblom M, Sachdeva R, Barde I, Verp S, Gentner B, Trono D, Jakobsson J. MicroRNA-124 is a subventricular zone neuronal fate determinant. J Neurosci. 2012;32:8879–8889. doi: 10.1523/JNEUROSCI.0558-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab. 2009;29:675–687. doi: 10.1038/jcbfm.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss JB, Eisenhardt SU, Stark GB, Bode C, Moser M, Grundmann S. MicroRNAs in ischemia-reperfusion injury. Am J Cardiovasc Dis. 2012;2:237–247. [PMC free article] [PubMed] [Google Scholar]